Abstract
Endothelial progenitor cells (EPCs) play a key role in tissue repair and regeneration. Previous studies have shown a positive correlation between the number of circulating EPCs and clinical outcomes of patients with traumatic brain injury (TBI). A recent study has further shown that intravenous infusion of human umbilical cord blood-derived endothelial colony-forming cells (ECFCs) improves outcomes of mice subjected to experimental TBI. This follow-up study was designed to determine whether intracerebroventricular (i.c.v.) infusion of ECFCs, which may reduce systemic effects of these cells, could repair the blood–brain barrier (BBB) and promote angiogenesis of mice with TBI. Adult nude mice were exposed to fluid percussion injury and transplanted i.c.v. with ECFCs on day 1 post-TBI. These ECFCs were detected at the TBI zone 3 days after transplantation by SP-DiIC18(3) and fluorescence in situ hybridization. Mice with ECFCs transplant had reduced Evans blue extravasation and brain water content, increased expression of ZO-1 and claudin-5, and showed a higher expression of angiopoietin 1. Consistent with the previous report, mice with ECFCs transplant had also increased microvascular density. Modified neurological severity score and Morris water maze test indicated significant improvements in motor ability, spatial acquisition and reference memory in mice receiving ECFCs, compared to those receiving saline. These data demonstrate the beneficial effects of ECFC transplant on BBB integrity and angiogenesis in mice with TBI.
Key words: : endothelial colony-forming cells, endothelial progenitor cells; transplantation; traumatic brain injury
Introduction
Traumatic brain injury (TBI) disrupts the blood–brain barrier (BBB) and induces neuronal death, resulting in neurological dysfunctions.1 Angiogenesis is a critical process for repairing TBI-induced cerebral damage and providing the neurovascular substrate necessary for neuronal remodeling.2–5 Endothelial progenitor cells (EPCs) are lineage-specific stem cells that are mobilized from the bone marrow (and possibly also from a number of other organs and tissues) to the peripheral blood in response to traumatic, inflammatory, ischemic injuries. They are capable of homing to the area of injury, differentiating into mature endothelial cells (ECs) and being incorporated into growing vessels (angiogenesis)/creating new vessels (vasculogenesis).6–11 Studies in animal models indicated that the transplantation of EPCs can improve functional recovery of limb ischemia,6,12 myocardial ischemia,13,14 and ischemic stroke.15–17 Studies further show that the level of EPCs in peripheral blood was first suppressed in the acute phase of TBI and then rapidly increased.18 This dynamic change is positively correlated with the clinical outcomes of patients with TBI.19 A recent study reports that a venous infusion of ex vivo expanded endothelial colony-forming cells (ECFCs) accelerated angiogenesis and improved cerebral functions after TBI in mice.20
In the present studies, we examine whether intracerebroventricular (i.c.v.) infusion of ECFCs, which could reduce systemic effects of these cells and require a smaller number of cells for transplant, could repair the BBB and promote angiogenesis in mice with TBI.
Methods
Ethics statement
The institutional review board of Tianjin Medical University General Hospital (Tianjin, China) approved all protocols, and informed consent was obtained from human EPC donors. Animal use was in accord with the National Institutes of Health's (Bethesda, MD) Guide for the Care and Use of Laboratory Animals.
Isolation and culture of endothelial colony-forming cells
Mononuclear cells isolated from human umbilical cord blood were cultured and expanded to ECFCs that were verified by staining with DiI-labeled acetylated low-density lipoprotein (acLDL) and fluorescein isothiocyanate (FITC)-conjugated Ulex europeus agglutinin (UEA), as described previously.20,21 For phenotypic characterization, ECFCs were incubated with primary antibodies (Abs) for CD34 (1:200, mouse monoclonal; Santa Cruz Biotechnology, Santa Cruz, CA), kinase domain receptor (KDR; 1:200, rabbit polyclonal; Santa Cruz Biotechnology), von Willebrand factor (vWF; 1:200, rabbit polyclonal; Santa Cruz Biotechnology), and vascular endothelial cadherin (VE-cadherin; 1:200, rabbit polyclonal; Santa Cruz Biotechnology). Human umbilical vein endothelial cells (HUVECs) were isolated by digesting umbilical cords with collagenase (Roche Diagnostics GmbH, Mannheim Germany)22 and used as control specifically for detecting the homing of ECFCs to injured cerebral tissue. Before transplants, ECFCs and HUVECs were labeled with the lipophilic tracer, SP-DiIC18(3) (D7777; Molecular Probes, Eugene OR), according to the manufacturer's protocol.
Experimental traumatic brain injury and endothelial colony-forming cell transplantation
Experimental TBI was induced in adult female BALB/C nude mice (8 weeks of age), as previously described.20 Immediately after TBI, the incision was closed and mice were allowed to recover from anesthesia. One day after TBI, mice were again anesthetized and received the stereotactic-guided transplantation of ECFCs (3×105/3 μL) into the contralateral lateral ventricle (1 mm left lateral from the sagittal suture, 0.5 mm posterior from the coronal suture, 2.5 mm deep from the surface of the cortex) through a 26-gauge Hamilton syringe at a rate of 1 μL/min. The needle was left in place for 5 min before withdrawal. The incision was closed after injection and mice were allowed to recover. Sham mice underwent the same surgical procedure without being exposed to percussion injury and received no treatment.
