Abstract
Human X-linked blue-cone monochromacy (BCM), a disabling congenital visual disorder of cone photoreceptors, is a candidate disease for gene augmentation therapy. BCM is caused by either mutations in the red (OPN1LW) and green (OPN1MW) cone photoreceptor opsin gene array or large deletions encompassing portions of the gene array and upstream regulatory sequences that would predict a lack of red or green opsin expression. The fate of opsin-deficient cone cells is unknown. We know that rod opsin null mutant mice show rapid postnatal death of rod photoreceptors. Using in vivo histology with high-resolution retinal imaging, we studied a cohort of 20 BCM patients (age range 5–58) with large deletions in the red/green opsin gene array. Already in the first years of life, retinal structure was not normal: there was partial loss of photoreceptors across the central retina. Remaining cone cells had detectable outer segments that were abnormally shortened. Adaptive optics imaging confirmed the existence of inner segments at a spatial density greater than that expected for the residual blue cones. The evidence indicates that human cones in patients with deletions in the red/green opsin gene array can survive in reduced numbers with limited outer segment material, suggesting potential value of gene therapy for BCM.
Introduction
The human retina has on average 100 million rod photoreceptors that subserve night vision and 6 million cone photoreceptors responsible for daylight, color, and fine spatial vision (Osterberg, 1935; Curcio et al., 1990). Three cone photoreceptor types with light-absorbing visual pigments of different wavelength sensitivity—named simply blue (short-wavelength sensitive, S), green (middle-wavelength sensitive, M), and red (long-wavelength sensitive, L)—give rise to trichromacy of normal human color vision (Young, 1802; von Helmholtz, 1866). The apoprotein opsins of rods and three types of cones are integral membrane proteins in the photoreceptor outer segments. Four kinds of pigments are thus formed when opsins are covalently linked to the 11-cis-retinal chromophore. Light absorbed by the pigments leads to isomerization of 11-cis to all-trans retinoid; the phototransduction cascade is activated in the corresponding rod or cone cell; and visual perception begins. At least two visual (retinoid) cycles are responsible for recycling the all-trans-retinal back to the 11-cis configuration in order to recharge the rod and cone pigments for continued vision (Nathans et al.,1986a,b; Saari, 2000; Palczewski, 2010; Neitz and Neitz, 2011; Wang and Kefalov, 2011).
The three cone subtypes differ in numbers and spatial distribution in adult human retina (Hofer et al., 2005). L and M cones are the most populous and peak density is in the fovea, the very central retina; S cones are only about 2–7% of the total cone population (Curcio et al., 1990, 1991; Mustafi et al., 2009). The gene encoding S-cone opsin (OPN1SW) is located on chromosome 7q31.3–q32. OPN1LW and OPN1MW, encoding the L- and M-cone opsins, respectively, are located on Xq28 and arranged as a head-to-tail tandem array, and expression is driven and regulated by specific promoters and a single upstream locus control region (LCR) (Nathans et al.,1986a,b; Neitz and Neitz, 2011).
X-linked eye disorders resulting from mutations in the L-/M-cone opsin gene array range from common color vision deficiencies to severe loss of day vision with nystagmus and macular degeneration (Nathans et al.,1986a, 1989; Neitz and Neitz, 2011). The latter disorder, named blue-cone monochromacy (BCM; OMIM #303700), is associated with three categories of mutations affecting OPN1LW and OPN1MW loci. Recent imaging analyses of the retina in subjects with two of the mutation types (missense mutations and L/M interchange mutations) reported differences in disease expression between them and raised potential issues with their candidacy for visual restoration by gene augmentation therapy (Carroll et al., 2012). The third category involving large deletions of the L/M pigment genes and/or deletions upstream of the L pigment gene containing the LCR, however, was not studied with modern methods. These mutations would be expected to result in complete absence of L-/M-cone opsin expression (Wang et al., 1992). A clue about possible effects of such mutations came with the finding of abnormal cone mosaics in carrier females (Carroll et al., 2010). We evaluated the retinal structure and function in BCM caused by such large deletion mutations, and weighed the value and goals of gene augmentation as a therapy for such patients.
Materials and Methods
Human subjects
Twenty patients (age range 5–58 years) with a clinical diagnosis of BCM and mutations in the X-chromosome visual pigment genes were included (Table 1). Informed consent or assent was obtained; procedures followed the Declaration of Helsinki and had institutional review board approval. All patients underwent a complete eye examination, genetic analyses, and specialized tests of retinal cross-sectional structure; subsets of patients were imaged with adaptive optics scanning laser ophthalmoscope (AOSLO) or evaluated with specialized visual function tests.
Table 1.
Clinical Characteristics of the Blue-Cone Monochromacy Patients
| |
|
|
|
|
|
|
|
ERGfamplitude |
|
|---|---|---|---|---|---|---|---|---|---|
| Patient/family | Age at visits (years) | Visual acuitya | Refractionb | Nystagmus (in early life)c | Light aversion | Color vision (confusion axis)d | S-cone functione | Rod b-wave | Cone flicker |
| P1/F1 |
5, 8 |
20/100 |
−12.50 |
+ (2–3 mos) |
+ |
Protan-deutan |
+ |
← np → |
|
| P2/F1 |
8, 10 |
20/80 |
−3.75 |
+ (2–3 mos) |
+ |
Protan-deutan |
+ |
← np → |
|
| P3/F1 |
11, 13 |
20/63 |
−12.75 |
+ (2–3 mos) |
+ |
Protan-deutan |
+ |
← np → |
|
| P4/F1 |
48, 50 |
20/100 |
−10.25 |
+ (infancy) |
+ (disabling) |
Protan-deutan |
+ |
N |
A |
| P5/F2 |
7 |
20/100 |
−3.25 |
+ (3–4 mos),↑(12 mos),↓(2.5 yr) |
+ |
Protan-deutan |
+ |
N(b) |
A |
| P6/F2 |
12 |
20/100 |
−5.00 |
+ (9 mos),↓(18 mos),↓↓ (4 yr) |
+ (not prominent) |
Protan-deutan |
+ |
← np → |
|
| P7/F3 |
5, 7 |
20/250 |
−0.50 |
+ |
No complaints |
All three axes |
+ |
N |
A |
| P8/F3 |
10, 19 |
20/100 |
−5.00 |
+ (4 mos) |
No complaints |
Protan-deutan |
+ |
← np → |
|
| P9/F3 |
16, 25 |
20/100 |
−6.00 |
+ (6 mos) |
+ (disabling) |
Protan-deutan |
np |
N |
A |
| P10/F3 |
19, 28 |
20/125 |
−6.50 |
+ (10 mos) |
+ |
Protan-deutan |
+ |
N |
A |
| P11/F4 |
7 |
20/80 |
+2.00 |
+ |
+ |
Protan-deutan |
np |
N |
A |
| P12/F4 |
41 |
20/160 |
−13.00 |
+ |
+ |
np |
np |
← np → |
|
| P13/F4 |
58 |
20/200 |
−10.00 |
+ |
+ |
np |
np |
N |
A |
| P14/F5 |
11 |
20/100 |
−7.50 |
+ (infancy) |
+ |
Protan-deutan |
+ |
N(b) |
A |
| P15/F6 |
14 |
20/100 |
−4.00 |
+ (3 mos) |
No complaints |
Protan-deutan |
+ |
N |
A |
| P16/F7 |
28 |
20/125 |
−8.50 |
+ (<1 yr),↓(later in life) |
+ (disabling) |
Protan-deutan |
+ |
N |
A |
| P17/F8 |
33 |
20/100 |
−3.75 |
+ |
+ (disabling) |
Protan-deutan |
+ |
← np → |
|
| P18/F9 |
35 |
20/63 |
−5.50 |
+ |
+ (disabling) |
Protan-deutan |
+ |
N(b) |
A |
| P19/F10 |
43 |
20/80 |
−6.00 |
+ (minimal) |
Glare |
Deutan |
+ |
N |
A |
| P20/F11 | 55 | 20/80 | −6.50 | + | + (disabling) | All three axes | + | ← np → | |
ERG, electroretinography; np, not performed; +, present.
Best corrected visual acuity at most recent visit; similar in the two eyes.
Spherical equivalent; average of both eyes.
Ages when nystagmus was first noted or when changed, amplifying the comment “in early life” when possible. The ages cited are from historical accounts by patients or parents (mos, months; yr, years). Arrows indicate whether there was recalled reduction or increase (and at what age).
Farnsworth D-15 color panel result.
