Abstract
Nigella sativa, commonly referred as black cumin, is a popular spice that has been used since the ancient Egyptians. It has traditionally been used for treatment of various human ailments ranging from fever to intestinal disturbances to cancer. This study investigated the apoptotic, antimetastatic, and anticancer activities of supercritical carbon dioxide (SC-CO2) extracts of the seeds of N. sativa Linn. against estrogen-dependent human breast cancer cells (MCF-7). Twelve extracts were prepared from N. sativa seeds using the SC-CO2 extraction method by varying pressure and temperature. Extracts were analyzed using FTIR and UV-Vis spectrometry. Cytotoxicity of the extracts was evaluated on various human cancer and normal cell lines. Of the 12 extracts, 1 extract (A3) that was prepared at 60°C and 2500 psi (∼17.24 MPa) showed selective antiproliferative activity against MCF-7 cells with an IC50 of 53.34±2.15 μg/mL. Induction of apoptosis was confirmed by evaluating caspases activities and observing the cells under a scanning electron microscope. In vitro antimetastatic properties of A3 were investigated by colony formation, cell migration, and cell invasion assays. The elevated levels of caspases in A3 treated MCF-7 cells suggest that A3 is proapoptotic. Further nuclear condensation and fragmentation studies confirmed that A3 induces cytotoxicity through the apoptosis pathway. A3 also demonstrated remarkable inhibition in migration and invasion assays of MCF-7 cells at subcytotoxic concentrations. Thus, this study highlights the therapeutic potentials of SC-CO2 extract of N. sativa in targeting breast cancer.
Key Words: : caspases, Hoechst 33258, MCF-7 cells, MTT, Ranunculaceae, supercritical fluid extraction
Introduction
Apoptosis, a process of programmed cell death characterized by activation of caspases, leads to the loss of mitochondrial membrane permeability, blebbing of cell membrane, nuclear chromatin condensation, shrinkage of cells, formation of apoptotic bodies, and phagocytosis of cell debris.1 In breast carcinoma, increased apoptosis leads to a low degree of differentiation, tumor aneuploidy, and ultimately decreased malignancy.2 A number of apoptosis inducers have been developed and successfully applied as breast cancer medicines, such as tocotrienols, RRR-α-tocopherol,3 and bisphosphonates.4
Nigella sativa Linn. (Ranunculaceae) is a well-known traditional medicinal plant, commonly known as black seed. Traditionally, the seeds of N. sativa have been used to treat various illnesses, including cancer, fever, infections, and intestinal disturbances.5 The seeds are also used as condiments, carminatives, appetizers, stimulants for menstrual flow (emmenagogue), tonics, and for increasing milk yield (galactagogue).6 A number of studies have validated the traditional uses of N. sativa, such as antibacterial,7 antihistamine,8 and antioxidant effects.9 N. sativa seed extracts and the isolated active principles, thymoquinone (TQ) and dithymoquinone showed cytotoxic activity against several tumor types and prevented tumor growth in mice10–13 and induced telomere attrition and apoptosis.14
Supercritical fluid extraction (SFE) has various industrial applications from pharmaceutical to food industries.15 SFE is preferred over conventional solvent extraction methods due to its characteristic lower viscosity, while its diffusivity is relatively high. It prevents secondary reactions in the extract that tend to occur during solvent extraction such as oxidation and hydrolysis.15 Supercritical carbon dioxide (SC-CO2) extraction is the most popular SFE method. It has many advantages, since it is efficient, fast and environmentally safe, nontoxic, nonexplosive, nonflammable, and inert to solutes. CO2 is gaseous at room temperature and pressure and this leads to a very simple recovery of the extract and results in solvent-free extracts.16 In the present study, the proapoptotic and antimetastatic activities of SC-CO2 extracts of N. sativa obtained from various extraction parameters (varying pressures and temperatures) were evaluated in a panel of human cancer cell lines.
Materials And Methods
Materials
The SC-CO2 extractor (SFX-220 SFE system) was obtained from ISCO. Dimethyl sulfoxide (DMSO), Folin-Ciocalteau reagent, TQ, tamoxifen, betulinic acid, Hoechst 33258 stain, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich. Caspases 3/7, 8, and 9 were purchased from Promega and Matrigel™ (10 mg/mL) was obtained from BD Bioscience.
Plant material
N. sativa seeds were purchased from a local market in Seiyun, Yemen. The plant was authenticated at the Herbarium Department, School of Biological Sciences, Universiti Sains Malaysia (USM) with a voucher number (11221—N. sativa—22/3/2011). The seeds were washed, dried, pulverized, and sieved into particles of 0.5 mm.
Cell culture and cell lines
Human cell lines, HCT 116 (colorectal carcinoma), MCF-7 (hormone sensitive and invasive breast cancer), MDA-MB-231 (hormone resistant breast cancer), Hep G2 (hepatocellular carcinoma), PC-3 (prostate carcinoma), and CCD-18Co (normal colonic fibroblasts) were obtained from ATCC. Cells were cultured at 5% CO2 humidified atmosphere at 37°C in a growth medium supplemented with 10% heat inactivated fetal bovine serum and 1% penicillin/streptomycin. HCT 116 and MCF-7 cells were cultured in RPMI-1640 and MEM media, respectively. PC-3 cells were cultured in F-12K, while MDA-MB-231, Hep G2, and CCD-18Co cells were grown in the DMEM.
SC-CO2 extraction
SC-CO2 extraction was employed using SFX-220, SFE system to obtain 12 extracts from N. sativa seeds (A1, A2, A3, B1, B2, B3, C1, C2, C3, D1, D2, and D3). Briefly, 1.2 g powder was extracted for 60 min with liquefied CO2 at various pressures (2500, 3000, 4500, and 6000 psi; ∼17.24, 20.68, 31.03, and 41.37 MPa) and temperatures (32°C, 45°C, and 60°C) at a CO2 flow rate of 2 mL/min.
Characterization and phytochemical analysis
FTIR
FTIR spectra were recorded at a wavelength ranging from 4000 to 400 cm−1 using an FTIR spectrometer (Thermo. Nicolet Nexus 670; Thermo Scientific) equipped with OMNIC application software (Thermo; Electron Corporation).