A total of 108 nude mice were tested for the study and were divided into three groups (n=36/group). Group 1 mice were exposed to TBI and transplanted with ECFCs, group 2 mice were exposed to TBI and infused with an equal volume of saline, and group 3 mice were subjected to sham surgery only. For each, 6 mice were evaluated for BBB permeability, brain water content, immunoblots for selective markers, quantitative reverse-transcription polymerase chain reaction (qRT-PCR), microvascular density (MVD), modified neurological severity score (mNSS), and Morris water maze (MWM).
Fluorescence in situ hybridization
The homing of transplanted ECFCs was also identified by gender-specific fluorescence in situ hybridization (FISH), as previously described.20 Briefly, on the third day after transplantation, mice were sacrificed to collect the brain, which was fixed in 4% paraformaldehyde at 4°C overnight. Brain sections (4 μm) were made through the TBI zone and processed for FISH.
Permeability of brain–blood barrier
BBB permeability was evaluated using Evans blue (EB) dye extravasation, as previously described, with minor modifications.23,24 On the third day after transplantation, mice were injected with EB (2% in saline; Sigma-AQldrich, St. Louis, MO) in a dose of 3 mL/kg through the tail vein and allowed to circulate for 1 h. Mice were then transcardially perfused with saline under deep anesthesia and sacrificed. The brain was removed and separated into the right and left hemispheres along the mid-line. Each hemisphere was weighted separately, homogenized in formamide (1 mL), and incubated at room temperature for 48 h. After centrifugation, the optical density (OD) of the supernatant was measured at 625 nm. The amount of EB (μg/g wet weight) was quantified according to a linear standard curve and expressed as relative amount.
Brain water content
Also on the third day after transplantation, mice were anesthetized and sacrificed to remove the brain. Brain water content was measured by a dry-wet method, as previously described,25 and expressed as a percentage of wet weight.
Immunoblots and quantitative real-time polymerase chain reaction
Mice were sacrificed to obtain the brain, which was quickly snap-frozen in liquid nitrogen on day 3 after transplant. The right cortex was dissected and stored at −80°C until analysis. Frozen brain tissue was solublized in a radioimmunoprecipitation assay buffer (Sigma-Aldrich) containing the protease inhibitor, phenylmethylsulfonyl fluoride (Roche), and separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and subsequently electrotransferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). After blocking with 5% nonfat milk for 2 h at room temperature, membranes were immunoblotted with each of the following Abs: ZO-1 (1:1000, rabbit polyclonal; Abcam, Cambridge, MA) and claudin-5 (1:500, rabbit polyclonal; Abcam) with gentle shaking at 4°C overnight. Bound Abs were recognized by a secondary horseradish peroxidase–conjugated goat anti-rabbit immunoglobulin G (IgG; 1:2000; room temperature, 2 h; Jackson ImmunoResearch Laboratories, PA), followed by incubation with the SuperSignal West Pico Chemiluminent Substrate (Pierce, Rockford, IL). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1000, rabbit monoclonal; Cell Signaling Technology, Irvine, CA) was used as an internal control. Protein level was reported as relative values normalized to the intensity of GAPDH.
For qRT-PCR, total RNA was isolated from a frozen brain with Trizol reagents (Invitrogen, Carlsbad, CA) and quantified by an OD of 260 nm on a Smart Spec™ plus spectrophotometer (Bio-Rad, Hercules, CA). RNA (2 μg) was reversely transcribed to complementary DNA using a Takara PrimeScript® RT reagent Kit (#DRR037; Takara, Dalian, China). PCR was performed using the SYBR Green Realtime PCR method (Toyobo Co., Ltd., Osaka, Japan) on an ABI 7500 instrument (Applied Biosystems, Foster City, CA). All reactions were performed in triplicate; a cycle threshold value was obtained by analysis software. GAPDH was again used as an internal control. Levels of cerebral messenger RNA (mRNA) were reported as a relative value that was normalized to GAPDH.
The following PCR primers were used for RT-PCR:
ANGPT1 (forward: 5’-CACATAGGGTGCAGCAACCA−3’, reverse: 5’-CGTCGTGTTCTGGAAGAATGA−3’);
ANGPT2(forward: 5’-GGAGACCGTCAACAGCTTG−3’, reverse: 5’- CTTCTTTACGGATAGCAACCGAG−3’);
GAPDH (forward: 5’-AGGTCGGTGTGAACGGATTTG−3’, reverse: 5’-TGTAGACCATGTAGTTGAGGTCA−3’)
Immunofluorescence staining
On days 3 and 7 after transplantation, anesthetized mice were perfused with saline transcardially before collecting the brain, which was quickly embedded in the optimal cutting temperature medium (Sakura Finetek USA Inc., Torrance, CA) to make 8-μm sections that were fixed in acetone at 4°C for 5 min.
Cell nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI) for 5 min. Slides were viewed under a fluorescence microscope to count SP-DiIC18(3)-positive cells (ECFCs) in the lesion boundary.
The remaining sections were incubated with a vWF antibody (1:200, rabbit polyclonal; Santa Cruz Biotechnology) overnight at 4°C, followed by a FITC/goat anti-rabbit IgG (1:500; Abcam) at 37°C in the dark for 1 h. Negative controls were identically treated without the primary Ab. vWF positive ECs and vessels with or without a lumen were defined as MVD and counted under a fluorescence microscope (200×) in three randomly selected fields from three separate sections of each sample. MVDs were quantified as the average number of microvessels per viewfield for statistical analyses.26
Modified neurological severity score and Morris water maze
mNSS20,26–28 was used to evaluate the postinjury neurologic functions of experimental mice in a blind manner before and 2, 7, 14, and 21 days after injury.