Detection of 440 nm target on 100 photċcd/m2 yellow background, or detectable S-cone ERG.
ERG was performed in different laboratories with different electrode types, protocols, and normal limits. A, abnormal; N, within normal limits of the laboratory; N(b), borderline normal—reports indicated blink or nystagmus artifact, imperfect cooperation, head not completely within ganzfeld stimulus, or other possible sources of reduced amplitude.
Genetic analyses
Whole venous blood samples were collected, and genomic DNA was extracted according to standard procedures. Details are provided in Supplementary Methods (Supplementary Data are available online at www.liebertonline.com/hum).
En face autofluorescence imaging and in vivo microscopy of human retina
Health of the retinal pigment epithelium (RPE) was evaluated with reduced-illuminance autofluorescence imaging (RAFI) as published (Cideciyan et al., 2007). Near-infrared (NIR) excitation was used to detect melanin granules and short-wavelength (SW) excitation was used to detect lipofuscin granules (Cideciyan et al., 2007; Gibbs et al. 2009). Cross-sectional images of the retina were obtained with a spectral-domain optical coherence tomography (OCT) instrument (RTVue-100; Optovue Inc., Fremont, CA). Details are provided in Supplementary Methods.
Adaptive optics imaging of the photoreceptor mosaic
A previously described adaptive optics scanning laser ophthalmoscope (AOSLO) was used to image each subject's photoreceptor mosaic (Dubra et al., 2011). Details are provided in Supplementary Methods.
Spectral sensitivity function
Spectral sensitivity functions were measured in normal subjects (n=3, age range 31–50) and BCM P15 using a modified perimeter with methods previously described (Cideciyan et al., 1998). Details are provided in Supplementary Methods.
Results
Genetic findings and clinical characteristics of the BCM patients
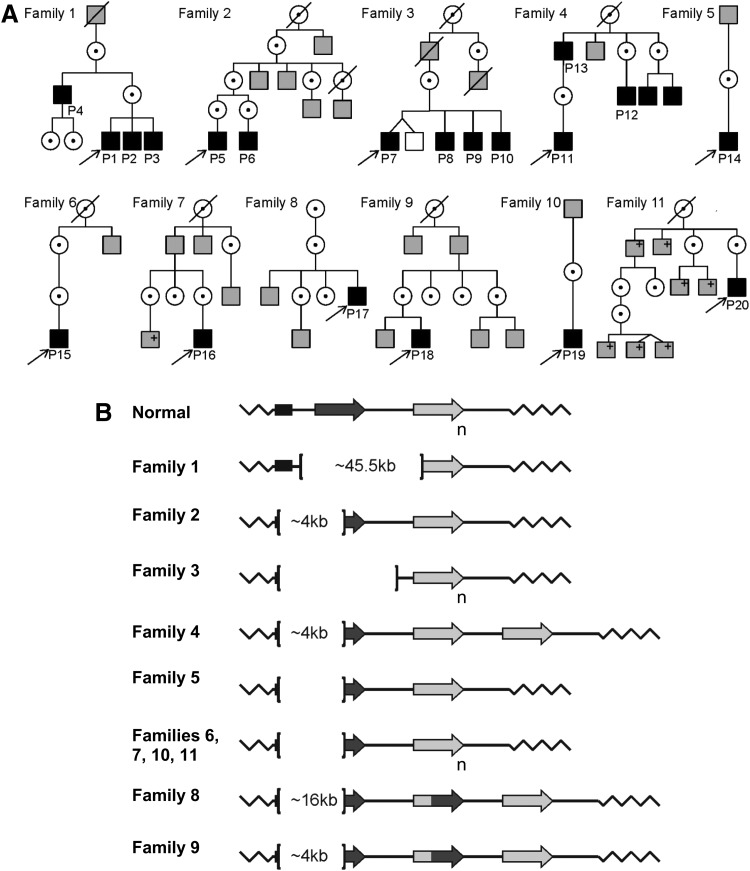
Pedigrees of the 11 families in this study suggested X-linked inheritance (Fig. 1A). The clinical diagnosis of all 20 patients was BCM. From early life, there was reduced visual acuity and nystagmus. When there was more specific information (by history) of age of onset or a decline or an increase in nystagmus, this was noted (Table 1). All but three of the patients described light aversion to some degree, leading to coping strategies such as almost continuous wear of sunglasses or squinting. Color vision was abnormal, indicating cone dysfunction, but there was detectable S-cone function. Electroretinography revealed normal or nearly normal rod waveforms but severely abnormal cone signals (Table 1). Genetic analyses identified genomic deletions of various extents at the OPN1MW/OPN1LW gene cluster in all 20 BCM patients (Fig. 1B). In 10 of the families, the deletions encompassed the LCR as well as the OPN1LW promoter. In Family 1, the LCR region was intact, but a large (over 45.5 kb) deletion covered all coding exons of the OPN1LW gene as well as the promoter and first coding exon of the OPN1MW gene (Fig. 1B). The types of deletions represented in the 11 families are considered sufficient for the complete loss of expression of the OPN1MW/OPN1LW genes on the X-chromosome (Wang et al., 1992).
FIG. 1.
Pedigrees and genotypes of BCM families. (A) Pedigrees of the 11 families. Black-filled squares, males diagnosed with BCM based on clinical evaluations and molecular testing; gray-filled squares, affected males by history;+, positive for familial BCM mutation; unfilled squares, unaffected males by history; dotted circles, carrier females. (B) Schematic representation of BCM genotypes. In individuals with normal color vision, the X-chromosomal OPN1LW/OPN1MW gene array consists of a proximal long-wavelength-sensitive opsin (OPN1LW) gene (dark-gray arrow), and one or more middle-wavelength-sensitive opsin (OPN1MW) genes (light-gray arrow) arranged in a head-to-tail tandem repeat. Subscript “n” indicates one or more M pigment genes. L/M hybrid genes are shown (dark-/light-gray arrow). Each gene is preceded by a proximal promoter and the expression is controlled by a single LCR upstream of the array (black rectangle). Genetic analyses implied that the OPN1LW/OPN1MW gene clusters in all patients of this study were impaired by large deletions affecting the LCR and varying parts of the opsin gene cluster with the exception of Family 1, which had an intact LCR with a deletion spanning the OPN1LW and OPN1MW genes. Brackets demarcate the deletions; deletion size is indicated inside the brackets where defined. BCM, blue-cone monochromacy; LCR, locus control region.
Near-normal retinal-macular appearance by en face viewing in most BCM eyes
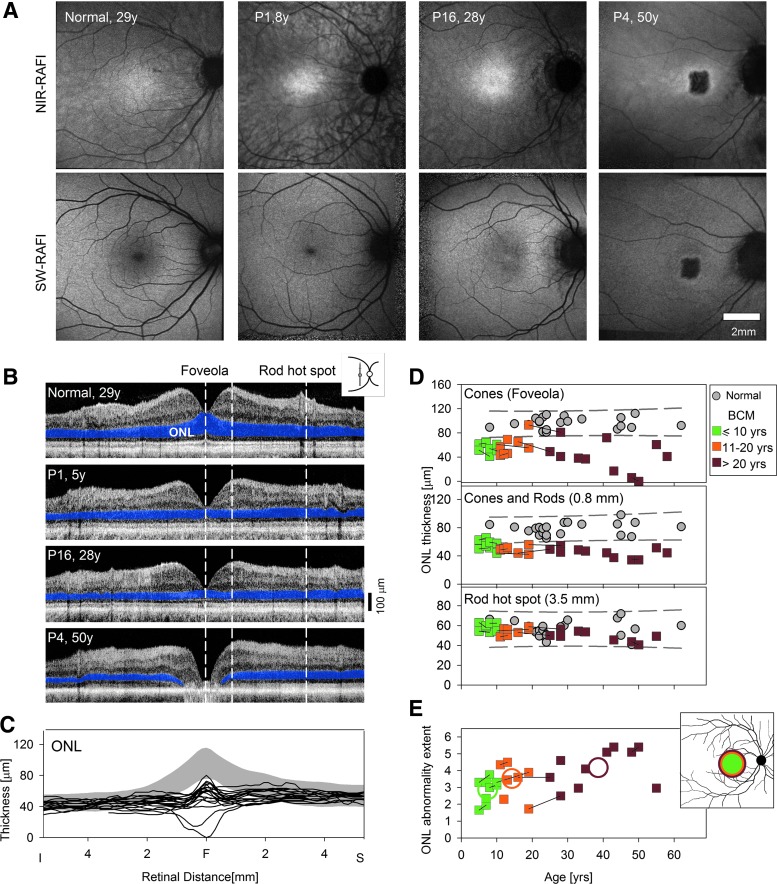
The traditional clinical view is that BCM is a congenital cone vision disturbance with normal retinal appearance in early life but the possibility of macular degeneration later in life (Weleber, 2002). En face imaging, performed with RAFI methods (Cideciyan et al., 2007), confirmed this clinical impression in our BCM cohort (Fig. 2A). RAFI assays the health of the RPE—the cellular layer adjacent to the photoreceptors—taking advantage of its naturally occurring fluorophores melanin and lipofuscin excited by NIR and SW lights (Gibbs et al., 2009). P1 (age 8) represents the majority of BCM patients who showed a macular appearance indistinguishable from normal with its characteristic higher central intensity on NIR-RAFI and lower central intensity on SW-RAFI (Fig. 2A). P16 (age 28) and two other BCM patients (P19, P20; age 43 and 55) represent a second group with minor abnormalities consisting of a lack of a distinct foveal hypo-autofluorescence on SW-RAFI and/or heterogeneity on NIR-RAFI (Fig. 2A). P4 (age 50) was the only BCM patient in this cohort showing a distinct region of foveal RPE atrophy apparent on both imaging modalities.