UV-Vis spectrophotometry
UV-Vis spectrophotometry was carried out using a Lambda25 UV/Vis spectrophotometer system operated with UV WinLab V2.85 software (Perkin Elmer). Samples were prepared in methanol at 100 μg/mL, and were scanned at the wavelength range from 500 to 200 nm.
Total phenolic and flavonoid contents
Total phenolics were determined using the Folin-Ciocalteau reagent with gallic acid as a standard and the result was expressed as mg of gallic acid equivalent.17 Total flavonoids were determined using the AlCl3 colorimetric method with quercetin as standard and the result was expressed as mg of quercetin equivalent.18
Cell viability
MTT assay19 was performed to assess the cytotoxicity of the extracts on various cancer cell lines (HCT 116, MCF-7, PC-3, MDA-MB-231, and Hep G2). CCD-18Co was used as the model cell line for normal cells. The assay plates were read using a microtiter plate reader (Hitachi U-2000) at 570 nm absorbance. DMSO (1%) was used as a negative control.
In vitro apoptotic and antitumorigenic activity of A3 on MCF-7 cells
Effect of A3 on caspases 3/7, 8, and 9
The assays were carried out according to the manufacturer's protocol (Promega). MCF-7 cells were treated with various concentrations of A3 (60–120 μg/mL) for different time intervals (3, 6, and 9 h). Tamoxifen (10 μg/mL) was used as a positive control, and DMSO (1%) as a negative control. Subsequently, an equal volume of freshly prepared caspase 3/7, 8, or 9 substrates were added, incubated at room temperature for 30 min, and luminescence was measured using the Infinite M200 PRO microplate reader (Tecan Group Ltd.). The results were expressed as the fold changes in the caspase activity relative to the negative control.
Nuclear chromatin condensation of MCF-7 cells
The effect of A3 on nuclear chromatin condensation in MCF-7 cells was quantified by fluorescence microscopy using Hoechst 33258 stain.20 The cells treated with A3 (20, 40, and 60 μg/mL) or 1% DMSO for 24 h, were fixed, stained, and photographed at 20×magnification, using a digital microscope (EVOS fl; Advanced Microscopy Group). The percentage of apoptotic index was presented as mean of experiments performed in triplicates.
Transmission electron microscope
MCF-7 cells were treated for 24 h with A3 (40 and 80 μg/mL) or 1% DMSO. Cells were fixed with 0.1 M McDowell–Trump and stained with 1% osmium tetroxide. The cells were then solidified in 2% agar, cut into small slides, and dehydrated in ethanol followed by acetone. The slides were embedded in resin and infiltrated five times in Suprr's mixture at 60°C for overnight. Subsequently, cells were molded in resin, sliced into semithin (1 μm) and ultrathin sections (0.1 μm). The semithin sections were stained with toluidine blue, whereas the ultrathin sections were collected in copper grids and stained with uranyl acetate and lead citrate. Finally, the cells were photographed using transmission electron microscope (TEM) at 1600×magnification.
Colony formation
The effect of A3 on clonogenicity of MCF-7 cells was investigated by the colony formation assay.21 Briefly, MCF-7 cells (500 cells/mL) were seeded in six-well plates and incubated for 12 h. Subsequently, cells were treated for 48 h with A3 (10, 20, 40, and 60 μg/mL) or tamoxifen (10 μg/mL) or 1% DMSO. The cells were maintained for 10 days until sufficiently large colonies (≥50 cells) were produced. The colonies were fixed, stained with 0.2% crystal violet, and counted under stereomicroscope. The percentage of plating efficiency (PE%) and percentage of surviving fraction were calculated.
Cell migration
Cell migration assay was conducted as described by Liang et al.22 MCF-7 cells were seeded in 24-well plates and incubated for 48 h to achieve an almost 100% confluent monolayer. A straight scratch was created using a 200-μL micropipette tip, and the cells were treated immediately with A3 (20 and 40 μg/mL) or 0.5% DMSO. The wound was photographed at 0, 12, and 24 h. The distance of the cell-free area was measured using Leica Quin software, and the results are presented as percentage of inhibition of migration in comparison to the negative control.
Cell invasion
Invasion assay was performed using Matrigel as an artificial basement membrane matrix.23 Matrigel in MEM (1:1) was pipetted and incubated for 45 min to solidify. MCF-7 cells (5×103 per well in 96-well plates) were treated for 24 h with 0.5% DMSO or A3 (concentrations of 20 and 40 μg/mL). Subsequently, the upper media were carefully aspirated and noninvading cells were washed off gently, and cells were photographed microscopically. Invading cells were counted and the result was reported as percentage inhibition of invasion relative to untreated cells.
Statistical analysis
Results were calculated as mean±SD of triplicates of three independent experiments and analyzed using SPSS 16.0 package. Differences were tested by one-way ANOVA followed by post hoc—Dunnett's or Tukey's—tests. Correlations were calculated by the bivariate, two-tailed Pearson's test; while R2 values were analyzed by the linear regression test. Significance was considered at P<.05.
