Abstract
Aim: This study explored the role of glycemic control on cardiac autonomic function, measured by heart rate variability (HRV), in youth with type 1 diabetes.
Patients and Methods: A retrospective cohort of 345 youth with type 1 diabetes (mean age, 18.5 years; duration, 10 years) participating in the SEARCH for Diabetes in Youth study were enrolled in the ancillary SEARCH Cardiovascular Disease (CVD) study. Anthropometric, metabolic, and HRV parameters were collected at the current research visit. Glycemic control over time was assessed by the mean glycated hemoglobin (A1c) levels collected over the past 6 years. Multiple linear regression analysis assessed the association between A1c over time and HRV parameters, independent of demographic and CVD risk factors. Participants were categorized into four glycemic control categories based on their mean A1c over time: Group 1, optimal (mean A1c, ≤7.4%); Group 2 (mean A1c, 7.5–8.4%); Group 3 (mean A1c, 8.5–9.4%), and Group 4, poor (mean A1c, ≥9.5%), and a linear trend was explored across these categories.
Results: For every 1% increase in the average A1c over 6 years there was a 5% decrease in the SD of the normal RR interval (SDNN) (P=0.02) and 7% decrease in the root mean square successive difference of the RR interval (RMSSD) (P=0.02), independent of demographic and traditional CVD risk factors. A dose–response relationship between worsening glucose control categories and measures of overall reduced HRV was found.
Conclusions: Chronic hyperglycemia is the main determinant of early cardiac autonomic dysfunction, manifested as reduced overall HRV and parasympathetic loss, among youth with type 1 diabetes.
Introduction
Cardiovascular autonomic neuropathy (CAN), one of the chronic complications of diabetes, carries a considerable risk of cardiac mortality and morbidity.1 Several studies among adults with type 1 diabetes (T1D) have demonstrated that poor glycemic control and increased duration of diabetes are independently related to the incidence of microvascular and macrovascular complications of diabetes.2–4 The Diabetes Control and Complications Trial (DCCT) has reported persistent benefits of tight glycemic control in reducing the incidence of CAN by 31% in the intensive treatment compared with the conventional treatment group.5 Previous studies have shown that strict glycemic control slows the deterioration of advanced CAN but fails to promote reversibility.5–7 However, in T1D patients with early CAN, improvement in the autonomic nerve function occurring as early as 1 year after the institution of strict glycemic control has been observed among 79 adolescents with T1D,8 suggesting that the benefits of glucose control on cardiac autonomic function among patients with T1D may be even greater during the early subclinical phase of CAN. Although there are several studies prospectively following adults with T1D to assess the long-term impact of glycemic control on the progression of CAN,5,7 few have systematically examined the impact of glycemic control on markers of subclinical cardiac autonomic dysfunction among youth with T1D.9,10
The aim of our study was to assess the impact of long-term glycemic control on various measures of cardiac autonomic function among youth with T1D, using data from the retrospective cohort component of the SEARCH Cardiovascular Disease (CVD) study.
Patients and Methods
SEARCH CVD is an ancillary study to the SEARCH for Diabetes in Youth, conducted in Colorado and Ohio. SEARCH is a multicenter study that conducts population-based ascertainment of nongestational cases of physician-diagnosed diabetes in youth <20 years old at diagnosis.11 In total, 406 SEARCH participants from Colorado and Ohio with physician-diagnosed T1D, a baseline SEARCH research visit completed between 2004 and 2005, and duration of diabetes of at least 5 years were enrolled in SEARCH CVD study. All SEARCH CVD participants had a baseline SEARCH visit at which data on demographic, anthropometric, and metabolic factors, including glycated hemoglobin (A1c), were collected, whereas 35% of them had additional SEARCH follow-up visits, on average about 12, 24, and 60 months following the baseline visit. All SEARCH CVD participants had a separate research visit between 2009 and 2011 during which assessment of cardiac autonomic function was conducted. The study was reviewed and approved by the local institutional review boards that had jurisdiction over the local study population, and all participants provided signed informed consent or assent.