MWM was performed to test spatial reference memory, as previously described.20,26,28,29 Briefly, a circular open-field tank (diameter, 122 cm; height, 50 cm) was filled with opaque water at 21±2°C, and a target platform was submerged 2 cm below the surface in the middle of the southwest quadrant. On day 14 after TBI, mice were placed in the maze without a platform 1 min/day for 3 days to adapt the trial. Spatial acquisition and reference memory trials were then performed in the next 6 consecutive days. For the first 5 days, mice were released into the water from one of four starting locations (north, east, northwest, and southeast) and allowed to swim freely until they reached and stayed on the platform. If the platform was found within 120 sec, mice were allowed to stay on it for 30 sec. If mice failed to find the platform within 120 sec, they were guided to, and placed on it, for 30 sec. The time of finding the platform (escape latency; EL) and swimming speed were recorded by a tracking system (Ethovision 3.0; Noldus Information Technology, Wageningen, the Netherlands). Twenty-four hours after the last spatial acquisition trial, mice were tested for reference memory. Briefly, after the target platform was removed, mice were put in the northeast position and allowed to swim for 60 sec. The time spent in the target quadrant and swimming speed were recorded.
Statistical analysis
Results were expressed as mean±standard deviation. Data from SP-DiIC18(3) tracking, BBB permeability, immunoblots, qRT-PCR, and mNSS were compared by an independent-samples t-test. Data from brain water content, MVD, and the reference memory trial of MWM were compared by one-way analysis of variance (ANOVA), followed by a least significant difference post-hoc analysis. Data from the spatial acquisition trial of MWM were made with repeated measures of two-way ANOVA. A statistical significance was defined at an error probability of <0.05. Data were analyzed using SPSS software (16.0; SPSS Inc., Chicago, IL).
Results
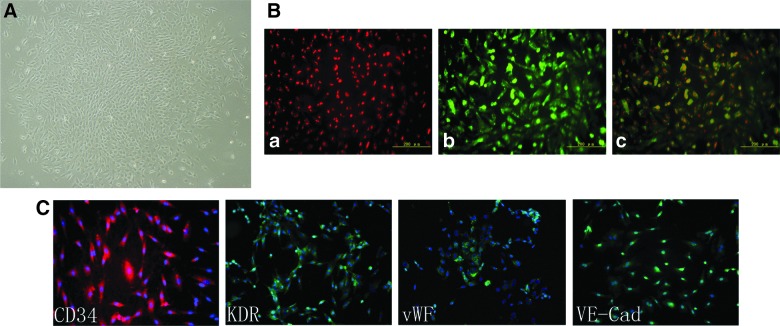
Mononuclear cells from umbilical cord blood were cultured for 5–8 days, as previously described21,30 and formed colonies at approximately 14 days in culture. These ECFCs were defined by their ability to take up DiI-acLDL, bound FITC/UEA-1, and expressed the stem cell marker, CD34, and the EC markers, KDR, VWF, and VE-cadherin (Fig. 1).
FIG. 1.
Generation of ECFCs. (A) Mononuclear cells from cord blood were transformed into spindle-like ECFCs after they were cultured for 14 days and formed colonies. (B) These ECFCs (a) uptake DiI-acLDL and (b) bind FITC-UEA-1 (c, two-color overlay). (C) ECFCs bind antibodies against the stem cell marker, CD34, and the endothelial cell markers, KDR, vWF, and VE-Cad. Nuclei were counterstained with DAPI. The image panels are representative of three separate experiments. ECFCs, endothelial colony-forming cells; Dil-acLDL, DiI-labeled acetylated low-density lipoprotein; FITC, fluorescein isothiocyanate; UEA, Ulex europeus agglutinin; KDR, kinase domain receptor; vWF, von Willebrand factor; VE-Cad, vascular endothelial cadherin; DAPI, 4’,6-diamidino-2-phenylindole. Color image is available online at www.liebertpub.com/neu
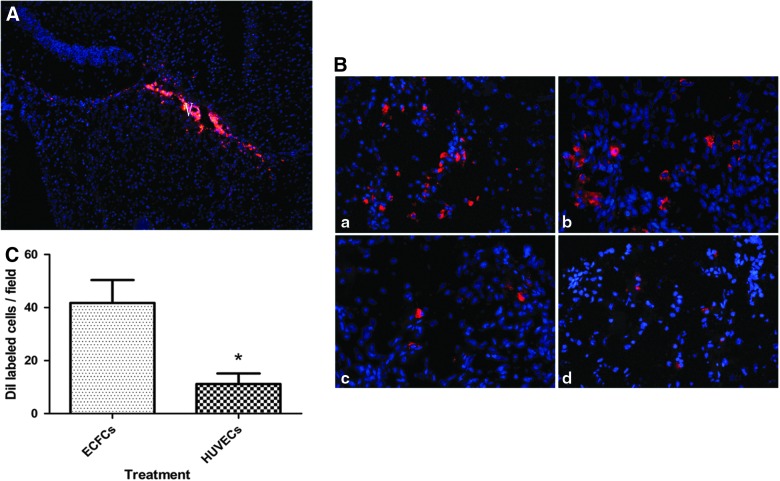
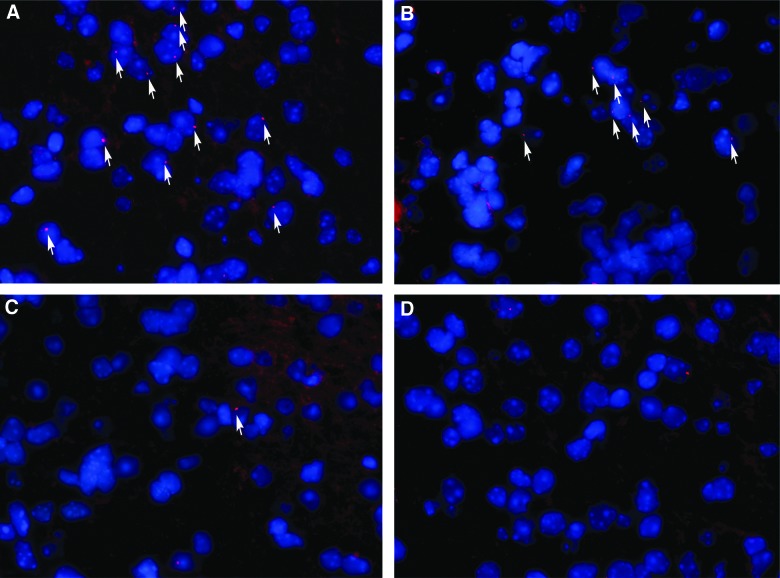