Results
Yield of SC-CO2 extracts of N. sativa seeds
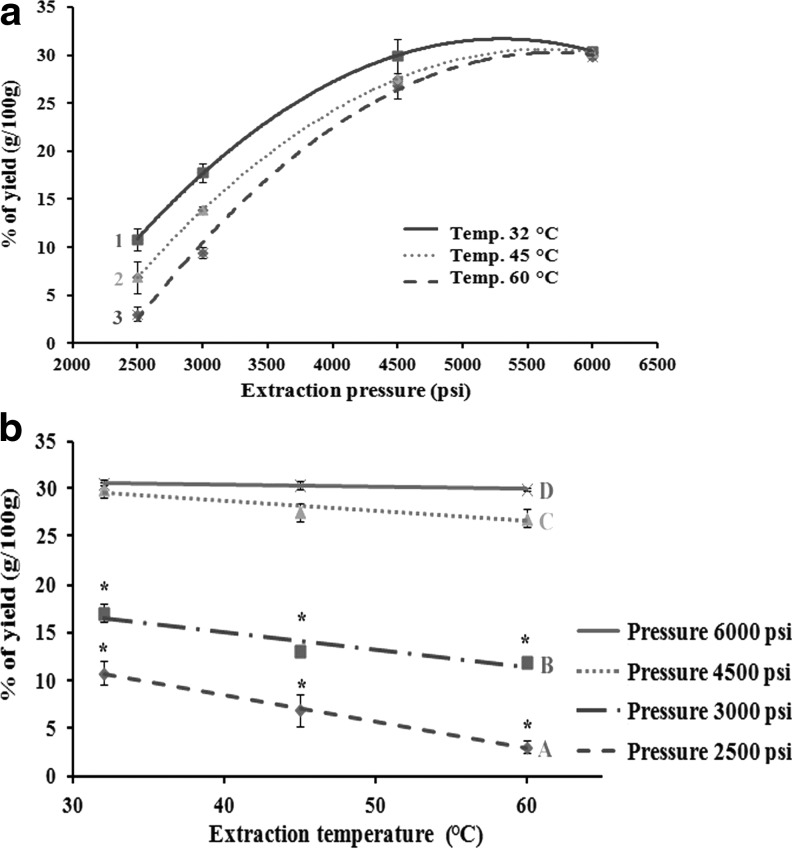
Cumulative percentage yields of the N. sativa seed extracts were calculated at intervals of 15 min. The percentage yield increased significantly with increasing extraction time. The results indicate that 60 min is the optimum extraction time of N. sativa seeds by SC-CO2 (Table 1). The extraction pressure showed a significant positive correlation with the percentage yield at 32°C, 45°C, and 60°C with R2=0.89, 0.94, and 0.96, respectively (Fig. 1a). On the contrary, the extraction temperature showed a negative correlation with the percentage yield at 2500 psi (R2=0.90) and 3000 psi (R2=0.98), whereas a lower effect of temperature on percentage yield was observed at 4500 psi (R2=0.64) and 6000 psi (R2=0.53) (Fig. 1b).
Table 1.
Percentage Yield of Supercritical Carbon Dioxide Extracts of Nigella sativa Seeds in Relation to the Extraction Time Interval
| |
|
|
Percentage yield (g/100 g) in the extraction time intervals |
|
|||
|---|---|---|---|---|---|---|---|
| Extract code | Pressure (psi) | Temperature (°C) | 15 min | 30 min | 45 min | 60 min | Correlation R2 value |
| A1 |
2500 |
32 |
02.58±0.24 |
06.57±0.02 |
09.04±0.11 |
10.81±1.18 |
0.95 |
| A2 |
2500 |
45 |
01.88±0.22 |
03.01±0.18 |
04.17±0.26 |
06.88±1.63 |
0.82 |
| A3 |
2500 |
60 |
01.43±0.06 |
02.60±0.06 |
02.65±0.08 |
03.06±0.85 |
0.59 |
| B1 |
3000 |
32 |
04.81±0.49 |
11.42±0.16 |
15.10±0.57 |
17.77±0.98 |
0.91 |
| B2 |
3000 |
45 |
03.72±0.27 |
07.46±0.47 |
11.08±0.94 |
13.89±0.33 |
0.97 |
| B3 |
3000 |
60 |
02.32±0.18 |
05.78±0.30 |
09.66±0.55 |
09.48±0.59 |
0.96 |
| C1 |
4500 |
32 |
05.72±0.23 |
18.49±0.37 |
25.34±0.23 |
30.41±1.09 |
0.95 |
| C2 |
4500 |
45 |
14.50±0.28 |
23.52±0.11 |
27.62±0.67 |
27.94±1.37 |
0.83 |
| C3 |
4500 |
60 |
17.34±0.04 |
24.34±0.11 |
25.44±0.69 |
26.86±0.15 |
0.82 |
| D1 |
6000 |
32 |
19.65±0.17 |
28.79±0.13 |
29.93±0.04 |
30.41±0.17 |
0.72 |
| D2 |
6000 |
45 |
15.17±0.11 |
26.75±0.28 |
29.85±0.06 |
30.27±0.41 |
0.78 |
| D3 | 6000 | 60 | 23.57±0.06 | 27.21±0.47 | 29.77±0.08 | 29.85±0.20 | 0.86 |
Results are represented as mean±SD (n=3), P<.05.
SC-CO2, supercritical carbon dioxide.
FIG. 1.
Percentage yield (g/100 g) of the 12 supercritical carbon dioxide (SC-CO2) extracts of Nigella sativa seeds at 60 min as a function of (a) pressure, at 1=32°C, 2=45°C, and 3=60°C; and (b) temperature, at various pressures, A=2500 psi, B=3000 psi, C=4500 psi, and D=6000 psi.
Phytochemical study of the extracts
FTIR analysis
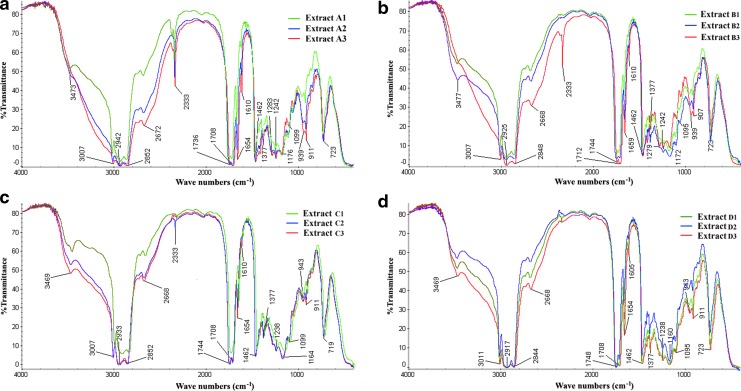
FTIR spectra of the extracts (Fig. 2 and Table 2) depict the related functional groups of transmittance bands of the corresponding wavelengths.24
FIG. 2.
FTIR spectra of the 12 SC-CO2 extracts of N. sativa seeds. (a) The extracts A1, A2, and A3 (pressure 2500 psi), (b) the extracts B1, B2, and B3 (pressure 3000 psi), (c) the extracts C1, C2, and C3 (pressure 4500 psi), and (d) the extracts D1, D2, and D3 (pressure 6000 psi). Color images available online at www.liebertpub.com/jmf
Table 2.