Anthropometric and metabolic measurements
SEARCH CVD participants were invited to attend an outpatient research visit after an 8-h overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit until after the blood draw was complete. All participants were asked to refrain from any strenuous exercise, smoking, or consumption of any caffeinated drinks 12 h prior to the visit. Race/ethnicity was self-reported, and participants were categorized into non-Hispanic white and other racial/ethnic group (including Hispanic, African-American, and Asian/Pacific Islander racial/ethnic groups). Participants completed standardized questionnaires including medical history, medication inventory, smoking status, daily insulin dose, and family history of CVD. Current cigarette smoking was defined as having smoked at least one cigarette on one or more of the 30 days preceding the survey.12 Youth who had never smoked a whole cigarette were considered nonsmokers. Individuals who had tried smoking or smoked regularly (at least one cigarette every day for 30 days) but were not current smokers were considered former smokers. Height was measured in centimeters using a stadiometer, and weight was measured in kilograms using a standardized weighing machine. Body mass index was calculated as weight (in kg) divided by the square of height (in m), and age- and sex-specific body mass index z-scores were derived based on the Centers for Disease Control and Prevention national standards.13 Waist circumference was measured to the nearest 0.1 cm with the National Health and Nutrition Examination Survey protocol.14 Resting systolic and diastolic blood pressures were measured three times, using an aneroid sphygmomanometer, while the subjects were seated for at least 5 min, and the average of the three measurements was taken.
Laboratory samples were obtained under conditions of metabolic stability, defined as no episode of diabetic ketoacidosis during the previous month. A fasting blood draw was done for the assessment of levels of the metabolic parameters: A1c, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, and triglycerides. High-performance liquid chromatography (TOSOH Bioscience, Inc., San Francisco, CA) was used to measure the A1c level. Measurements of levels of triglycerides and high-density lipoprotein-cholesterol were performed enzymatically on a Roche Modular P chemistry autoanalyzer (Roche Diagnostics Inc., Indianapolis, IN). Low-density lipoprotein-cholesterol was calculated by the Friedewald equation for individuals with a triglyceride concentration of <400 mg/dL and by the beta quantification procedure for those with triglyceride levels of ≥400 mg/dL. Urinary albumin and creatinine levels were measured from overnight timed urine samples. The albumin level was measured by using Siemens reagent on a BNII nephelometer (Siemens Healthcare Diagnostics Inc., Newark, DE), and the creatinine level was measured using the Creatinine Plus enzymatic method with Roche reagent on a Roche Modular P chemistry autoanalyzer. The values thus obtained were used to compute albumin/creatinine ratio. A value of >3.5 mg/mmol was used to define an elevated albumin/creatinine ratio.
Assessment of cardiac autonomic function
The assessment of cardiac autonomic function was performed in the fasting state to prevent the possible influence of a surge in acute ambient glucose levels after a meal on the cardiac function. Heart rate variability (HRV) was measured in the morning between 7 and 11 a.m. in a room with a stable room temperature, with the participant lying in the resting supine position for 10 min, using the SphygmoCor® device (AtCor Medical, Sydney, Australia). The device takes into account the normal heart beats, ignoring the ectopic beats, to derive the statistical parameters of the normal R-R intervals (NN intervals) of the electrocardiogram and estimates several time and frequency domain HRV indices.15 The time domain indices of HRV used in these analyses were the SD of the NN intervals (SDNN) and the root mean square differences of successive NN intervals (RMSSD). SDNN is a measure of overall HRV, so lower SDNN levels indicate reduced overall HRV. Parasympathetic loss is quantified by a reduction in the RMSSD. The frequency domain parameter of HRV that was measured was the low frequency (LF):high frequency (HF) ratio, which represents the balance of sympathetic/parasympathetic activity. Thus an increased LF:HF ratio denotes sympathetic override. The HRV data were edited, and any records with extreme outlying values were visually inspected to ensure that HRV values were not influenced by artifacts in the electrocardiogram tracings.