The homing of transplanted ECFCs through the lateral ventricle was evaluated 1 day after transplantation. We used two markers (SP-DiIC18(3) and FISH targeting on Y chromosome from the donor) to track transplanted ECFCs in nude mice to ensure accuracy. Some SP-DiIC18(3)-labeled ECFCs migrated across the corpus callosum toward the TBI zone, whereas others displayed a undirectional diffusion from the lateral ventricle (Fig. 2A). On day 3 after transplantation, ECFCs were detected close to the TBI zone (Fig. 2B, a and b), whereas the number of HUVECs detected in the TBI zone (Fig. 2B, c and d) was significantly lower than that of ECFCs (Fig. 2C, 11.2±3.97/field for HUVECs vs. 41.7±8.67/field for ECFCs; p<0.05). Human Y chromosome in female mice brain tissue was probed 3 days after transplantation by FISH (Fig. 3) as a marker for ECFC homing. The results were consistent with that by SP-DiIC18(3) labeling (Fig. 2).
FIG. 2.
SP-DiIC18(3)-labeled transplanted cells in the TBI zone. (A) Photomicrographs show a distribution pattern of infused SP-DiIC18(3)-positive ECFCs in the lateral ventricle 1 day after transplantation. (B) SP-DiIC18(3)-labeled ECFCs (a and b) and HUVECs (c and d) migrated into the TBI zone on day 3 after transplantation. (C) The number of SP-DiIC18(3)-labeled ECFCs and HUVECs that were homed to the TBI zone are shown (*p<0.05; n=6/group). TBI, traumatic brain injury; ECFCs, endothelial colony-forming cells; HUVECs, human umbilical vein endothelial cells. Color image is available online at www.liebertpub.com/neu
FIG. 3.
FISH detection of transplanted cells. The numbers of ECFCs (A and B) and HUVECs (C and D) in the TBI zone 3 days after transplantation are shown (600×). The panel images are representative of three separate experiments. FISH, fluorescence in situ hybridization; ECFCs, endothelial colony-forming cells; HUVECs, human umbilical vein endothelial cells; TBI, traumatic brain injury. Color image is available online at www.liebertpub.com/neu
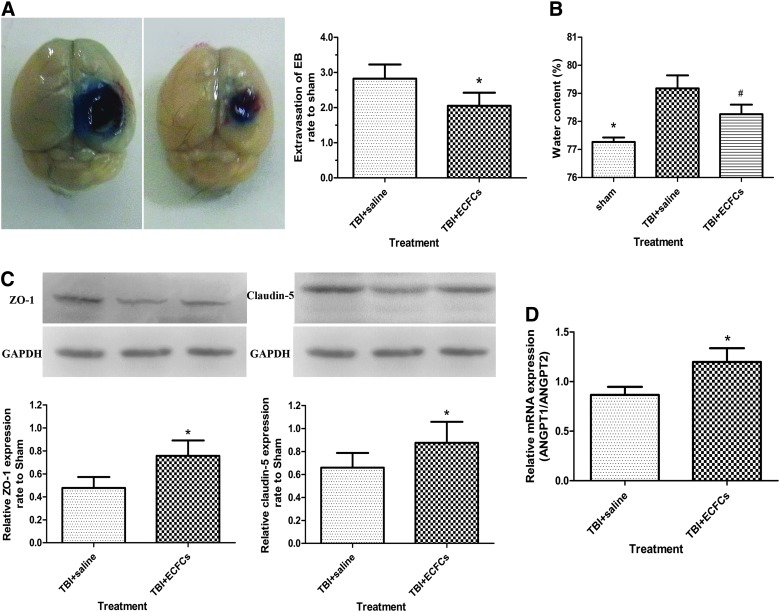
The effect of ECFCs transplant on BBB repair was evaluated by several techniques. First, BBB permeability was assessed by measuring the extravasation of the EB dye 3 days after i.c.v. injection and found to be significantly reduced in mice receiving ECFC transplant, compared to mice receiving saline (Fig. 4A; P<0.05). Second, brain water content was used as a measure of edema, which is associated with the state of BBB integrity. Consistent with results from EB extravasation, edema was significantly less in mice receiving ECFC transplant (Fig. 4B; p<0.05). Third, the expression of the tight-junction–associated proteins, ZO-1 and claudin-5, which are known for promoting the development of tight junctions,31 were increased in mice receiving ECFCs (Fig. 4C; p<0.05). Finally, angiopoietin (Ang)1 is known to stimulate Tie2 phosphorylation and promote angiogenesis and vascular stability, whereas Ang2 is a natural antagonist of Ang1, an Ang1/Ang2 ratio is indicative of the state of angiogenesis, and a low ratio would indicate vascular leakage.32 In mice receiving ECFCs, this ratio was significantly higher than those receiving saline (Fig. 4D; p<0.05) (Supplementary Fig. 1 is available at www.liebertpub.com/neu). Together, these changes are consistent with an improved BBB integrity.