Infrared Transmittance Bands Correlated to the Corresponding Wavenumbers Representing the Functional Organic Groups Present in the Supercritical Carbon Dioxide Extracts of Nigella sativa seeds
| Vibrational frequency (cm−1) | Extract | Corresponding organic groups |
|---|---|---|
| 3477–3469 (broad) |
A1, B1, B2, C1, C2, C3, D1, D2, and D3 |
O-H stretching |
| 3015–2844 |
All 12 extracts |
C-H stretching |
| 1748–1708 (strong) |
All 12 extracts |
C=O stretching |
| 1659–1654 (weak) |
All 12 extracts |
C=C stretching |
| 1610–1605 |
All 12 extracts |
NO2− stretching |
| 1471–1462 |
All 12 extracts |
C-H bending (deformation) |
| 1381–1373 |
All 12 extracts |
C-C, C-H, O-H, and C-O bending |
| 1242–1160 |
All 12 extracts |
C-C, C-O, C-N stretching |
| 1099–1094 |
All 12 extracts |
C-C, C-O, C-N stretching |
| 1050–1046 |
A2, A3, B3 |
C-C, C-O, C-N stretching |
| 960–907 |
All 12 extracts |
C-H bending |
| 723–719 | All 12 extracts | C-H bending |
UV-Vis spectrophotometry
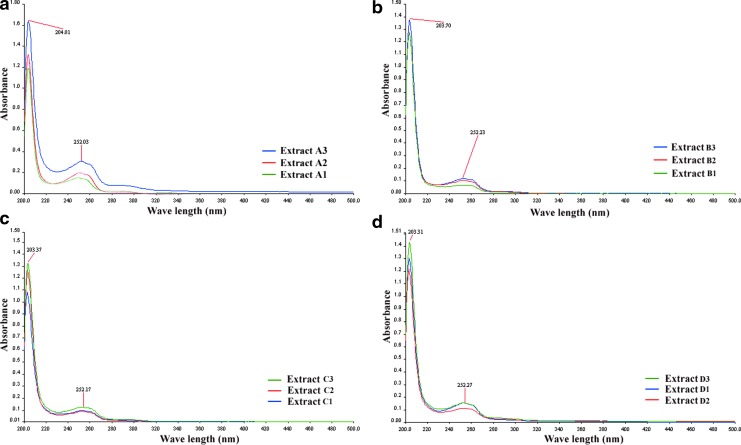
Figure 3 shows that all of the 12 extracts have absorbances at 252.03–252.32 nm at different intensities, which refer to the presence of conjugated homoannular diene. This region of λmax also indicates the conjugation of diketones (such as p-benzoquinone) and aromatic carbonyl compounds, an aromatic characteristic of the oil of N. sativa.25
FIG. 3.
UV-Vis absorption spectra of the 12 SC-CO2 extracts N. sativa seeds. (a) The extracts A1, A2, and A3 (pressure 2500 psi), (b) the extracts B1, B2, and B3 (pressure 3000 psi), (c) the extracts C1, C2, and C3 (pressure 4500 psi), and (d) the extracts D1, D2, and D3 (pressure 6000 psi). Color images available online at www.liebertpub.com/jmf
Total phenolic and total flavonoid content
The result of quantitative analysis of total phenolic and total flavonoid content of N. sativa seed extracts is presented in Table 3.
Table 3.
Total Phenolic and Total Flavonoid Contents of the Supercritical Carbon Dioxide Extracts of Nigella sativa Seeds
| Extract | Total phenolic content (mg GAE/g of sample) | Total flavonoids content (mg QE/g of sample) |
|---|---|---|
| A1 |
2.80±0.10 |
13.54±1.29 |
| A2 |
2.68±0.05 |
11.63±0.22 |
| A3 |
2.60±0.05 |
11.43±1.16 |
| B1 |
3.19±0.03 |
09.18±0.78 |
| B2 |
3.14±0.11 |
07.90±1.45 |
| B3 |
2.59±0.08 |
06.15±1.42 |
| C1 |
2.54±0.04 |
12.22±1.21 |
| C2 |
2.61±0.06 |
11.53±1.29 |
| C3 |
2.70±0.13 |
10.90±0.53 |
| D1 |
2.93±0.09 |
15.85±1.25 |
| D2 |
2.76±0.07 |
14.24±1.26 |
| D3 | 2.59±0.05 | 12.20±1.46 |
Results are represented as mean±SD (n=3).
GAE, gallic acid equivalent; QE, quercetin equivalent.
Effect on cell viability
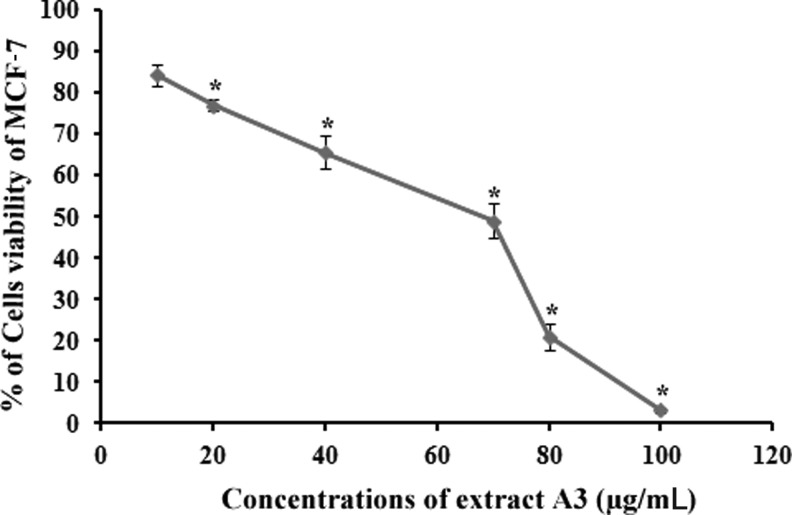
Table 4 shows the effect of different extracts of N. sativa on various human cell lines after 48 h of treatment. Among all extracts, the extract prepared at 2500 psi and 60°C (A3) exhibited the greatest selective cytotoxicity against MCF-7 cells with the cell viability of 0.07%±0.12% at 100 μg/mL. A3 exhibited dose-dependent cytotoxicity toward MCF-7 cells with IC50 53.34±2.15 μg/mL, whereas A3 showed poor antiproliferative activity toward other tested cell lines. IC50 values of TQ and tamoxifen were 5.33±0.29 and 10.51±0.38 μg/mL, respectively (Fig. 4).