FIG. 2.
Younger BCM patients show normal RPE but abnormal thinning of the photoreceptor nuclear layer. (A) En face imaging of melanin and lipofuscin fluorophores of the RPE using NIR-RAFI and SW-RAFI, respectively, in a normal subject (age 29; myope, −6D) and three BCM patients. The small dark foveal region in the normal and P1 on SW-RAFI is the result of absorption of the excitation light by dense macular pigment; the larger dark region in the central macula of P4 on NIR-RAFI and SW-RAFI represents RPE atrophy. Retinal blood vessels and the optic nerve head also appear darker than the background. All images are shown contrast stretched for visibility of features. (B) Cross-sectional scans along the vertical meridian crossing the fovea in the same four eyes as shown in (A). ONL, where the photoreceptor nuclei reside, is highlighted (blue). Vertical dashed lines delineate the foveola dominated by cone photoreceptors, 0.8 mm superior retina with cone and rod photoreceptors, and the rod hot spot dominated by rod photoreceptors. (C) ONL thickness vertically across the central 10 mm of retina is graphically displayed for a group of normal subjects (gray represents mean±2SD; n=22; age range 8–62 years), and the BCM data (lines). (D) ONL thickness at the foveola (upper), 0.8 mm superior retina (middle), and the rod hot spot (lower) as a function of age in patients (colored symbols: green, age <10; orange, age range 10–20; brown, age >20 years) and normal controls (gray symbols). The 95% prediction interval of linear regression fit to the normal data is shown (gray dashed lines). Symbols connected with lines represent patients with multiple visits. (E) The extent of ONL abnormality along the vertical meridian as a function of age. Individual patient data are displayed as in (D). Average results for the three age groups are shown with colored circles. Inset, schematic of the average extent of ONL abnormality for the three age groups overlaid to scale on a representative retinal view. ONL, outer nuclear layer; RPE, retinal pigment epithelium.
Cone photoreceptor cells in BCM diminished already in childhood
Using retinal cross-sectional imaging, we examined the hypothesis that BCM is simply an early-onset cone visual disturbance and only older patients may show macular atrophy. The layer corresponding to photoreceptor nuclei was measured to determine whether the photoreceptor cells were intact (Fig. 2B–E). Scans crossing the fovea along the vertical meridian are shown in a representative normal subject and three BCM patients (Fig. 2B). The photoreceptor outer nuclear layer (ONL), which includes the photoreceptor nuclei and their axons (Lujan et al., 2011), is highlighted. P1 (age 5) and P16 (age 28) showed some differences from the normal (age 29), whereas P4 (age 50) had foveal degeneration with a ∼2 mm region missing all photoreceptor nuclei (Fig. 2B). Quantitation of ONL thickness in all BCM patients indicated that there was a central region of abnormal thinning surrounded by normal or near-normal thickness at greater eccentricities (Fig. 2C). At the foveola, which normally is dominated by L/M cones and lacks rods (Borwein et al., 1980; Youdelis and Hendrickson, 1986), the ONL was abnormally thin in all patients but one (Fig. 2D, upper panel). Younger BCM patients between the ages of 5 and 20 showed a mean foveolar ONL thickness of 60.7 μm (SD=13 μm; n=12), which is about 60% of normal (mean±SD, 95.7±9.5 μm; n=22, age range 8–62). Older BCM patients, with some exceptions, tended to show increased thinning of foveolar ONL with age (Fig. 2D, upper panel). In a subset of 8 patients (P1–P4, P7–P10), longitudinal data spanning 2–9 years (mean, 5 years) were suggestive of a slowly progressive disease averaging 11.9 μm/decade thinning of the ONL at the foveola.
Immediately surrounding the foveola (∼0.8 mm eccentric), rod and cone nuclei normally occupy the ONL. All but the youngest patients showed abnormal ONL thickness at this location (Fig. 2D, middle panel), implying potential involvement of macular rods in addition to cones in the BCM pathology. The highest density of rods in the human retina is located outside the macula at the “rod hot spot” in the superior retina (∼3.5 mm from the foveola), and the ONL at this location is expected to be dominated by rod photoreceptor nuclei (Curcio et al., 1990). All BCM patients had normal ONL thickness in this rod-dense zone (Fig. 2D, lower panel). When the spatial extent of the central retinal region with abnormal ONL thickness was quantified along the vertical meridian, there was an age-related expansion from ∼3 mm extent (corresponding to ∼1.5 mm eccentricity) in the first decade of life to ∼4 mm extent in the fourth decade of life (Fig. 2E). Assuming circularly isotropic distribution, the ONL abnormality would be expected to cover a substantial proportion of the macula (Fig. 2E, inset).
Abnormal but detectable cone photoreceptor outer segments in BCM
Is the BCM disease sequence similar to that in human rhodopsin (rod opsin) mutations with photoreceptor outer segment shortening accompanying or preceding the cell loss (Milam et al., 1998)? The answer was sought by analyses of the OCT signals originating from subcellular components of photoreceptors in our BCM cohort.
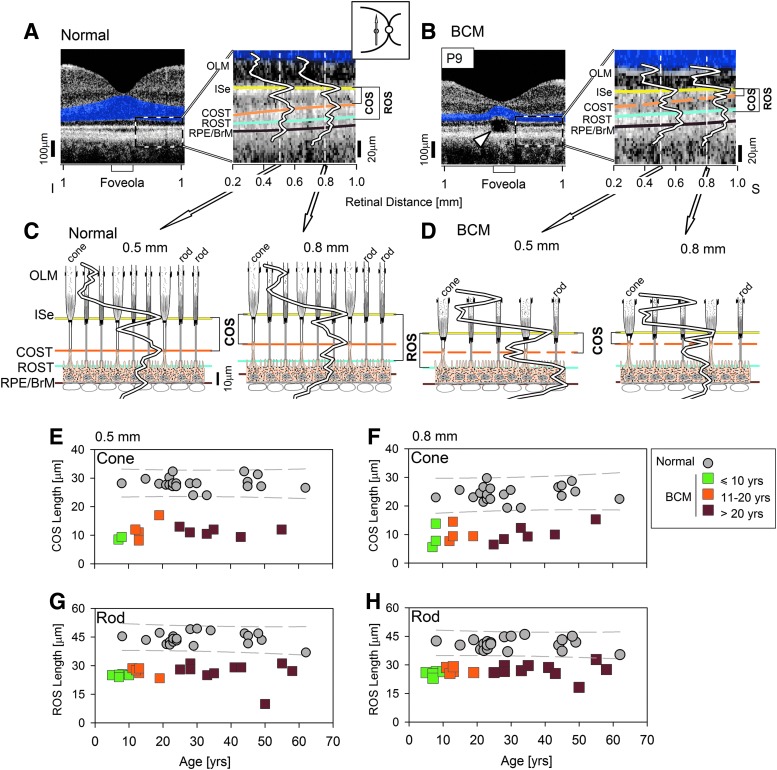
In the normal central retina immediately eccentric to the foveola, there are five distinct hyperscattering peaks (Srinivasan et al., 2008; Kocaoglu et al., 2011; Mustafi et al., 2011; Sakami et al., 2011; Spaide and Curcio, 2011; Syed et al., 2013) (Fig. 3A). These peaks are thought to correspond to the outer limiting membrane (OLM), ellipsoid region of photoreceptor inner segments (ISe), the contact cylinder between the RPE apical processes and the cone outer segments tips (COST), apical RPE microvilli and rod outer segment tips (ROST), and basal RPE and Bruch's membrane (RPE/BrM). Comparison of the longitudinal reflectivity profiles (LRPs), the signal underlying the scans, with a previously published model using quantitative histological measurements (redrawn from Spaide and Curcio, 2011) shows the expected correspondence of scattering peaks and subcellular rod and cone structures at 0.5 and 0.8 mm superior to the foveola (Fig. 3C).