FIG. 4.
Measurements of BBB integrity. (A) The extravasation of the EB dye in brain samples from saline or ECFC-transplanted mice is shown (*p<0.05; n=6/group). (B) Brain water contents in three groups of mice are (*p<0.05, sham group vs. saline group; #p<0.05, saline group vs. ECFC group; n=6/group). (C) The expression of ZO-1 and claudin-5 in mice receiving different treatments is shown (*p<0.05; n=6/group). (D) mRNA of Ang1:Ang2 in mice receiving different treatments (*p<0.05; n=6/group). BBB, blood–brain barrier; EB, Evans blue; TBI, traumatic brain injury; ECFCs, endothelial colony-forming cells; mRNA, messenger RNA; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; Ang, angiopoietin. Color image is available online at www.liebertpub.com/neu
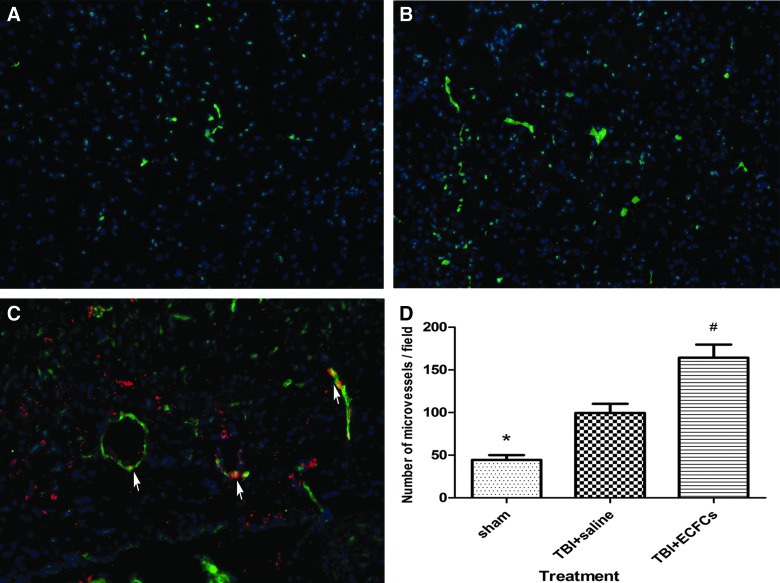
We have also measured the rate of angiogenesis in the TBI zone by quantifying MVD using vWF as a marker on day 7 after transplantation. MVD was significantly higher in ECFC-transplanted mice (164.44±15.27/field) compared to those infused with saline (99.67±10.79/field) or underwent sham surgery (44.56±5.55/field; p<0.05). Double immunostaining of brain tissue further showed that SP-DiIC18(3) labeling was colocalized with vWF positivity in cells at the lesion boundary, suggesting that ECFCs have integrated into vessel-like structures during angiogenesis (Fig. 5).
FIG. 5.
Quantitation of MVD. MVD was defined as vWF-positive cells and vessel-like structures and quantitatively compared among mice that underwent sham surgery (A), received saline (B), and received ECFCs (C) 7 days after transplant (red, SP-DiIC18(3); green, vWF; blue, nuclei. Arrows indicate SP-DiIC18(3)-positive cells that are also positive for VWF, 200×). (D) The number of MVD in three groups of mice are shown (*p<0.05, sham group vs. saline group; #p<0.05, saline group vs. ECFC group; n=6/group). MVD, microvascular density; vWF, von Willebrand factor; ECFCs, endothelial colony-forming cells. Color image is available online at www.liebertpub.com/neu
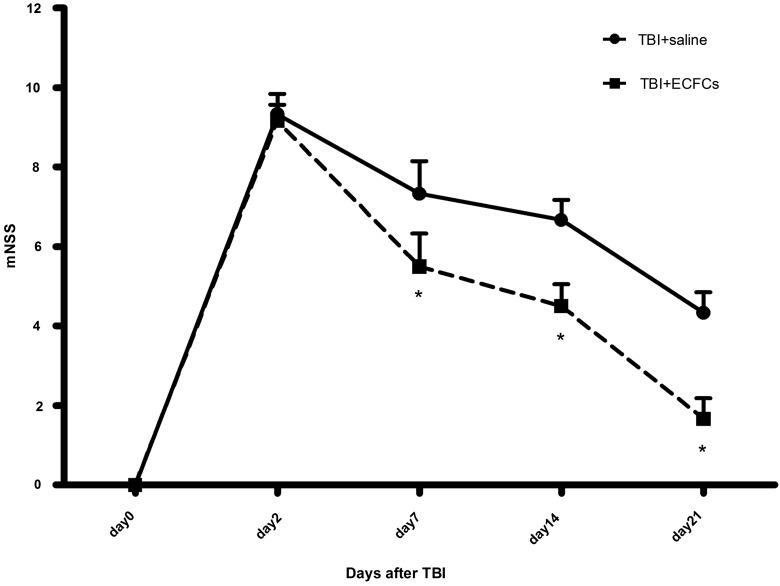
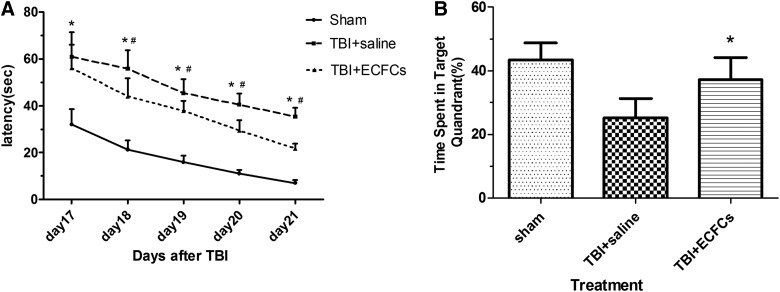
To test whether transplanted ECFCs improved outcomes of mice after TBI, mNSS and MWM were performed. The mNSS score was found to be significantly improved in ECFC-transplanted mice, compared to those infused with an equal volume of saline on days 7, 14, and 21 after TBI (Fig. 6; p<0.05). MWM was used to detect the spatial reference memory for 6 consecutive days, starting on day 17 after TBI. On spatial acquisition trials, the EL was significantly shortened in mice transplanted with ECFCs, compared to those infused with saline at all time points except day 17 after TBI (Fig. 7A; p<0.05). On subsequent reference memory trials, ECFC-treated mice spent more time in the target quadrant, as compared to saline-treated mice (Fig. 7B; p<0.05).
FIG. 6.
mNSS. Score was assigned 7, 14, and 21 days after mice were subjected to TBI and received either ECFCs or saline (*p<0.05; n=6/group). mNSS, modified neurological severity score; TBI, traumatic brain injury; ECFCs, endothelial colony-forming cells.
FIG. 7.
MWM. (A) EL was evaluated 17–21 days after TBI in mice transplanted with ECFCs, saline, or received sham surgery (*p<0.05, sham group vs. saline group; #p<0.05, saline group vs. ECFCs group). (B) Mice transplanted with ECFCs or saline (*p<0.05; n=6/group) or underwent sham surgery on a probe trial. MWM, Morris water maze; EL, escape latency; TBI, traumatic brain injury; ECFCs, endothelial colony-forming cells.
Discussion