Table 4.
Percentage of Cell Viability of Seven Human Cell Lines Treated with 100 μg/mL of Supercritical Carbon Dioxide Extracts of Nigella sativa Seeds
| |
% of cell viability (mean±SD) on different cell lines treated with 100 μg/mL of extracts |
|||||
|---|---|---|---|---|---|---|
| Extract | HCT 116 | MCF-7 | MDA-MB-231 | Hep G2 | PC-3 | CCD-18Co |
| A1 |
107.05±1.29 |
83.95±3.31a |
105.53±3.04 |
126.58±3.44 |
90.08±2.27a |
101.74±3.92 |
| A2 |
109.46±3.59 |
67.90±5.93a |
93.13±4.05 |
111.08±8.55 |
101.03±3.42 |
88.70±4.29a |
| A3 |
88.80±1.03a |
0.07±0.12a |
103.23±3.97 |
101.44±6.87 |
108.12±2.34 |
69.70±8.78a |
| B1 |
93.16±2.06 |
100.97±2.29 |
88.22±1.83a |
122.71±4.51 |
96.52±3.21 |
78.20±6.00a |
| B2 |
84.08±2.26a |
107.12±1.90 |
91.26±5.29 |
121.80±4.36 |
104.10±5.44 |
83.60±7.49a |
| B3 |
86.52±2.13a |
70.70±5.39a |
85.58±4.99a |
119.59±4.67 |
119.26±6.52 |
96.21±4.91 |
| C1 |
79.47±1.86a |
103.72±2.96 |
96.19±2.44 |
107.52±6.86 |
98.27±4.34 |
107.51±6.74 |
| C2 |
65.62±1.06a |
108.61±3.17 |
101.70±2.04 |
96.94±1.52 |
115.463.73 |
105.16±3.39 |
| C3 |
61.11±3.62a |
75.21±1.80a |
110.53±3.67 |
91.59±8.38a |
108.56±5.07 |
98.88±6.47 |
| D1 |
86.49±3.19a |
66.47±4.64a |
92.57±3.91 |
114.18±5.93 |
86.05±3.79a |
95.82±7.38 |
| D2 |
73.61±1.70a |
71.42±2.64a |
106.71±2.60 |
129.96±3.56 |
88.79±4.27a |
88.01±5.01a |
| D3 | 69.89±2.29a | 69.97±2.29a | 116.19±1.41 | 127.49±4.21 | 92.29±3.52 | 92.66±7.97 |
P<.05 compared to the negative control (DMSO ≤1%). Results are represented as mean±SD (n=3).
DMSO, dimethyl sulfoxide.
FIG. 4.
Concentration–activity curve, obtained using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The graph depicts the dose-dependent antiproliferative effect of the extract A3 on MCF-7 cells for 48 h, IC50=53.34±2.15 μg/mL, *P<.05.
In vitro proapoptotic activity of A3 on MCF-7
Activation of caspases 3/7, 8, and 9
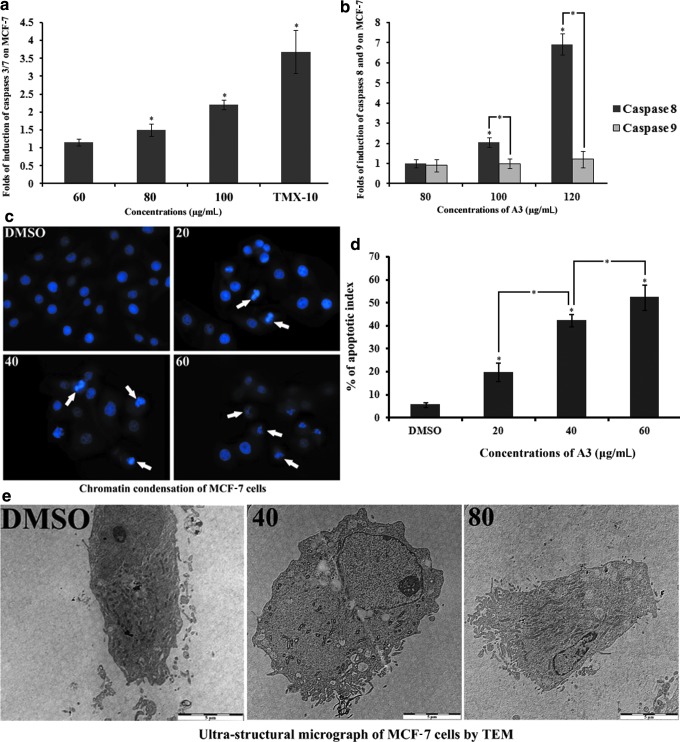
The cellular levels of apoptotic markers, caspases, were estimated to explore the mechanism of action of A3 by which the extract induced toxicity in MCF-7 cells. The effect of A3 was studied at three different time intervals (3, 6, and 9 h). Treatment of MCF-7 cells with A3 for either 3 or 6 h did not elevate caspase levels, whereas the treatment for 9 h caused significant activation of caspases 3/7 at 100 and 80 μg/mL by 2- and 1.5-fold, respectively. At 60 μg/mL, there was insignificant elevation of the level of caspases when compared to the negative control (Fig. 5a). A3 also activated caspase 8 at 100 and 120 μg/mL by two- and sevenfold. On the other hand, A3 did not show significant elevation in the level of caspase 9 (Fig. 5b).
FIG. 5.