FIG. 3.
Abnormal cone and rod photoreceptor outer segments in BCM. (A and B) Left panels: An optical coherence tomography along the vertical meridian in a representative normal (A) and BCM patient P9 (B). Right panels: Enlarged view of the region immediately superior to the foveola. Four of the five hyperscattering bands are painted for visibility: ISe (yellow), COST (orange), ROST (cyan), and RPE/BrM (brown). OLM layer is also labeled. LRPs are shown at 0.5 and 0.8 mm eccentricity. Dashed orange line in the BCM patient represents intermittent visibility of the COST peak. Cavitation in P9 causes structural loss of the ISe and distal layers at the foveola (arrowhead). The lengths of ROS and COS are bracketed. (C and D) LRPs from the two regions (0.5 and 0.8 mm superior) are overlaid onto models of retina showing the region at rod and cone inner and outer segments and the RPE. Axial dimensions are to scale, but lateral dimensions are not. (E–H) COS and ROS lengths at the 0.5 and 0.8 mm superior retina as a function of age in patients. Individual patient symbols and colors as in Fig. 2. Individual normal controls (gray symbols), and the 95% prediction interval of linear regression fit to the normal data (gray dashed lines) are shown. BrM, Bruch's membrane; COS, cone outer segment; COST, COS tips; ISe, ellipsoid region of photoreceptor inner segments; LRP, longitudinal reflectivity profile; OLM, outer limiting membrane; ROS, rod outer segment; ROST, ROS tips; RPE, retinal pigment epithelium.
The BCM foveola often shows discrete discontinuities along the ISe band, suggesting abnormality at the inner and outer segment regions of the photoreceptor cells (Fig. 3B, arrowhead). Existence of these cavitations (Carroll et al., 2012; Leng et al., 2012) does not allow quantitative analyses to be performed in some of the patients at the foveola. Thus, LRPs were analyzed first at 0.5 and 0.8 mm superior to the foveola (Fig. 3B and D). These locations are still within the foveal region of the human macula. At 0.5 and 0.8 mm, all BCM patients showed hyperscattering peaks identifiable as OLM, ISe, ROST, and RPE/BrM. In addition, the majority of BCM patients, exemplified by P9, showed an intermittently identifiable hyperscattering peak (Fig. 3B, dashed orange bands between ISe and ROST). This peak is where the COST peak would be expected. A model of the BCM retina at the level of the photoreceptor inner and outer segments (using the identities of hyperscattering peaks derived from normal retina) schematizes the interpretation most consistent with the data at the 0.5 and 0.8 mm superior loci (Fig. 3D). The distance from the ISe peak to the COST peak, representing the length of cone outer segments (COS), was quantifiable in many BCM patients. At 0.5 mm eccentricity, the mean COS length in BCM was 11.2 μm (SD=2.4 μm; n=12), representing ∼40% of normal value (28.1±2.1 μm) (Fig. 3E). At 0.8 mm eccentricity, the mean COS length in BCM was 10.0 μm (SD=3.1 μm; n=13), representing ∼41% of the normal value (24.2±2.7 μm) (Fig. 3F). There was no obvious relationship between COS length and age at either eccentricity (Fig. 3E and F). In some BCM patients, a COST peak may not have been identifiable either because of a lack of cones or because COS were shorter than the axial resolution of the instrument. There was no obvious age relationship to the identifiability of the COST peak; there were both younger and older patients without a COST peak.
Abnormally short rod photoreceptor outer segments in BCM
Rod and cone outer segment lengthening tend to be temporally synchronized during human retinal development (Hendrickson and Drucker, 1992). We questioned whether the abnormal congenital shortening of the COS in BCM patients could have secondary consequences on the developmental elongation of ROS length. The distance between hyperscattering LRP peaks ISe and ROST was used to estimate the ROS length in BCM (Fig. 3A–D).
At 0.5 mm eccentricity, all BCM patients showed abnormally short ROS length; the mean was 26.1 μm (SD=4.5 μm; n=19), representing ∼59% of normal (44.2±3.1 μm) (Fig. 3G). Similarly at 0.8 mm eccentricity, the mean ROS length was 26.7 μm (SD=3.0 μm; n=19), representing ∼65% of normal (41.0±2.9 μm) (Fig. 3H). There was no obvious age relationship; both the youngest patients in the first decade of life as well as older patients in the fourth and fifth decades of life showed ROS length shortening of similar extent with the exception of P4, the patient with foveal atrophy. At 3.5 mm superior retina—a locus within the rod hot spot—ROS thickness straddled the lower limit of normal (71–90% of mean normal, data not shown), and again there was no obvious age relationship, except for P4.
Dark cones in BCM: remnant L/M cones with impaired reflectivity
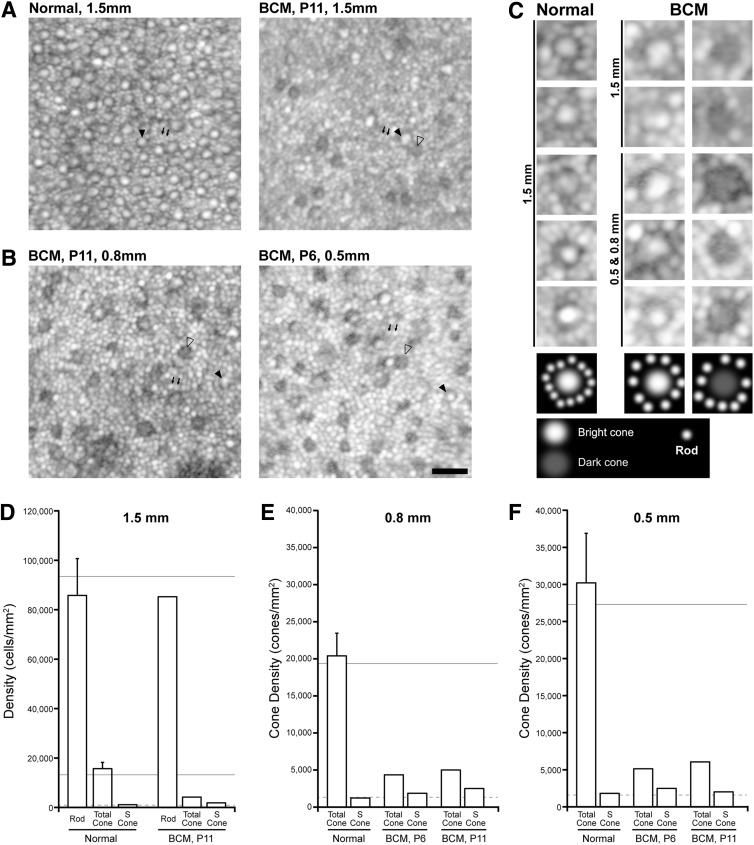
To define the identity and spatial density of photoreceptors in BCM, AOSLO was used to image along a plane tangential to the retina at the level of the inner segments (Dubra et al., 2011). The normal photoreceptor mosaic at 1.5 mm eccentricity contains regularly spaced larger and smaller structures (Fig. 4A, left). Under high magnification (Fig. 4C), the larger structures have a central reflective core surrounded by a darker ring, consistent with normally waveguiding and reflecting bright cones, and the smaller structures are consistent with adjacent rods (Cooper et al., 2011; Dubra et al., 2011; Carroll et al., 2012). The BCM patient P11 had a different pattern compared with normal at the same eccentricity (Fig. 4A). Under high magnification, two types of larger structures with interleaving smaller structures could be distinguished in the BCM retina. Larger structures retaining a central reflective core would be consistent with normal waveguiding and reflecting bright cones, whereas larger structures missing the central reflective component would correspond to dark cones with present inner segments but missing shortened or otherwise abnormal outer segments (Fig. 4C). The altered outer segment morphology would be expected to interfere with normal waveguiding and/or reflection of light. The smaller structures are presumed to be rods. Closer to the foveola, at 0.8 and 0.5 mm, the mosaics of BCM patients P6 and P11 showed a similar pattern to each other and to the images at 1.5 mm consisting of bright and dark cones, and interleaved rods (Fig. 4B and C) that appeared larger in diameter than normal rods and more distinct. Taken together with the evidence of shortened COS length (Fig. 3) and the underlying molecular defect in L-/M-cone opsin, a parsimonious hypothesis is that in BCM the bright cones represent the S-cone population, whereas the dark cones represent remnant L/M cones.