In the present study, we demonstrated that ECFCs transplanted by an i.c.v. route were able to home to the TBI zone. ECFC transplant is associated with the increased formation of new vessels and improved neurological functions in immune-deficient nude mice subjected to TBI. The finding is consistent with a previous report on intravenous (i.v.) transfusion of ECFCs.20 But, we further show that i.c.v. transplant requires a small number of cells and provide the evidence that ECFC transplant improved BBB integrity.
The finding demonstrates that transplanted ECFCs were viable and able to home to sites of injury. They were able to differentiate into mature ECs, as demonstrated by the positive staining for vWF. Ingram and colleagues21 and Yoder and colleagues33 found that mononuclear cells cultured for 2 weeks generate highly proliferating endothelial-like cells. These cells have a high potential for proliferation and self-renewal and are capable of forming vascular structures.34–36 It has also been shown that ECFCs cultured from the umbilical cord blood have a greater number and telomerase activity than those from the peripheral blood.21 In addition to the source of cells, transplant routes may also influence the survival of these cells. I.c.v. injection permits a more efficient delivery of donor cells to an injury site because it allows a greater concentration of transplanted cells in the brain, as compared to i.v. injection, which distributes cells systemically. In addition, an i.c.v. injection requires a relatively small number of cells, compared to i.v. injection.
Once injected into the ventricle, ECFCs were found to rapidly migrate to cerebral tissue in the TBI zone, as identified by a newly developed FISH technology to detect transplanted gender-mismatched cells. Detecting the male Y chromosome from a male donor in a female recipient has conclusively demonstrated the homing of transplanted cells to the site of injury as early as the third day after transplant. This homing efficiency was much greater than mature ECs from human umbilical veins.
These transplanted ECFCs were not only homed to the site of injury, but also functional in repairing the BBB disrupted by TBI, as indicated by the reduction in dye extravasations and cerebral edema, and improved endothelial cell tight junction (increased expression of ZO-1 and claudin-5). The BBB is a physical barrier that is crucial for maintaining homoeostasis of the central nervous system and consists of ECs, basal lamina, and astrocytic foot processes.37 The disruption of this protective BBB is a hallmark event of severe TBI and contributes to such pathologies as brain edema, inflammation, and loss of neuronal viability or function after brain injury.38,39 We also found that ECFC transplant was associated with a higher Ang1/Ang2 ratio, which stimulates Tie2 phosphorylation, reduces endothelial permeability, and enhances vascular stabilization and maturation, as reported previously.40–42 As a result of these changes, mice receiving ECFCs have a higher number of vWF-positive ECs and vasculature, as compared to mice receiving an equal value of saline, indicating that these cells have integrated into existing vasculature or generated new vessel-like structures at the site of injury. This finding is consistent with previous reports that ECFCs are capable of enhancing neovascularization in injured tissue and were found to reduce ischemic events secondary to a primary effect to the brain, such as vascular injury and tissue edema.43,44 Further, transplanted cells may also secrete or stimulate ambient cells to secrete vascular growth factors, such as vascular endothelial growth factor, granuclocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, and stromal-derived factor 1, to promote angiogenesis.45,46 Increasing angiogenesis and repairing BBB integrity could restore local blood flow to promote cerebral repairs and improve neurological and memory function of a TBI brain.