Statistical analyses
Statistical analyses were performed using SAS for Windows software (version 9.2; SAS Institute, Cary, NC). SDNN, RMSSD, triglycerides, and albumin/creatinine ratio were log-transformed to meet model assumptions (e.g., homogeneity of variance). Characteristics of study participants were expressed as mean and SD for continuous variables and as percentages for categorical variables. Because all SEARCH CVD participants had at least two A1c measures and some had at least one additional measure (135 had three measures and 91 had all four measures of A1c) over a time period of approximately 6 years, we created an aggregated average measure of A1c over time. We used multiple linear regression to test for the independent association between this average measure of A1c over time and each of the HRV measures: SDNN, RMSSD, and LF:HF ratio. Models were adjusted for demographic characteristics (age, sex, race/ethnicity, and diabetes duration) and traditional CVD risk factors (body mass index z-score, systolic blood pressure, diastolic blood pressure, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, triglycerides, albumin/creatinine ratio, and smoking). In addition, participants were categorized into four glycemic control categories based on their average A1c over time: Group 1, optimal glucose control (mean A1c, ≤7.4%; n=66); Group 2, suboptimal control (mean A1c, 7.5–8.4%; n=120); Group 3, intermediate control (mean A1c, 8.5–9.4%; n=74), and Group 4, poor control (mean A1c, ≥9.5%; n=85). PROC GLM was used to compute mean levels of SDNN, RMSSD, and LF:HF ratio, adjusted for age, sex, race/ethnicity, and diabetes duration, corresponding to each glucose control group, and an orthogonal polynomial linear trend test was used to explore whether a linear trend exists across these categories.
Results
Table 1 shows characteristics of study participants at the SEARCH CVD research visit. In total, 345 youth with T1D who had complete HRV data were included in the analyses: 46% were females, and 87% were non-Hispanic white. The mean age and duration were 18.5±3.3 and 10±3.5 years, respectively, and the current A1c level was 8.9%. The lipid and blood pressure levels were within normal ranges for their age.16 The prevalence of an elevated albumin/creatinine ratio was 7%. The proportion of former and current smokers was 23% and 20%, respectively. Based on our measure of average glucose control, 85 participants (25%) had been in poor glycemic control (A1c, ≥9.5%) over the past 6 years.
Table 1.
Characteristics of Study Participants
| Variable | Value at current visit |
|---|---|
| Number of patients |
345 |
| Age (years) |
18.5 (3.3) |
| Duration of diabetes (years) |
10.0 (3.5) |
| Female sex |
158 (46%) |
| Race/ethnicity | |
| Non-Hispanic white |
300 (87%) |
| Other |
45 (13%) |
| A1c | |
| IFCC units (mmol/L) |
74 (19.6) |
| NGSP units (%) |
8.9 (1.8) |
| BMI z-score |
0.5 (0.9) |
| Waist circumference (cm) |
84.9 (12.8) |
| LDL-c (mmol/L) |
2.5 (0.7) |
| HDL-c (mmol/L) |
1.3 (0.3) |
| Triglycerides (mmol/dL) |
1.0 (0.6) |
| Blood pressure (mm Hg) | |
| Systolic |
110.7 (9.8) |
| Diastolic |
70.1 (8.7) |
| Elevated ACR >3.5 mg/mmol |
23 (7%) |
| Smokers | |
| Never |
197 (57%) |
| Former |
79 (23%) |
| Current |
69 (20%) |
| SDNN (ms) |
70.4 (35.4) |
| RMSSD (ms) |
64.2 (41.5) |
| LF:HF ratio | 1.3 (1.3) |
Data are mean (SD) values or number (%) as indicated.
A1c, glycated hemoglobin; ACR, albumin/creatinine ratio; BMI, body mass index; HDL-c, high-density lipoprotein-cholesterol; IFCC, International Federation of Clinical Chemistry; LDL-c, low-density lipoprotein-cholesterol; LF:HF ratio, low frequency:high frequency ratio; NGSP, National Glycohemoglobin Standardization Program; RMSSD, root mean square difference of successive RR intervals; SDNN, SD of normal RR intervals.
Table 2 lists the demographic and clinical characteristics of the youth stratified by their mean A1c status. Except for age, A1c, SDNN, RMSSD, triglycerides, and systolic blood pressure, no significant differences were observed among the groups.
Table 2.