Apoptotic effect of A3 in MCF-7 cells. The level of caspases in cells was measured at 3, 6, and 9 h after the treatment. (a) The dose-dependent stimulatory effect of A3 on the caspase 3/7 level after 9 h of treatment. (b) After 9 h of treatment, A3 increased the level of caspases 8, whereas the level of caspase 9 was unaltered. (c) MCF-7 cells with DNA Hoechst 33258 stain. The picture reveals the proapoptotic properties of A3, as there is evidence of chromatin condensation in treated (for 24 h) cells, which is considerably significant when compared to the untreated cells (P<.05). It can be seen clearly that higher concentrations of A3 rendered the cells with crescent shaped nuclei, which is a characteristic features of apoptosis. (d) The percentage of apoptotic indices of chromatin condensation assay. (e) Ultrastructural micrographs using the transmission electron microscope (TEM) reveal further supportive information of the apoptotic property of A3. The pictures at magnification 1600× showed all characteristic features of the apoptosis in MCF-7 cells treated with A3 for 24 h. *P<.05; **P<.01. Color images available online at www.liebertpub.com/jmf
Nuclear morphology and chromatin condensation
The effect of A3 on nuclear morphology of MCF-7 cells was investigated by staining the nucleus with Hoechst 33258 stain. Figure 5c illustrates that the untreated cells displayed evenly stained nuclei, whereas cells treated with A3 (20, 40, and 60 μg/mL for 24 h) exhibited dose-dependent effects with clear signs of nuclear shrinkage, chromatin condensation, and nuclear fragmentation. At higher concentrations, a few cells also revealed the characteristic crescent-shaped nuclei, which is a typical apoptotic nuclear morphology. The apoptotic index of the negative control was 5.65%±1.13%, which was increased to 19.86%±3.93%, 42.43%±2.71%, and 52.37%±5.46% following treatment with A3 at 20, 40, and 60 μg/mL, respectively (Fig. 5d).
Ultrastructural morphology by TEM
Figure 5e shows that untreated MCF-7 cells had intact cell membranes with dense cellular contents and prominent nuclei with conspicuous nucleoli. The cells treated with 40 and 80 μg/mL of A3 for 24 h displayed clear signs of apoptosis, accompanied with cell membrane disruption, blebbing, and apoptotic morphological alterations such as formation of various sized vacuoles and chromatin condensation. The nuclei of the cells treated with 40 μg/mL were smaller than the untreated cells and contain clear nucleoli, whereas at 80 μg/mL, the nucleolus was no longer apparent in the cells (arrows in Fig. 5e).
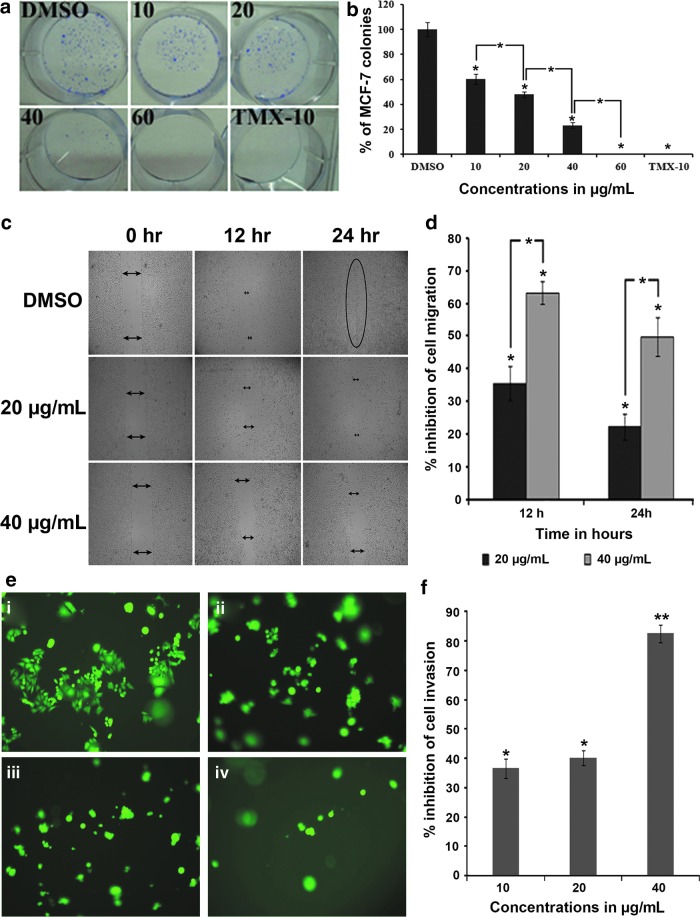
Inhibition of clonogenicity of MCF-7 cells
In this assay, MCF-7 cells were treated with A3 for 48 h. The clonogenicity study on MCF-7 cells indicated A3 to be cytotoxic at higher concentrations, while cytostatic at its lower concentrations, as evidenced by the decrease in the survival fraction (SF). The PE% was 24.13%±3.46%, SF at 10, 20, 40, and 60 μg/mL was 60.08%±4.07%, 48.7%±2.31%, 23.34%±2.28%, and 0%, respectively. The result is comparable with the standard reference, tamoxifen (10 μg/mL), which inhibited colony formation completely (Fig. 6a).
FIG. 6.
Antitumorigenic and metastatic activities of A3 on MCF-7 cells. (a) The effect of A3 on the survival of MCF-7 colonies in colony formation assay. The picture clearly depicts the cytotoxic as well as cytotostatic effects of A3 on MCF-7 cells. (b) The dose-dependent inhibitory effect of A3 on colony formation of MCF-7 cells after 48 h. (c) Due to the successful migration of endothelial cells in the untreated group, the wound is almost closed after 24 h, whereas in the A3 treated group, the wound remained open even after 24 h of incubation. A3 (20 μg/mL) caused significant inhibition of endothelial cell migration (P<.05). At a concentration of 40 μg/mL, A3 caused dislodgement of monolayer of endothelial cells with almost complete inhibition of migration. (d) The time- and dose-dependent inhibitory effect of A3 on the migration property of the MCF-7 cells, which is a characteristic of metastasis of the cancer cells. (e) Invasion of the tumor cells through the Matrigel barrier. It can be seen clearly that, in untreated wells (i), the population of MCF-7 cells that invaded through the Matrigel was more compared with the treated wells. The effect of A3 on MCF-7 cells was obvious with significant (P<.05) inhibition of 31.9% at 10 μg/mL concentration (ii), 35.57% at 20 μg/mL concentration (iii), and 63.22% at 40 μg/mL concentration (iv). (f ) The dose-dependent inhibition of MCF-7 cell migration. The result is expressed as the mean number of cells invading per field of view, after counting 10 fields of view for triplicate wells (P<.05). *P<.05; **P<.01. Color images available online at www.liebertpub.com/jmf
Inhibition of cell migration
Cell migration assay represents an important step in the metastasis of cancer cells. The results are presented as percentage inhibition of migrating cells relative to untreated cells (Fig. 6b, c). Significant reduction in MCF-7 cell motility was achieved at subcytotoxic concentrations of 20 and 40 μg/mL with 35.57%±5.11% and 63.22%±3.47% inhibition, respectively, at 12 h of treatment. At 24 h, the percentage inhibition was 22.33%±4.09% and 49.80%±5.89% at 20 and 40 μg/mL, respectively.