In summary, we found that i.c.v.-transplanted ECFCs homed to the TBI zone and contribute to the improvement of neurological functions, potentially by repairing disrupted BBB and enhancing angiogenesis in the host brain. Our findings suggest that ECFC-based cell therapy may improve recovery of patients with TBI. Additional studies may be needed to elucidate the mechanism of enhanced angiogenesis and neurogenesis after ECFC transplantation.
Supplementary Material
Acknowledgments
The authors acknowledge Wei-yun Cui, Li Liu, and Lei Zhou for their excellent technical support. The work was supported by the National Natural Science Foundation of China (grant no.: 81271361) and the Tianjin Health Bureau Technology Foundation (grant no.: 07KZ26).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Werner C., and Engelhard K. (2007). Pathophysiology of traumatic brain injury. Br. J. Anaesth. 99, 4–9 [DOI] [PubMed] [Google Scholar]
- 2.Murasawa S., and Asahara T. (2005). Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 20, 36–42 [DOI] [PubMed] [Google Scholar]
- 3.Noguchi C.T., Asavaritikrai P., Teng R., and Jia Y. (2007). Role of erythropoietin in the brain. Crit. Rev. Oncol. Hematol. 64, 159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong Y., Mahmood A., and Chopp M. (2010). Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs 11, 298–308 [PMC free article] [PubMed] [Google Scholar]
- 5.Hayward N.M., Immonen R., Tuunanen P.I., Ndode-Ekane X.E., Grohn O., and Pitkanen A. (2010). Association of chronic vascular changes with functional outcome after traumatic brain injury in rats. J. Neurotrauma 27, 2203–2219 [DOI] [PubMed] [Google Scholar]
- 6.Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., and Isner J.M. (1997). Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M., Magner M., Isner J.M., and Asahara T. (1999). Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 [DOI] [PubMed] [Google Scholar]
- 8.Walter D.H., Rittig K., Bahlmann F.H., Kirchmair R., Silver M., Murayama T., Nishimura H., Losordo D.W., Asahara T., and Isner J.M. (2002). Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 105, 3017–3024 [DOI] [PubMed] [Google Scholar]
- 9.Gill M., Dias S., Hattori K., Rivera M.L., Hicklin D., Witte L., Girardi L., Yurt R., Himel H., and Rafii S. (2001). Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ. Res. 88, 167–174 [DOI] [PubMed] [Google Scholar]
- 10.Lev E.I., Kleiman N.S., Birnbaum Y., Harris D., Korbling M., and Estrov Z. (2005). Circulating endothelial progenitor cells and coronary collaterals in patients with non-ST segment elevation myocardial infarction. J. Vasc. Res. 42, 408–414 [DOI] [PubMed] [Google Scholar]
- 11.Khoo C.P., Pozzilli P., and Alison M.R. (2008). Endothelial progenitor cells and their potential therapeutic applications. Regen. Med. 3, 863–876 [DOI] [PubMed] [Google Scholar]
- 12.Murohara T., Ikeda H., Duan J., Shintani S., Sasaki K., Eguchi H., Onitsuka I., Matsui K., and Imaizumi T. (2000). Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Invest. 105, 1527–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon Y.S., Lee N., and Scadova H. (2005). Myocardial regeneration with bone-marrow-derived stem cells. Biol. Cell 97, 253–263 [DOI] [PubMed] [Google Scholar]
- 14.Kawamoto A., Tkebuchava T., Yamaguchi J., Nishimura H., Yoon Y.S., Milliken C., Uchida S., Masuo O., Iwaguro H., Ma H., Hanley A., Silver M., Kearney M., Losordo D.W., Isner J.M., and Asahara T. (2003). Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation 107, 461–468 [DOI] [PubMed] [Google Scholar]
- 15.Taguchi A., Soma T., Tanaka H., Kanda T., Nishimura H., Yoshikawa H., Tsukamoto Y., Iso H., Fujimori Y., Stern D.M., Naritomi H., and Matsuyama T. (2004). Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 114, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Z.G., Zhang L., Jiang Q., and Chopp M. (2002). Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ. Res. 90, 284–288 [DOI] [PubMed] [Google Scholar]
- 17.Fan Y., Shen F., Frenzel T., Zhu W., Ye J., Liu J., Chen Y., Su H., Young W.L., and Yang G.Y. (2010). Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann. Neurol. 67, 488–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L., Liu H., Jiao J., Bergeron A., Dong J.F., and Zhang J. (2007). Changes in circulating human endothelial progenitor cells after brain injury. J. Neurotrauma 24, 936–943 [DOI] [PubMed] [Google Scholar]
- 19.Liu L., Wei H., Chen F., Wang J., Dong J.F., and Zhang J. (2011). Endothelial progenitor cells correlate with clinical outcome of traumatic brain injury. Crit. Care Med. 39, 1760–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Li Y., Wang S., Han Z., Huang X., Li S., Chen F., Niu R., Dong J.F., Jiang R., and Zhang J. (2013). Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J. Surg. Res. June11. 10.1016/j.jss.2013.05.073. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Ingram D.A., Mead L.E., Tanaka H., Meade V., Fenoglio A., Mortell K., Pollok K., Ferkowicz M.J., Gilley D., and Yoder M.C. (2004). Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104, 2752–2760 [DOI] [PubMed] [Google Scholar]