Characteristics of Study Participants by Their Glycemic Status
| Group 1 (mean A1c ≤7.4%) | Group 2 (mean A1c 7.5–8.4%) | Group 3 (mean A1c 8.5–9.4%) | Group 4 (mean A1c ≥9.5%) | P | |
|---|---|---|---|---|---|
| Number of patients |
66 |
120 |
74 |
85 |
NA |
| Age (years) |
19.4 (3.3) |
18.1 (3.0) |
18.2 (3.4) |
19.2 (3.1) |
0.02 |
| Duration of diabetes (years) |
9.5 (3.7) |
9.5 (3.0) |
10.7 (3.9) |
10.2 (3.7) |
0.1 |
| Female sex |
28 (42%) |
54 (45%) |
37 (50%) |
48 (56%) |
0.1 |
| Race/ethnicity |
|
|
|
|
0.18 |
| Non-Hispanic white |
58 (88%) |
106 (88%) |
61 (82%) |
54 (63%) |
|
| Other |
8 (12%) |
14 (12%) |
13 (18%) |
31 (37%) |
|
| A1c (%) |
6.6 (0.7) |
7.6 (0.8) |
8.3 (1.0) |
9.2 (1.8) |
<0.0001 |
| BMI z-score |
0.49 (0.8) |
0.40 (0.9) |
0.43 (0.93) |
0.46 (1.08) |
0.95 |
| Waist circumference (cm) |
75.0 (12.0) |
70.6 (11.6) |
71.3 (15.0) |
72.9 (12.8) |
0.15 |
| LDL-c (mmol/L) |
2.3 (0.5) |
2.5 (0.6) |
2.4 (0.5) |
2.5 (0.5) |
0.37 |
| HDL-c (mmol/L) |
1.3 (0.3) |
1.4 (0.3) |
1.5 (0.3) |
1.4 (0.3) |
0.10 |
| Triglycerides (mmol/dL) |
0.6 (0.2) |
0.7(0.3) |
0.6 (0.2) |
0.8 (0.5) |
0.0006 |
| Blood pressure (mm Hg) | |||||
| Systolic |
104.1 (10.4) |
102.4 (9.6) |
99.6 (9.9) |
103.4 (9.7) |
0.052 |
| Diastolic |
65.1 (9.7) |
65.1 (8.5) |
65.4 (7.6) |
68.0 (8.9) |
0.12 |
| Elevated ACR >3.5 mg/mmol |
1 (%) |
7 (7%) |
3 (6%) |
8 (14%) |
0.1 |
| Smokers |
|
|
|
|
0.09 |
| Never |
39 (80%) |
78 (95%) |
38 (81%) |
50 (85%) |
|
| Former |
7 (14%) |
3 (4%) |
8 (17%) |
8 (14%) |
|
| Current |
3 (6%) |
1 (1%) |
1 (2%) |
1 (1%) |
|
| SDNN (ms) |
77.9 (33.0) |
75.9 (36.5) |
67.6 (7.6) |
55.14 (25.2) |
0.0002 |
| RMSSD (ms) |
70.9 (33.0) |
70.7 (43.7) |
58.8 (44.5) |
49.4 (29.9) |
0.0018 |
| LF:HF ratio | 1.1 (0.9) | 1.2 (1.1) | 1.6 (2.0) | 1.2 (1.1) | 0.17 |
Data are mean (SD) values or number (%) as indicated.
A1c, glycated hemoglobin; ACR, albumin/creatinine ratio; BMI, body mass index; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; LF:HF ratio, low frequency:high frequency ratio; NA, not applicable; RMSSD, root mean square difference of successive RR intervals; SDNN, SD of normal RR intervals.
Table 3 shows the associations between HRV parameters and continuous mean A1c over time adjusted for demographic and current CVD risk factors. For every 1% increase in the mean A1c over time, there was a 5% reduction in the SDNN (P=0.02) and a 7% reduction in the RMSSD (P=0.02), independent of demographic characteristics and traditional CVD risk factors. However, the association between mean A1c and LF:HF ratio was not significant (P=0.9). Older age was a significant independent predictor of lower SDNN and RMSSD (P=0.01 and 0.001, respectively). For every 1 year increase in age, there was a 1% reduction in SDNN and a 5% reduction in RMSSD. Current triglyceride levels were also significantly associated with lower SDNN (P=0.0004) and lower RMSSD (P=0.0007). Duration of diabetes was not independently related to any of the HRV parameters. Moreover, we did not find any significant interaction between average glycemic control over time and diabetes duration, suggesting that, in our population, diabetes duration does not influence the relationship between glycemic control and HRV.