Inhibition of cell invasion
Cell invasion assay was performed on the Matrigel matrix and the results are shown as a percentage inhibition of invading cells. After a 24-h treatment period, the percentage inhibition was 79.29%±2.09% and 43.04%±2.25% following treatment with A3 at 40 and 20 μg/mL, respectively (Fig. 6d).
Discussion
The findings of the present study showed that, out of the 12 extracts, A3 (prepared at 2500 psi and 60°C) showed considerable antiproliferative activity against MCF-7 cells, whereas no cytotoxic effect was observed on other tested cancer cells, including the normal CCD-18Co cells. This indicates that A3 has potential selective cytotoxicity against MCF-7 cells. Apoptotic effects of A3 were investigated to further elucidate its mode of action. Caspases are a family of enzymes that have important roles in cell apoptosis cascades. Their activation occurs at the early steps of apoptosis.26 This subsequently activates other apoptosis triggering signals and leads to cell shrinkage, chromatin condensation, and fragmentation. Finally, the cascade culminates in the formation of apoptotic bodies.27 A3 significantly stimulates the apoptosis activator (caspase 8) in MCF-7 cells, which is consequently coincided by the activation of apoptosis executioner (caspases 3/7). This results in a significant induction of apoptosis and survival suppression of MCF-7 cells, which further contributes to counteracting the breast cancer development.28 The activation of caspases leads to nuclear chromatin condensation and DNA degradation. The segmentation of chromatin and formation of apoptotic bodies are indicators of apoptosis in the cells. The ultrastructural alterations of the A3 treated breast cancer cells were demonstrated using TEM, which showed obvious apoptotic signs. The shrinkage of nucleus and disappearance of nucleoli at 80 μg/mL of A3 indicated an advanced stage of apoptosis.29
The induction of apoptosis controls various regulators that abrogate tumorigenesis and metastasis.30 Tumor cells are shed daily as part of their movement in blood circulation, and the cells continue their extravasations and proliferation into the secondary sites, despite the daily hemodynamic stresses and body immunity. This is based on the cell migration and invasion cascades.31 A3 suppressed colonization of MCF-7 cells in a dose-dependent manner. It is cytotoxic against MCF-7 cells at 60 μg/mL, where it irreversibly damaged the cell integrity, whereas at 10 and 20 μg/mL, it showed cytostatic effects. Cell migration and invasion are crucial processes that control tumorigenesis and metastasis. Inhibition of MCF-7 cell migration in vitro by A3 indicates that the extract could probably inhibit cell metastasis.32 Treatment of MCF-7 cells with A3 resulted in a potent inhibition of cell migration toward the closure of the wound scratch, even after 12 and 18 h, indicating that the extract has a capability to suppress the motility of the breast cancer cells, whereas the wound was totally closed after 24 h in untreated cells. Cell invasion is one of the main hallmarks of metastatic cascade.33 Although induction of caspase 8 activity in the early steps of apoptosis alters survival, it also inhibits cell invasion into the basement membranes.33 The extract A3 activated caspase 8 in the MCF-7 cell line; thus it can be postulated that this leads to its inhibitory effect on invasion of MCF-7 cells. A3 significantly restricted invasion of MCF-7 cells into the Matrigel basement. This further technically supports the antimetastatic potential of A3 against breast cancer.
FTIR and UV-Vis spectrometric analysis of the extracts of N. sativa seeds resulted in obvious differences in their chemical constituents, which were related to the different bioactivities observed for the extracts. The antiproliferative, proapoptotic, and antimetastatic effects of A3 may be due to the collective contribution of its antioxidant-rich polyphenolic contents, particularly, TQ, thymohydroquinone, and alpha-hederin. 10,13 Phenolic compounds counteract cancer either by means of antioxidant effect or by inhibiting the formation of carcinogenic metabolites that damage the vital biomolecules.33,34 Studies have shown that flavonoids have potent cytotoxic activities by induction of the apoptosis pathway.35 In this study, the A3 extract was rich in total flavonoids and polyphenols, which might have contributed toward its overall anticancer effects observed in MCF-7 cells.
Conclusion
In conclusion, the present work provides good supporting evidence that the SC-CO2 extract A3, prepared using 60°C and 2500 psi, interferes with the several physiological properties of MCF-7 breast cancer cells. The antitumorigenic effect of A3 may be due to the collective contributions of phytochemicals, particularly, TQ and thymohydroquinone. The findings reveal that SC-CO2 extraction can be useful to produce potent extracts from N. sativa seeds that can induce apoptosis. It was found that the anticancer potency of this extract is highly dependent on extraction temperature and pressure. Higher extraction temperature (60°C) and lower extraction pressure (2500 and 3000 psi) produced the most potent extracts. The MCF-7 cell line was the most sensitive. A3 halted the proliferation and invasion of MCF-7 cells and caused significant apoptosis in the cell line by activating caspase 3/7 and 8.