FIG. 4.
Residual cone structure in BCM. (A) The photoreceptor mosaic visible with AOSLO at superior retina 1.5 mm eccentric to the fovea in a normal subject and P11. Normal (left) shows larger regularly spaced spots representing cones (filled arrowhead) with more numerous, smaller rods (small arrows) interleaved across the mosaic. In comparison, P11 (right) shows a different pattern of bright cones with normal reflectivity (filled arrowhead) and dark cones with impaired/diminished reflectivity (unfilled arrowhead), and more numerous smaller rods (small arrows). (B) The photoreceptor mosaic in two BCM patients at 0.8 and 0.5 mm eccentricity also showed rods along with two populations of cones. Scale bar=20 μm. (C) Enlarged examples of normal and BCM structures at different eccentricities. Schematic drawings (lowest panel) define the interpretation assigned to the intensity patterns observed. Normally waveguiding/reflecting bright cones have a characteristic reflectance profile (bright center with a dark ring and adjacent rods). Dark cones have a substantially reduced or missing central reflective component. (D–F) Quantitative measurements of rod and/or cone density derived from the AOSLO images (open bars), compared with previous estimates from histology (solid horizontal lines). Error bars are 1 SD. The dashed line indicates the expected (mean normal) S-cone density, assuming that S cones comprise 6–7% of the total normal cone population. In all cases, the patients with BCM had a lower cone density than that seen in normal subjects, but a higher total cone density than would be expected for just the S-cone submosaic, indicating that at least some of the cones in these images are L/M cones. AOSLO, adaptive optics scanning laser ophthalmoscope.
To support this interpretation, quantitative analyses of the spatial density of photoreceptors were performed at three eccentricities. At 1.5 mm, P11 had 85,734 rods/mm2, which was normal by imaging (85,785±14,914 rods/mm2) as well as by published histology (94,079 rods/mm2) (Curcio et al., 1990) (Fig. 4D). At 0.8 and 0.5 mm, normal rod inner segments are difficult to distinguish individually by current noninvasive AOSLO imaging methods because of their size, packing, and relatively wide reflectivity profile (Dubra et al., 2011). Larger rod inner segments combined with lower cone densities in BCM allow quantitation of rod densities near the fovea in these subjects. Rod densities of P6 (62,812 and 57,344 rods/mm2 at 0.8 and 0.5 mm, respectively) and P11 (70,156 and 53,594 rods/mm2 at 0.8 and 0.5 mm, respectively) were normal or near-normal based on published histology (73,600 and 51,853 rods/mm2 at 0.8 and 0.5 mm, respectively; Curcio et al., 1990).
The spatial density of all cones (both bright and dark) in BCM (4,351 and 5,128 cones/mm2 in P6 at 0.8 and 0.5 mm, respectively; 4,194, 4,999, and 6,065 cones/mm2 in P11 at 1.5, 0.8, and 0.5 mm, respectively) were ∼25% of normal by imaging or by histology (Curcio et al., 1990) (Fig. 4D–F). Consistent with the assumption that a healthier S-cone population corresponds to the bright cones in BCM, their spatial densities (1,865 and 2,486 cones/mm2 in P6 at 0.8 and 0.5 mm, respectively; 1,864, 2,499, and 2,022 cones/mm2 in P11 at 1.5, 0.8, and 0.5 mm, respectively) were similar to that expected from a 7% estimate of the total cone population (Hofer et al., 2005) imaged by AO or direct S-cone density estimates from histology (Curcio et al., 1991) (Fig. 4D–F). Three other BCM patients (P12, P14, and P18) imaged with AOSLO had qualitatively similar findings, although greater instability of fixation did not allow reconstruction of a wide-angle montage required to make quantitative measurements at known retinal eccentricities. AOSLO imaging results, taken together with OCT, provided further evidence that BCM patients have a lower cone density than that seen in normal subjects, but a higher total cone density than would be expected for just the S-cone submosaic, indicating the existence of a remnant L-/M-cone population in BCM.
Retinal structure at the BCM foveola
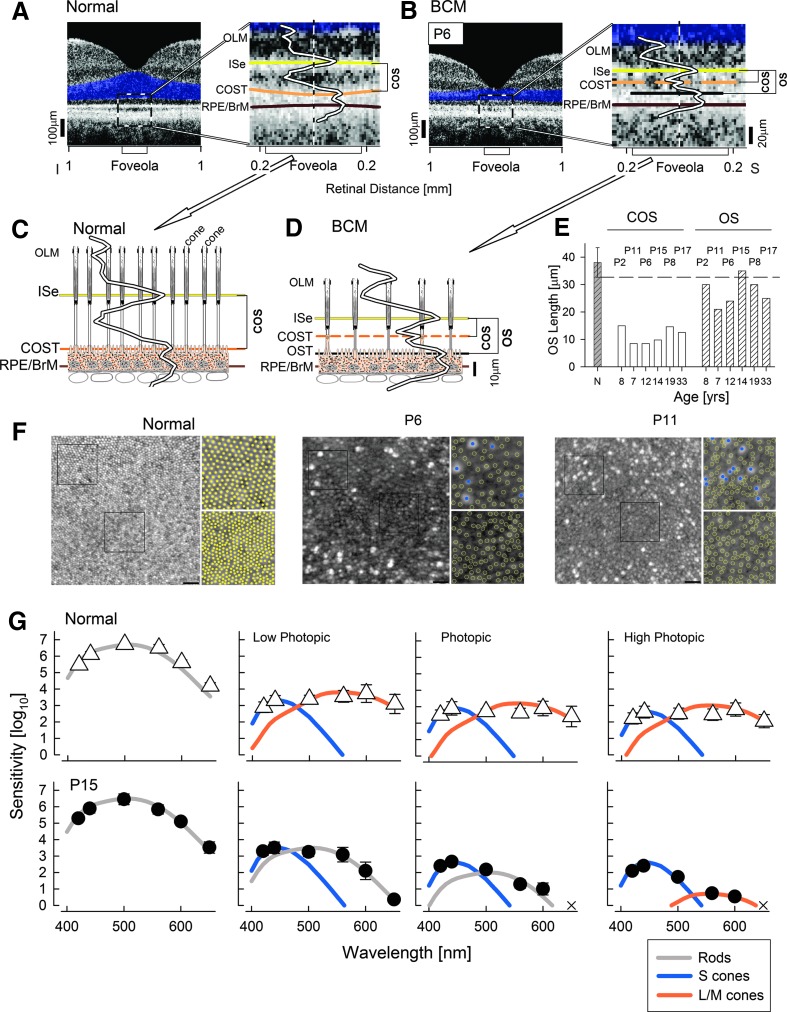
The foveola refers to a region at the center of the fovea packed with L/M cones and lacking rod photoreceptors (Borwein et al., 1980; Youdelis and Hendrickson, 1986). Foveola is thus a key retinal location to answer questions about L/M cones in BCM. On optical cross section of the normal foveola, the number of hyperscattering peaks distinguishable distal to the hyposcattering ONL trough are reduced from five to four, consistent with the lack of the ROST peak (Fig. 5A); notably, this feature is observed only when the scan is obtained through the exact cone-only region. Comparison of the normal foveolar LRP with a previously published model using quantitative histological measurements (redrawn from Spaide and Curcio, 2011) shows the correspondence of scattering peaks and subcellular structures at the foveola (Fig. 5C). The peaks are thought to correspond to the OLM, ISe, the interface at or near the apical RPE microvilli and COST, and the interface near the basal RPE and BrM.
FIG. 5.