Table 3.
The Association Between Average Glycated Hemoglobin over Time and Heart Rate Variability Parameters in Youth with Type 1 Diabetes, Adjusted for Demographics and Traditional Cardiovascular Disease Risk Factors
| |
SDNN |
RMSSD |
LF:HF ratio |
|||
|---|---|---|---|---|---|---|
| Covariate | β (SEM) | P | β (SEM) | P | β (SEM) | P |
| Average A1c over time |
−0.05 (0.02) |
0.02 |
−0.07 (0.03) |
0.02 |
0.003 (0.07) |
0.9 |
| Age (per 1 year) |
−0.01 (0.01) |
0.01 |
−0.05 (0.1) |
0.001 |
0.04 (0.02) |
0.07 |
| Sex (male vs. female) |
0.16 (0.06) |
0.01 |
0.15 (0.6) |
0.7 |
−0.31 (0.1) |
0.06 |
| Race (NHW vs. others) |
0.04 (0.09) |
0.6 |
0.05 (0.1) |
0.4 |
0.2 (0.2) |
0.2 |
| Duration (per 1 year) |
−0.009 (0.008) |
0.2 |
−0.8 (0.3) |
0.2 |
0.01 (0.02) |
0.4 |
| BMI z-score (per unit) |
0.02 (0.03) |
0.4 |
0.008 (0.04) |
0.8 |
0.07 (0.09) |
0.4 |
| LDL-c (per 1 mg/dL) |
−0.03 (0.04) |
0.3 |
−0.03 (0.05) |
0.4 |
−0.002 (0.002) |
0.4 |
| HDL-c (per 1 mg/dL) |
−0.02 (0.09) |
0.9 |
−0.05 (0.1) |
0.6 |
0.0002 (0.006) |
0.9 |
| TG (per 1 unit) |
−0.18 (0.05) |
0.0004 |
−0.23 (0.06) |
0.0007 |
0.17 (0.2) |
0.4 |
| SBP (per 1 mm Hg) |
−0.0004 (0.003) |
0.9 |
0.001 (0.004) |
0.8 |
−0.002 (0.009) |
0.8 |
| DBP (per 1 mm Hg) |
−0.004 (0.003) |
0.9 |
−0.005 (0.005) |
0.3 |
0.006 (0.009) |
0.4 |
| ACR (per unit) |
−0.04 (0.03) |
0.1 |
−0.03 (0.04) |
0.4 |
0.04 (0.09) |
0.5 |
| Smoking (yes/no) | 0.01 (0.04) | 0.6 | 0.009 (0.05) | 0.8 | 0.14 (0.1) | 0.1 |
Significant associations depicted in bold.
A1c, glycated hemoglobin; ACR, albumin/creatinine ratio; BMI, body mass index; DBP, diastolic blood pressure; HDL-c, high-density lipoprotein-cholesterol; LDL-c, low-density lipoprotein-cholesterol; LF:HF ratio, low frequency:high frequency ratio; NHW, non-Hispanic white; RMSSD, root mean square difference of successive RR intervals; SBP, systolic blood pressure; SDNN, SD of normal RR intervals; TG, triglycerides.
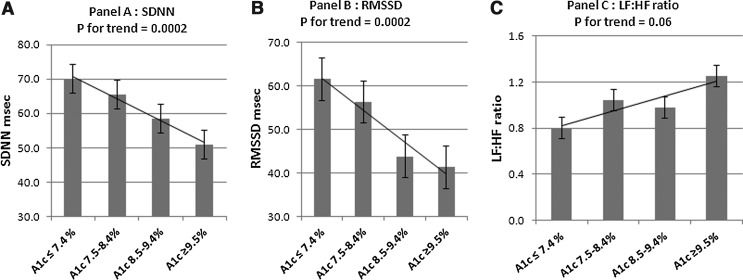
Figure 1 shows the mean SDNN, RMSSD, and LF:HF ratio adjusted for age, sex, race/ethnicity, and diabetes duration across the four categories of average glucose control over time. A significant inverse trend was evident for SDNN, a measure of overall HRV, with the lowest SDNN found among youth with worst glycemic control over time (mean A1c over time, ≥9.5%) compared with those with optimal glycemic control (mean A1c over time, <7.4%) (P for trend=0.0002). Similarly, RMSSD, a surrogate for parasympathetic dysfunction, was lowest in the group with the worst glycemic control and highest among the group with optimal glycemic control (P for trend=0.0002). A marginally significant positive trend (P=0.06) was evident for the LF:HF ratio, which was highest among those with A1c ≥9.5% and lowest among those with A1c <7.4%, suggesting that sympathovagal balance may also be altered with loss of parasympathetic tone and surge of sympathetic tone.