- 22.Baudin B., Bruneel A., Bosselut N., and Vaubourdolle M. (2007). A protocol for isolation and culture of human umbilical vein endothelial cells. Nat. Protoc. 2, 481–485 [DOI] [PubMed] [Google Scholar]
- 23.Zhao J., Pati S., Redell J.B., Zhang M., Moore A.N., and Dash P.K. (2012). Caffeic acid phenethyl ester protects blood-brain barrier integrity and reduces contusion volume in rodent models of traumatic brain injury. J. Neurotrauma 29, 1209–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taya K., Marmarou C.R., Okuno K., Prieto R., and Marmarou A. (2010). Effect of secondary insults upon aquaporin-4 water channels following experimental cortical contusion in rats. J. Neurotrauma 27, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott K.A., and Jasper H. (1949). Measurement of experimentally induced brain swelling and shrinkage. Am. J. Physiol. 157, 122–129 [DOI] [PubMed] [Google Scholar]
- 26.Li S., Wei M., Zhou Z., Wang B., Zhao X., and Zhang J. (2012). SDF-1alpha induces angiogenesis after traumatic brain injury. Brain Res. 1444, 76–86 [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Sanberg P.R., Li Y., Wang L., Lu M., Willing A.E., Sanchez-Ramos J., and Chopp M. (2001). Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 32, 2682–2688 [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Wang B., Kan Z., Zhang B., Yang Z., Chen J., Wang D., Wei H., Zhang J.N., and Jiang R. (2012). Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J. Neurotrauma 29, 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vorhees C.V., and Williams M.T. (2006). Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 1, 848–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y., Weisdorf D.J., Solovey A., and Hebbel R.P. (2000). Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 105, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao H., Wang Z., Liu Y., Wang P., and Xue Y. (2011). Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 44, 130–139 [DOI] [PubMed] [Google Scholar]
- 32.Hansen T.M., Moss A.J., and Brindle N.P. (2008). Vascular endothelial growth factor and angiopoietins in neurovascular regeneration and protection following stroke. Curr. Neurovasc. Res. 5, 236–245 [DOI] [PubMed] [Google Scholar]
- 33.Yoder M.C., Mead L.E., Prater D., Krier T.R., Mroueh K.N., Li F., Krasich R., Temm C.J., Prchal J.T., and Ingram D.A. (2007). Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109, 1801–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson M.R., and Yoder M.C. (2011). Endothelial progenitor cells: quo vadis? J. Mol. Cell. Cardiol. 50, 266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melero-Martin J.M., Khan Z.A., Picard A., Wu X., Paruchuri S., and Bischoff J. (2007). In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109, 4761–4768 [DOI] [PubMed] [Google Scholar]
- 36.Mukai N., Akahori T., Komaki M., Li Q., Kanayasu-Toyoda T., Ishii-Watabe A., Kobayashi A., Yamaguchi T., Abe M., Amagasa T., and Morita I. (2008). A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp. Cell Res. 314, 430–440 [DOI] [PubMed] [Google Scholar]
- 37.Janzer R.C., and Raff M.C. (1987). Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325, 253–257 [DOI] [PubMed] [Google Scholar]
- 38.Shear D.A., Lu X.C., Pedersen R., Wei G., Chen Z., Davis A., Yao C., Dave J., and Tortella F.C. (2011). Severity profile of penetrating ballistic-like brain injury on neurofunctional outcome, blood-brain barrier permeability, and brain edema formation. J. Neurotrauma 28, 2185–2195 [DOI] [PubMed] [Google Scholar]
- 39.Unterberg A.W., Stover J., Kress B., and Kiening K.L. (2004). Edema and brain trauma. Neuroscience 129, 1021–1029 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z.G., Zhang L., Tsang W., Soltanian-Zadeh H., Morris D., Zhang R., Goussev A., Powers C., Yeich T., and Chopp M. (2002). Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 22, 379–392 [DOI] [PubMed] [Google Scholar]
- 41.Pfaff D., Fiedler U., and Augustin H.G. (2006). Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems as regulators of cell trafficking. J. Leukoc. Biol. 80, 719–726 [DOI] [PubMed] [Google Scholar]
- 42.Suri C., Jones P.F., Patan S., Bartunkova S., Maisonpierre P.C., Davis S., Sato T.N., and Yancopoulos G.D. (1996). Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 [DOI] [PubMed] [Google Scholar]
- 43.Greve M.W., and Zink B.J. (2009). Pathophysiology of traumatic brain injury. Mt. Sinai J. Med. 76, 97–104 [DOI] [PubMed] [Google Scholar]
- 44.Khan M., Im Y.B., Shunmugavel A., Gilg A.G., Dhindsa R.K., Singh A.K., and Singh I. (2009). Administration of S-nitrosoglutathione after traumatic brain injury protects the neurovascular unit and reduces secondary injury in a rat model of controlled cortical impact. J. Neuroinflammation 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman J., Li J., Orschell C.M., and March K.L. (2003). Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107, 1164–1169 [DOI] [PubMed] [Google Scholar]
- 46.Rouhl R.P., van Oostenbrugge R.J., Damoiseaux J., Tervaert J.W., and Lodder J. (2008). Endothelial progenitor cell research in stroke: a potential shift in pathophysiological and therapeutical concepts. Stroke 39, 2158–2165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.