Photoreceptor inner and outer segments at the BCM foveola, and evidence of red/green cone-mediated visual function. (A and B) Left panels: An optical coherence tomography along the vertical meridian in a representative normal subject (A) and BCM P6 (B). Right panels: Enlarged view of the foveolar region. Hyperscattering bands are painted for visibility as in Fig. 3. The additional hyperscattering band at the BCM foveola is painted black. LRPs are overlaid. The lengths of COS and the additional OS in the BCM patient are bracketed. (C and D) LRPs are overlaid onto models of foveolar retina showing only cones in the normal (C) and a mixture of cones and another cell type in the BCM (D). Axial dimensions are to scale, but lateral dimensions are not. (E) Histograms of OS length measurements at the foveola of six BCM patients who lacked large cavitations. Shorter COS lengths and longer OS lengths are grouped; age and patient numbers are specified. Hashed gray bar to the left represents mean (±2SD) COS length for the normal (N). (F) The photoreceptor mosaic visible with AOSLO at the foveola in a normal subject compared with BCM patients P6 and P11. Normal (left) shows a contiguous mosaic of cones decreasing in diameter and increasing in density toward the center of the foveola. In comparison, P6 and P11 show a disrupted foveal mosaic made up of a central region of photoreceptors with a reduced density and reflectivity compared with normal and a surrounding ring of highly reflective bright photoreceptors. Square insets for each panel show an image from ∼0.1 mm from the foveal center (top) and an image at the foveal center (bottom). In the normal, it is not possible to determine the spectral subtype of the cones in these images, which are marked as filled yellow circles. In P6 and P11, the S cones are marked as filled blue circles, while the weakly reflective structures are marked as open yellow circles. Scale bar=20 μm. (G) Spectral sensitivity functions in normal subjects and BCM patient P15 at a retinal location centered at 0.6 mm eccentric to the foveola. Scotopic conditions recorded under dark adaptation; photopic conditions recorded on steady achromatic lights increasing from 1 to 20 cd/m2. Theoretical functions describing rod (gray), S-cone (blue), and L-/M-cone (orange) sensitivities are shown after vertical shifts to fit relevant normal and BCM data. X marks the stimuli not seen by the patient. OS, outer segment.
Quantitative analyses of the foveola were possible in six BCM patients (P2, P6, P8, P11, P15, and P17; age range 8–33) who demonstrated a partially intact ISe band at this location (Fig. 3B). Similar to the 0.5 and 0.8 mm loci in BCM and normal subjects, but unlike the foveolar scans in normal individuals, there were five hyperscattering peaks detectable at the foveola of these BCM patients. Of note was the small but identifiable hyperscattering peak immediately distal to ISe band in addition to the pair of larger hyperscattering peaks (Fig. 5B). Such a scattering signature would be consistent with an admixture of cone photoreceptors with shorter COS as well as a second type of photoreceptors (likely rods) with longer outer segments (Fig. 5D). In the six BCM patients with retained foveolar structure, the COS length ranged from 8.5 to 14.5 μm, which is ∼30% of normal COS length at the foveola (38.1±2.7 μm). The longer outer segment length ranged from 21 to 35 μm (Fig. 5E).
AOSLO images of the normal fovea show a contiguous mosaic of cones that decrease in diameter and increase in density toward the foveola (Fig. 5F) as expected from cone packing (Curcio et al., 1990). BCM patients showed a severely disrupted foveal mosaic, consisting of a ring of sparsely packed, highly reflective bright cones and a second population of weakly reflecting photoreceptors, which were present throughout the foveola (Fig. 5F). The bright cones presumably correspond to S cones, and the central region lacking bright cones defines the extent of the S-cone-free zone at the center of the foveola; this has been noted in other patients with BCM (Carroll et al., 2012). The weakly reflecting cells may comprise both remnant L/M cones and rods, though it was not possible to differentiate subtypes within this population. The density of these cells at the foveola (38,400 and 42,400 cells/mm2 for P6 and P11, respectively) was ∼25% of the normal (162,000 cells/mm2).
Evidence for abnormal but detectable L-/M-cone function in BCM
Lastly, we asked whether a subset of structurally retained L/M cones with shortened outer segments could make a contribution to visual perception in BCM patients. Visual function was assessed in P15 at fixation, which was located at 0.6 mm superotemporal to the foveola. P15 was able to view a bright ambient light without squinting. Many BCM patients squint in even modest lighting presumably in order to limit desensitization of their rods by ambient light levels.
Spectral sensitivity was determined under scotopic and photopic conditions. This was achieved by testing under dark-adapted as well as a range of ambient light conditions designed to increasingly desensitize the dominant rod photoreceptor function in order to uncover cone photoreceptor function (Fig. 5G). Under scotopic conditions, normal and BCM vision were spectrally consistent with rod photoreceptor mediation (Fig. 5G, far left panel). Under low photopic (mesopic) conditions using a dim (1 cd/m2) achromatic background light, sensitivities in normal subjects are mediated by S cones and L/M cones when probed with short-, middle-, and long-wavelength stimuli, respectively. In BCM P15 under low photopic conditions, sensitivity was mediated by S cones and rods; there was no spectral evidence of L-/M-cone function. Under higher photopic conditions, sensitivities in normal subjects were dominated by S and L/M cones when probed with short- and long-wavelength stimuli, respectively. In BCM P15, under photopic conditions, spectral sensitivities again were consistent with S-cone and rod function. Under high photopic conditions, however, the spectral sensitivity function of P15 was consistent with L-/M-cone function about 1.5 log units reduced compared with normal.
Discussion
Consequences of congenital cone opsin deficiency on cones
A congenital reduction in density or absence of rod opsin in the rod outer segment, as in the rhodopsin knockout mouse, causes loss of outer segment integrity and rapid rod cell death (Humphries et al., 1997; Lem et al., 1999; Liang et al., 2004; Makino et al., 2012). Should we expect the same consequences for L-/M-cone photoreceptors as for rods when there is congenital opsin deficiency, such as in BCM with large deletions at the OPN1LW / OPN1MW gene cluster? The many differences between structure and function of rods versus cones make such an extrapolation uncertain (Mustafi et al., 2009). Cones contain about half as many visual pigment molecules as do rods (Tachibanaki et al., 2001; Mustafi et al., 2009). Cones, in contrast to rods, do not have separate stacked membranous discs but have a lamellated continuously connected membrane. The turnover rate of COS is thought to be less rapid than in rods (Mustafi et al., 2009; Jonnal et al., 2010). The expectation is that the effects of opsin deficiency on cones could be different than on rods, and this has been bolstered by the clinical impression that BCM may simply be a congenital stationary cone dysfunction with some patients having a degenerative component in later life.
From the present studies of BCM, we know that there is not only severe congenital cone visual dysfunction but also a reduced number of cones even in the youngest patients studied (as early as age 5 years). Evidence of progressive central retinal cone loss was present in some patients and macular atrophy in later stages, thereby confirming previous observations (e.g., Ayyagari et al., 1999; Nathans et al., 1989; Kellner et al., 2004). This disease pattern does show some resemblance to that in the rhodopsin knockout mouse, but it is less cataclysmic. The cone cell reduction detected in early life could represent the end of a rapid postnatal phase of degeneration, and this may be followed by a slowly progressive decades-long phase of further cone loss. Alternatively, congenital cone opsin deficiency may interfere with the protracted postnatal maturation of the human fovea and the BCM retinas may not complete normal high-density cone packing in the fovea, which takes years to achieve (Hendrickson et al., 2012).
In BCM patients with normal retinal lamination at the foveola, there was evidence for some photoreceptors retaining outer segments. At least a subset of these photoreceptors likely corresponds to remnant L/M cones with abnormally shortened outer segments. A small number of rod cells with longer outer segments may also have migrated into the foveola during an incomplete foveal maturation process. Surrounding the foveola, there were shortened but detectable COS, corresponding to weakly reflecting dark cone inner segments (Carroll et al., 2004). These likely represent remnant L/M cones. Also surrounding the foveola, intermingled with the cones, were slightly shortened rod outer segments and swollen rod inner segments; rod cell density was near normal. It is tempting to attribute the minor rod inner and outer segment abnormalities in the central BCM retina to the “stress” from neighboring abnormal cones (Milam et al., 1998; Rossi et al., 2013), and to make analogies to the secondary cone loss known to occur in rod-specific diseases. The effects of rod loss on cones continue to be explored (Humphries et al., 2012) and a rod-derived cone viability factor has been identified (Leveillart and Sahel, 2010), but little is known of the effects of cone loss on rods (Cho et al., 2013). It is also possible that defective development is at play because the rod abnormalities are present as early as we measured (Hendrickson et al., 2012).