FIG. 1.
Heart rate variability parameters by average glucose control category over time adjusted for age, sex, race/ethnicity, and diabetes duration: (A) SD of normal RR intervals (SDNN), (B) root mean square difference of successive RR intervals (RMSSD), and (C) low frequency:high frequency (LF:HF) ratio. A1c, glycated hemoglobin.
Discussion
This study confirms that poor glycemic control over a time period of approximately 6 years is an important determinant of early autonomic dysfunction among contemporary U.S. youth with T1D. This relationship is independent of traditional CVD risk factors and does not vary with diabetes duration. Increasing age and higher triglyceride levels are other independent correlates of lower HRV among youth with T1D. Our results highlight the crucial role of optimal glycemic control in the functioning and regulation of cardiac autonomic function in youth with T1D.
The DCCT17 and the Stockholm Diabetes Intervention Study18 have conclusively documented that tight glycemic control, as a consequence of intensive insulin therapy, can slow down the development and progression of abnormal autonomic function among adults with T1D. In the DCCT, adults with T1D in the intensive therapy arm had a substantial reduction in incident CAN (odds ratio=0.69; 95% confidence interval 0.51–0.93) and incident abnormal R-R variation (odds ratio=0.70; 95% confidence interval 0.51–0.96), which was still apparent 14 years after the trial, suggesting that the benefits of strict glycemic control may be sustained for a long period of time.17 Similar findings were reported by Larsen et al.19 in a smaller cohort of 39 adults with T1D on intensive insulin treatment followed for 18 years in which they found that cardiac autonomic function was preserved among those with a mean A1c <8.4% over 18 years and impaired in the group with mean A1c ≥8.4%. In the European Diabetes Prospective Complications (EURODIAB) study, Witte et al.20 prospectively followed 1,172 adults with T1D for approximately 7 years and found that the incidence of CAN was strongly related to rate of deterioration of glycemic control during follow-up. This prospective study also reported that, in addition to glycemic control, the incidence of neuropathy was associated with other potentially modifiable CVD risk factors, including raised triglyceride levels, obesity, smoking, and hypertension. Our findings of an association between reduced HRV and elevated triglyceride levels are in concordance with those reported by EURODIAB, albeit in a younger cohort with a shorter duration of diabetes and a higher mean A1c. Although smoking has consistently emerged as a risk factor for CAN among adults,20 it was not associated with reduced HRV in our study.
Few studies have examined the impact of glycemic control and duration of diabetes on cardiac autonomic dysfunction in pediatric populations with T1D.21,22 Young et al.21 reported that the deterioration of motor, sensory, and autonomic nerve function over 2.5 years among 75 adolescents with T1D was related to poor glycemic control. In a small clinic-based cross-sectional study of 79 children with T1D, Chen et al.22 found that both A1c and duration were significant predictors of reduced HRV as measured by HF power. Children with an A1c level of ≥8% and duration of diabetes of ≥4.5 years had significantly lower HRV parameters compared with those with an A1c level of <8% and duration of <4.5 years (P=0.001).22 We have recently reported that youth with T1D have significantly worse HRV parameters reflective of overall reduced HRV (SDNN) and parasympathetic loss (HF power) compared with the age-matched healthy counterparts.23 Furthermore, youth with T1D and A1c levels of ≥7.5% had significantly worse HRV parameters than control subjects; however, in youth with optimal glycemic control (A1c, <7.5%), HRV parameters did not differ significantly from those of age-matched healthy control subjects. Although these associations were cross-sectional in nature, we are now able to demonstrate that optimal glycemic control over time is also predictive of abnormal autonomic function at this young age.