Acknowledgments
This study was supported by Natureceuticals Sdn Bhd, Malaysia and the research grant (Ref. no. 1001/PFARMASI/834052) at Universiti Sains Malaysia. It was also funded from the Institute of Health Sciences in Seiyun–Hadramaut and Ministry of Health, Yemen.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.O'Driscoll L, O'Connor R, Clynes M: Methods in apoptosis. In: Cell Biology, 3rd edition. (Julio EC.) Academic Press, Burlington, MA, USA, 2006, pp. 335–342 [Google Scholar]
- 2.Lipponen P, Aaltomaa S, Kosma VM, Syrjanen K: Apoptosis in breast cancer as related to histopathological characteristics and prognosis. Eur J Cancer 1994;30A:2068–2073 [DOI] [PubMed] [Google Scholar]
- 3.Yu W, Simmons MM, Gapor A, Sanders BG, Kline K: Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr Cancer 1999;33:26–32 [DOI] [PubMed] [Google Scholar]
- 4.Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW: Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 2000;82:1459–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MA, Chen H, Tania M, Zhang D: Anticancer activities of Nigella sativa (Black cumin). Afr J Tradit Complement Altern Med 2011;8:226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nadkarni KM: Indian Materia Medica, Vol. 2, 3rd edition. Popular Prakashan Pvt. Ltd, Mumbai, 1976, pp. 956 [Google Scholar]
- 7.Ferdous AJ, Islam SN, Ahsan M, Hasan CM, Ahmed ZU: In vitro antibacterial activity of the volatile oil of Nigella sativa seeds against multiple drug-resistant isolates of Shigella spp. and isolates of Vibrio cholerae and Escherichia coli. Phytother Res 1992;6:137–140 [Google Scholar]
- 8.Chakravarty N: Inhibition of histamine release from mast cells by nigellone. Ann Allergy Asthma Immunol 1993;70:237–242 [PubMed] [Google Scholar]
- 9.Burits M, Bucar F: Antioxidant activity of Nigella sativa essential oil. Phytother Res 2000;14:323–328 [DOI] [PubMed] [Google Scholar]
- 10.Worthen DR, Ghosheh OA, Crooks PA: The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res 1998;18:1527–1532 [PubMed] [Google Scholar]
- 11.Kumara SS, Huat BT: Extraction, isolation and characterisation of antitumor principle, alpha-hederin, from the seeds of Nigella sativa. Planta Med 2001;67:29–32 [DOI] [PubMed] [Google Scholar]
- 12.Ivankovic S, Stojkovic R, Jukic M, Milos M, Jurin M: The antitumor activity of thymoquinone and thymohydroquinone in vitro and in vivo. Exp Oncol 2006;28:220–224 [PubMed] [Google Scholar]
- 13.Salomi NJ, Nair SC, Jayawardhanan KK, Varghese CD, Panikkar KR: Antitumour principles from Nigella sativa seeds. Cancer Lett 1992;63:41–46 [DOI] [PubMed] [Google Scholar]
- 14.Gurung RL, Lim SN, Khaw AK, Soon JFF, Shenoy K, Ali SM, Jayapal M, Sethu S, Baskar R, Hande MP: Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS One 2010;5:e12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahena F, Zaidul ISM, Jinap S, Karim AA, Abbas KA, Norulaini NAN, Omar AKM: Application of supercritical CO2 in lipid extraction—a review. J Food Eng 2009;95:240–253 [Google Scholar]
- 16.Brunner G: Supercritical fluids: technology and application to food processing. J Food Eng 2005;67:21–33 [Google Scholar]
- 17.Lizcano LJ, Bakkali F, Ruiz-Larrea MB, Ruiz-Sanz JI: Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food Chem 2010;119:1566–1570 [Google Scholar]
- 18.Chang C, Yang M, Wen H, Chern J: Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002;10:178–182 [Google Scholar]
- 19.Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63 [DOI] [PubMed] [Google Scholar]
- 20.Cheah YH, Azimahtol HL, Abdullah NR: Xanthorrhizol exhibits antiproliferative activity on MCF-7 breast cancer cells via apoptosis induction. Anticancer Res 2006;26:4527–4534 [PubMed] [Google Scholar]
- 21.Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C: Clonogenic assay of cells in vitro. Nat Protoc 2006;1:2315–2319 [DOI] [PubMed] [Google Scholar]
- 22.Liang CC, Park AY, Guan JL: In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007;2:329–333 [DOI] [PubMed] [Google Scholar]
- 23.Shaw LM: Tumor cell invasion assays. Meth Mol Biol 2005;294:97–105 [DOI] [PubMed] [Google Scholar]
- 24.Pavia DL, Lampman GM, Kriz GS, Engel RG: A Small Scale Approach to Organic Laboratory Techniques, 3rd edition. Brooks/Cole, Belmont, CA, USA, 2009 [Google Scholar]
- 25.Mistry BD: A Handbook of Spectroscopic Data Chemistry. Oxford Book Company, Gujrat, India, 2009 [Google Scholar]
- 26.Eguchi K: Apoptosis in autoimmune diseases. Intern Med 2001;40:275–284 [DOI] [PubMed] [Google Scholar]
- 27.Kass GE, Eriksson JE, Weis M, Orrenius S, Chow SC: Chromatin condensation during apoptosis requires ATP. Biochem J 1996;318:749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan TJ, Han LH, Cong RS, Liang J: Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–727 [DOI] [PubMed] [Google Scholar]
- 29.John FRK: History of the events leading to the formulation of the apoptosis concept. Toxicology 2002;181–182:471–474 [DOI] [PubMed] [Google Scholar]
- 30.Zhang YA, Nemunaitis J, Scanlon KJ, Tong AW: Anti-tumorigenic effect of a K-ras ribozyme against human lung cancer cell line heterotransplants in nude mice. Gene Ther 2000;7:2041–2050 [DOI] [PubMed] [Google Scholar]
- 31.Bockhorn M, Jain RK, Munn LL: Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol 2007;8:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M: Cell migration and invasion assays. Methods 2005;37:208–215 [DOI] [PubMed] [Google Scholar]
- 33.Hocman G: Chemoprevention of cancer: phenolic antioxidants (BHT,BHA). Int J Biochem 1988;20:639–651 [DOI] [PubMed] [Google Scholar]
- 34.Ahamed MB, Aisha AF, Nassar ZD, Siddiqui JM, Ismail Z, Omari SM, Parish CR, Majid AM: Cat's whiskers tea (Orthosiphon stamineus) extract inhibits growth of colon tumor in nude mice and angiogenesis in endothelial cells via suppressing VEGFR phosphorylation. Nutr Cancer 2012;64:89–99 [DOI] [PubMed] [Google Scholar]
- 35.Ramos S: Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem 2007;18:427–442 [DOI] [PubMed] [Google Scholar]