Residual L-/M-cone function in BCM caused by a deletion at the L/M opsin gene cluster
Vision in BCM is classically dominated by rod and S-cone photoreceptors (Blackwell and Blackwell, 1957, 1961; Nathans et al., 1989), but existence of a residual amount of L/M-cone-driven vision has been demonstrated in patients preceding the molecular era (Smith et al., 1983). Detailed visual function studies defining the extent of residual L-/M-cone function in molecularly characterized patients have been rare. Consistent with the classical point of view has been the lack of detectable L-/M-cone function in three patients: one with a deletion genotype, and two patients with C203R missense mutations (Stockman et al., 1999). On the other hand, residual L- or M-cone function was found in a 23-year-old patient with the LIAVA type of mutation (Crognale et al., 2004), which is thought to cause abnormal splicing of exon 3 of the cone opsin gene (Ueyama et al., 2012), and in several young affected males in a family with a deletion genotype (Mizrahi-Meissonnier et al., 2010) very similar to the genotype of our Family 1. In patient P15, central vision was mediated by rods and S cones under dark-adapted or standard ambient light conditions. Upon desensitization of the rods by a brighter background light, however, residual vision originating from L/M cones was uncovered (Fig. 5). Molecular analysis showed that this subject carries a deletion that encompasses the LCR and parts of the proximal L opsin gene. However, at least one intact M opsin gene with a functional proximal promoter is still present. We hypothesize that low levels of M opsin are expressed in this BCM patient's cone photoreceptors with abnormally shortened outer segments and this enables cone vision to be detected psychophysically.
Searching for an animal model with cone opsin deficiency and cone cell loss for proof-of-concept studies of therapy in BCM
The experimental literature on the role of congenital cone opsin deficiencies in mammals is limited and complex. Ideally, there should be a primate model with a human-like fovea with a high density of cones. Some monkeys are dichromats and may model color vision abnormalities (Neitz and Neitz, 2011), but there are no reports of cone cell reduction as we observed in our BCM cohort. An M-opsin null mutant rat has deficient cone function, but there is neither cone loss nor outer segment abnormalities up to 12 months of age. This rat has also been suggested to be a model for common human color blindness (Xie et al., 2010). Mice are dichromats with cones that may coexpress both opsins (Applebury et al., 2000). An S-cone opsin knockout mouse showed cone dysfunction and disordered or absent COS in the ventral retina (Daniele et al., 2011). A conclusion in this study of possible relevance to BCM is that cone photoreceptors, despite reduced cone opsin expression and abnormal COS, can survive for at least 1.5 years of this animal's life (Daniele et al., 2011). The results of the current human study point to the need for an animal model that would share sufficient characteristics with the BCM patients for proof-of-concept experiments en route to therapy.
Considerations for future clinical trials in BCM
A gene augmentation therapeutic approach for BCM caused by deletion mutations must consider not only the potential for efficacy but also the fragility of these central retinas with residual foveal cone photoreceptors. Previous human trials using gene delivery by subretinal injection have documented loss of foveal outer segments and cone cells after this procedure (Hauswirth et al., 2008; Maguire et al., 2008; Jacobson et al., 2012). With the specter of further cone cell loss from subfoveal injections or even uncertainty about loss related to the procedure, it may be prudent to consider intravitreal administration of novel vectors, such as has been shown to be effective at transducing photoreceptors in recent reports in mice and at the fovea in a primate (Dalkara et al., 2013; Kay et al., 2013).
Candidate selection based on pre-enrollment imaging would also be necessary, considering the need to exclude those with macular atrophy. Further research work on human BCM is also needed. It must be clarified whether BCM patients with other than deletion mutations are appropriate candidates to be included in future clinical trials aimed at treating residual central cones. A question not posed in the present study is whether the reduced cone cell layer and limited number of COS in the foveola of our patients can account for the reduced visual acuity. If there is a disproportionate vision loss for the number of residual cone photoreceptors, there is the possibility that the early loss of vision may also have a component of amblyopia or there may be abnormalities of postreceptor wiring in the retina and higher visual pathways (Baseler et al., 2002). Expectations for efficacy based on a treatment-related increase in outer segment length or simply increased visual pigment content in cones with reduced outer segment length may have to be recalibrated adding in the uncertainty of a possible postreceptoral developmental acuity disturbance.
Adding further to the complexity of developing and interpreting outcomes of a BCM clinical trial are the constraints that light aversion imposes on psychophysical testing of cone function, except for measurement of absolute thresholds using chromatic stimuli. A potentially quantifiable parameter that may decrease with treatment is light aversion (e.g., Gawande et al., 1989), and this may provide not only an outcome measure but also a practical benefit to these patients, who otherwise show varying degrees of disability under ambient lights.
Supplementary Material
Acknowledgments
Supported by grants from BCMfamilies; Macula Vision Research Foundation; NIH P30EY001931, C06RR016511, R01EY019304, R01EY013203, and R01EY017607; Foundation Fighting Blindness (USA); Research to Prevent Blindness; Fight for Sight (UK); UK National Institute for Health Research (Moorfields Eye Hospital BRC); and Moorfields Special Trustees.
Author Disclosure Statement
No competing financial interests exist.
References
- Applebury M.L., Antoch M.P., Baxter L.C., et al. (2000). The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27, 513–523 [DOI] [PubMed] [Google Scholar]
- Ayyagari R., Kakuk L.E., Coats C.L., et al. (1999). Bilateral macular atrophy in blue cone monochromacy (BCM) with loss of the locus control region (LCR) and part of the red pigment gene. Mol. Vis. 5, 13. [PubMed] [Google Scholar]
- Baseler H.A., Brewer A.A., Sharpe L.T., et al. (2002). Reorganization of human cortical maps caused by inherited photoreceptor abnormalities. Nat. Neurosci. 5, 364–370 [DOI] [PubMed] [Google Scholar]
- Blackwell H.R., and Blackwell O.M. (1957). Blue mono-cone monochromacy: a new color vision defect. J. Opt. Soc. Am. 47, 338 [Google Scholar]
- Blackwell H.R., and Blackwell O.M. (1961). Rod and cone receptor mechanisms in typical and atypical congenital achromatopsia. Vis. Res. 1, 62–107 [Google Scholar]
- Borwein B., Borwein D., Medeiros J., and McGowan J.W. (1980). The ultrastructure of monkey foveal photoreceptors, with special reference to the structure, shape, size, and spacing of the foveal cones. Am. J. Anat. 159, 125–146 [DOI] [PubMed] [Google Scholar]
- Carroll J., Neitz M., Hofer H., et al. (2004). Functional photoreceptor loss revealed with adaptive optics: an alternate cause of color blindness. Proc. Natl. Acad. Sci. USA 101, 8461–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J., Rossi E.A., Porter J., et al. (2010). Deletion of the X-linked opsin gene array locus control region (LCR) results in disruption of the cone mosaic. Vis. Res. 50, 1989–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J., Dubra A., Gardner J.C., et al. (2012). The effect of cone opsin mutations on retinal structure and the integrity of the photoreceptor mosaic. Invest. Ophthalmol. Vis. Sci. 53, 8006–8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.I., Haque M., Wang J., et al. (2013). Distinct and atypical intrinsic and extrinsic cell death pathways between photoreceptor cell types upon specific ablation of Ranbp2 in cone photoreceptors. PLoS Genet. 9, e1003555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V., Zhao X., Nielsen L., et al. (1998). Mutation in the rhodopsin kinase gene slows recovery kinetics of rod and cone phototransduction in man. Proc. Natl. Acad. Sci. USA 95, 328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V., Swider M., Aleman T.S., et al. (2007). Reduced-illuminance autofluorescence imaging in ABCA4-associated retinal degenerations. J. Opt. Soc. Am. A. Opt. Image Sci. Vis. 24, 1457–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R.F., Dubis A.M., Pavaskar A., et al. (2011). Spatial and temporal variation of rod photoreceptor reflectance in the human retina. Biomed. Opt. Express 2, 2577–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crognale M.A., Fry M., Highsmith J., et al. (2004). Characterization of a novel form of X-linked incomplete achromatopsia. Vis. Neurosci. 21, 197–203 [DOI] [PubMed] [Google Scholar]
- Curcio C.A., Sloan K.R., Kalina R.E., and Hendrickson A.E. (1990). Human photoreceptor topography. J. Comp. Neurol. 292, 497–523 [DOI] [PubMed] [Google Scholar]