There is ample biologic plausibility for the causal role of hyperglycemia in the development of micro- and macrovascular complications and progression of cardiac autonomic dysfunction. Hyperglycemia promotes accumulation of advanced glycation end products, causing cross-linking and polymerization of collagen molecules within the vessel wall,24 which leads to loss of elasticity and subsequent reduction in arterial and myocardial compliance. Hyperglycemia also induces abnormal signaling of the autonomic neurons via accumulation of advanced glycation end products and microangiopathy, causing ischemic atrophy of the autonomic nerve fibers innervating cardiac and vascular tissues.25 In our study nearly 81% of youth with T1D had A1c levels >7.5%, thus exceeding the levels recommended by the American Diabetes Association16 and indicating an urgent need for efforts focused at improving glycemic control among contemporary cohorts of youth with T1D, to mitigate the elevated risk of early CVD events seen in this population.
Our study has several limitations. First, the retrospective cohort design of the study limits our ability to evaluate the temporal trend in the development and progression of CAN among youth with T1D because HRV tests were not conducted previously. We therefore intend to follow this cohort in the future to better understand the progression of CAN over time and the role of glycemic control in the progression of cardiac autonomic dysfunction. Although we were able to derive an average measure of glucose control over time, it was mostly based on two A1c values measured on average 6 years apart, and only a fraction of the study participants (39%) contributed more than two measures. However, similar results were seen in analyses restricted to 135 SEARCH CVD participants with at least three A1c levels measured longitudinally. Finally, the HRV measures used in our study are derived from a 10-min recording of the baseline electrocardiogram. Although this is a relatively short length of recording, it is considered standard practice for clinical and research purposes and is advocated by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology,15 as opposed to the HRV measures derived from the 24-h Holter recordings. The major strengths of our study are the large and diverse sample of contemporary youth with T1D, the simple noninvasive bedside assessment of multiple measures of HRV, and the longitudinal assessment of glycemic control over time.
In conclusion, our study demonstrates that chronic hyperglycemia is an important determinant of early cardiac autonomic dysfunction, manifested as reduced overall HRV and parasympathetic loss, among youth with T1D. This association is graded and independent of traditional cardiovascular risk factors. Because cardiac autonomic dysfunction is associated with an increased risk of cardiovascular mortality and morbidity later in life, the study underscores the vital role of glycemic control among adolescents and youth with T1D in the promotion and preservation of optimal cardiovascular health throughout the life course.
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their healthcare providers, whose participation made this study possible. The SEARCH CVD study was funded by the National Institute for Diabetes and Digestive and Kidney Diseases (grant RO1DK078542; Principal Investigator, Dana Dabelea).
Author Disclosure Statement
No competing financial interests exist.
M.J. analyzed the data and wrote the manuscript. D.D., R.P.W., and E.M.U. helped in the research and writing of the manuscript. T.E.F. helped in the analyses of the data. R.F.H., T.E.F., R.B.D., Jr., S.R.D., L.M.D., and S.M.M. contributed to the discussion and reviewed/edited the manuscript. J.W.T. helped in the research of data. D.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Maser RE, Mitchell BD, Vinik AI, Freeman R: The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 2003;26:1895–1901 [DOI] [PubMed] [Google Scholar]
- 2.Stettler C, Allemann S, Jüni P, Cull CA, Holman RR, Egger M, Krähenbühl S, Diem P: Glycemic control and macrovascular disease in type 1 and 2 diabetes mellitus: meta-analysis of randomized studies. Am Heart J 2006;152:27–38 [DOI] [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4.Reichard P, Nilsson BY, Rosenqvist U: The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 1993;329:304–309 [DOI] [PubMed] [Google Scholar]
- 5.The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) The Diabetes Control and Complications Trial Research Group. Diabetologia 1998;41:416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichard P, Berglund B, Britz A, Cars I, Nilsson BY, Rosenqvist U: Intensified conventional insulin treatment retards the microvascular complications of insulin-dependent diabetes mellitus (IDDM): the Stockholm Diabetes Intervention Study (SDIS) after 5 years. J Intern Med 1991;230:101–108 [DOI] [PubMed] [Google Scholar]
- 7.Ziegler D, Dannehl K, Wiefels K, Gries FA: Differential effects of near-normoglycaemia for 4 years on somatic nerve dysfunction and heart rate variation in type 1 diabetic patients. Diabet Med 1992;9:622–629 [DOI] [PubMed] [Google Scholar]
- 8.Young RJ, Ewing DJ, Clarke BF: Nerve function and metabolic control in teenage diabetics. Diabetes 1983;32:142–147 [DOI] [PubMed] [Google Scholar]
- 9.Burger AJ, Weinrauch LA, D'Elia JA, Aronson D: Effect of glycemic control on heart rate variability in type I diabetic patients with cardiac autonomic neuropathy. Am J Cardiol 1999;84:687–691 [DOI] [PubMed] [Google Scholar]
- 10.Jakobsen J, Christiansen JS, Kristoffersen I, Christensen CK, Hermansen K, Schmitz A, Mogensen CE: Autonomic and somatosensory nerve function after 2 years of continuous subcutaneous insulin infusion in type I diabetes. Diabetes 1988;37:452–455 [DOI] [PubMed] [Google Scholar]
- 11.SEARCH Study Group: SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 12.Kann L, Kinchen SA, Williams BI, Ross JG, Lowry R, Grunbaum JA, Kolbe LJ; State and Local YRBSS Coordinators. Youth Risk Behavior Surveillance System: Youth risk behavior surveillance—United States, 1999. MMWR CDC Surveill Summ 2000;49:1–32 [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention: The Third National Health and Nutrition Examination Survey (NHANES III 1988–94) Reference Manuals and Reports [CD ROM]. Bethesda, MD: National Center for Health Statistics, 2005 [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;(246):1–190 [PubMed] [Google Scholar]
- 15.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–1065 [PubMed] [Google Scholar]
- 16.American Diabetes Association: Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, Sommer C, Cleary PA, Lachin JM, Herman WH; DCCT/EDIC Research Group: Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation 2009;119:2886–2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichard P, Jensen-Urstad K, Ericsson M, Jensen-Urstad M, Lindblad LE: Autonomic neuropathy—a complication less pronounced in patients with type 1 diabetes mellitus who have lower blood glucose levels. Diabet Med 2000;17:860–866 [DOI] [PubMed] [Google Scholar]
- 19.Larsen JR, Sjøholm H, Berg TJ, Sandvik L, Brekke M, Hanssen KF, Dahl-Jørgensen K: Eighteen years of fair glycemic control preserves cardiac autonomic function in type 1 diabetes. Diabetes Care 2004;27:963–966 [DOI] [PubMed] [Google Scholar]
- 20.Witte DR, Tesfaye S, Chaturvedi N, Eaton SE, Kempler P, Fuller JH; EURODIAB Prospective Complications Study Group: Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia 2005;48:164–171 [DOI] [PubMed] [Google Scholar]
- 21.Young RJ, McIntyre CCA, Martin CN, Prescott RJ, Ewing DJ, Smith AF, Viberti G, Clarke BF: Progression of subclinical polyneuropathy of young patients with type I diabetes: association with glycemic control and microangiopathy. Diabetologia 1986;29:156–161 [DOI] [PubMed] [Google Scholar]
- 22.Chen SR, Lee YJ, Chiu HW, Jeng C: Impact of glycemic control, disease duration, and exercise on heart rate variability in children with type 1 diabetes mellitus. J Formos Med Assoc 2007;106:935–942 [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal M, Urbina EM, Wadwa RP, Talton JW, D'Agostino RB, Jr, Hamman RF, Fingerlin TE, Daniels S, Marcovina SM, Dolan LM, Dabelea D: Reduced heart rate variability among youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care 2013;36:157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad A, Bekker P, Tsimikas S: Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev 2012;20:177–183 [DOI] [PubMed] [Google Scholar]
- 25.Vinik AI: The conductor of the autonomic orchestra. Front Endocrinol (Lausanne) 2012;3:71. [DOI] [PMC free article] [PubMed] [Google Scholar]