- Curcio C.A., Allen K.A., Sloan K.R., et al. (1991). Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J. Comp. Neurol. 312, 610–624 [DOI] [PubMed] [Google Scholar]
- Dalkara D., Byrne L.C., Klimczak R.R., et al. (2013). In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5, 189ra76. [DOI] [PubMed] [Google Scholar]
- Daniele L.L., Insinna C., Chance R., et al. (2011). A mouse M-opsin monochromat: retinal cone photoreceptors have increased M-opsin expression when S-opsin is knocked out. Vis. Res. 51, 447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubra A., Sulai Y., Norris J.L., et al. (2011). Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed. Opt. Express 2, 1864–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawande A.A., Donovan W.J., Ginsburg A.P., and Marmor M.F. (1989). Photoaversion in retinitis pigmentosa. Br. J. Ophthalmol. 73, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D., Cideciyan A.V., Jacobson S.G., and Williams D.S. (2009). Retinal pigment epithelium defects in humans and mice with mutations in MYO7A: imaging melanosome-specific autofluorescence. Invest. Ophthalmol. Vis. Sci. 50, 4386–4393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W.W., Aleman T.S., Kaushal S., et al. (2008). Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A., and Drucker D. (1992). The development of parafoveal and mid-peripheral human retina. Behav. Brain Res. 49, 21–31 [DOI] [PubMed] [Google Scholar]
- Hendrickson A., Possin D., Vajzovic L., and Toth C.A. (2012). Histologic development of the human fovea from midgestation to maturity. Am. J. Ophthalmol. 154, 767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer H., Carroll J., Neitz J., et al. (2005). Organization of the human trichromatic cone mosaic. J. Neurosci. 25, 9669–9679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M.M., Rancourt D., Farrar G.J., et al. (1997). Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat. Genet. 15, 216–219 [DOI] [PubMed] [Google Scholar]
- Humphries M.M., Kenna P.F., Campbell M.et al. (2012). C1q enhances cone photoreceptor survival in a mouse model of autosomal recessive retinitis pigmentosa. Eur. J. Hum. Genet. 20, 64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S.G., Cideciyan A.V., Ratnakaram R., et al. (2012). Gene therapy for Leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 130, 9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnal R.S., Besecker J.R., Derby J.C., et al. (2010). Imaging outer segment renewal in living human cone photoreceptors. Opt. Express 18, 5257–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay C.N., Ryals R.C., Aslanidi G.V., et al. (2013). Targeting photoreceptors via intravitreal delivery using novel, capsid-mutated AAV vectors. PLoS One 8, e62097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner U., Wissinger B., Tippmann S., et al. (2004). Blue cone monochromatism: clinical findings in patients with mutations in the red/green opsin gene cluster. Graefes Arch. Clin. Exp. Ophthalmol. 242, 729–735 [DOI] [PubMed] [Google Scholar]
- Kocaoglu O.P., Lee S., Jonnal R.S., et al. (2011). Imaging cone photoreceptors in three dimensions and in time using ultrahigh resolution optical coherence tomography with adaptive optics. Biomed. Opt. Express 2, 748–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lem J., Krasnoperova N.V., Calvert P.D., et al. (1999). Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc. Natl. Acad. Sci. USA 96, 736–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng T., Marmor M.F., Kellner U., et al. (2012). Foveal cavitation as an optical coherence tomography finding in central cone dysfunction. Retina 32, 1411–1419 [DOI] [PubMed] [Google Scholar]
- Leveillard T., and Sahel J.-A. (2010). Rod-derived cone viability factor for treating blinding diseases: from clinic to redox signaling. Sci. Transl. Med. 2, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Fotiadis D., Maeda T., et al. (2004). Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J. Biol. Chem. 279, 48189–48196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan B.J., Roorda A., Knighton R.W., and Carroll J. (2011). Revealing Henle's fiber layer using spectral domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52, 1486–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A., et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino C.L., Wen X.H., Michaud N.A., et al. (2012). Rhodopsin expression level affects rod outer segment morphology and photoresponse kinetics. PLoS One 7, e37832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam A.H., Li Z.Y., and Fariss R.N. (1998). Histopathology of the human retina in retinitis pigmentosa. Prog. Retin. Eye Res. 17, 175–205 [DOI] [PubMed] [Google Scholar]
- Mizrahi-Meissonnier L., Merin S., Banin E., and Sharon D. (2010). Variable retinal phenotypes caused by mutations in the X-linked photopigment gene array. Invest. Ophthalmol. Vis. Sci. 51, 3884–3892 [DOI] [PubMed] [Google Scholar]
- Mustafi D., Engel A.H., and Palczewski K. (2009). Structure of cone photoreceptors. Prog. Retin. Eye Res. 28, 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D., Kevany B.M., Genoud C., et al. (2011). Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration. FASEB J. 25, 3157–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J., Piantanida T.P., Eddy R.L., et al. (1986a). Molecular genetics of inherited variation in human color vision. Science 232, 203–210 [DOI] [PubMed] [Google Scholar]
- Nathans J., Thomas D., and Hogness D.S. (1986b). Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science 232, 193–202 [DOI] [PubMed] [Google Scholar]
- Nathans J., Davenport C.M., Maumenee I.H., et al. (1989). Molecular genetics of human blue cone monochromacy. Science 245, 831–838 [DOI] [PubMed] [Google Scholar]
- Neitz J., and Neitz M. (2011). The genetics of normal and defective color vision. Vis. Res. 51, 633–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg G. (1935). Topography of the layer of rods and cones in the human retina. Acta Ophthal. Suppl. 6, 1–103 [Google Scholar]
- Palczewski K. (2010). Retinoids for treatment of retinal diseases. Trends Pharmacol. Sci. 31, 284–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi E.A., Achtman R.L., Guidon A., et al. (2013). Visual function and cortical organization in carriers of blue cone monochromacy. PLoS One 8, e57956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari J.C. (2000). Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 41, 337–348 [PubMed] [Google Scholar]
- Sakami S., Maeda T., Bereta G., et al. (2011). Probing mechanisms of photoreceptor degeneration in a new mouse model of the common form of autosomal dominant retinitis pigmentosa due to P23H opsin mutations. J. Biol. Chem. 286, 10551–10567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V.C., Pokorny J., Delleman J.W., et al. (1983). X-linked incomplete achromatopsia with more than one class of functional cones. Invest. Ophthalmol. Vis. Sci. 24, 451–457 [PubMed] [Google Scholar]
- Spaide R.F., and Curcio C.A. (2011). Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina 31, 1609–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V.J., Monson B.K., Wojtkowski M., et al. (2008). Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 49, 1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman A., Sharpe L.T., and Fach C. (1999). The spectral sensitivity of the human short-wavelength sensitive cones derived from thresholds and color matches. Vis. Res. 39, 2901–2927 [DOI] [PubMed] [Google Scholar]
- Syed R., Sundquist S.M., Ratnam K., et al. (2013). High-resolution images of retinal structure in patients with choroideremia. Invest. Ophthalmol. Vis. Sci. 54, 950–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibanaki S., Tsushima S., and Kawamura S. (2001). Low amplification and fast visual pigment phosphorylation as mechanisms characterizing cone photoresponses. Proc. Natl. Acad. Sci. USA 98, 14044–14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama H., Muraki-Oda S., Yamade S., et al. (2012). Unique haplotype in exon 3 of cone opsin mRNA affects splicing of its precursor, leading to congenital color vision defect. Biochem. Biophys. Res. Commun. 424, 152–157 [DOI] [PubMed] [Google Scholar]
- Von Helmholtz H. (1866). Concerning the perceptions in general. In Treatise on Physiological Optics, III. Southall J.P.C., ed. (Dover, New York, NY: ) pp. 1–18 [Google Scholar]
- Wang J.S., and Kefalov V.J. (2011). The cone-specific visual cycle. Prog. Retin. Eye Res. 30, 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Macke J.P., Merbs S.L., et al. (1992). A locus control region adjacent to the human red and green visual pigment genes. Neuron 9, 429–440 [DOI] [PubMed] [Google Scholar]
- Weleber R.G. (2002). Infantile and childhood retinal blindness: a molecular perspective (The Franceschetti Lecture). Ophthalmic Genet. 23, 71–97 [DOI] [PubMed] [Google Scholar]
- Xie B., Nakanishi S., Guo Q., et al. (2010). A novel middle-wavelength opsin (M-opsin) null-mutation in the retinal cone dysfunction rat. Exp. Eye Res. 91, 26–33 [DOI] [PubMed] [Google Scholar]
- Youdelis C., and Hendrickson A.H. (1986). A qualitative and quantitative analysis of the human fovea during development. Vis. Res. 26, 847–855 [DOI] [PubMed] [Google Scholar]
- Young T. (1802). The Bakerian Lecture: on the theory of light and colours. Phil. Trans. R. Soc. 92, 12–48 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.