Abstract
Gene transfer to both cone and rod photoreceptors (PRs) is essential for gene therapy of inherited retinal degenerations that are caused by mutations in genes expressed in both PR types. Vectors based on the adeno-associated virus (AAV) efficiently transduce PRs of different species. However, these are predominantly rods and little is known about the ability of the AAV to transduce cones in combination with rods. Here we show that AAV2/8 transduces pig cones to levels that are similar to AAV2/9, and the outer nuclear layer (mainly rods) to levels that are on average higher, although not statistically significant, than both AAV2/5 and AAV2/9. We additionally found that the ubiquitous cytomegalovirus (CMV), but not the PR-specific GRK1 promoter, transduced pig cones efficiently, presumably because GRK1 is not expressed in pig cones as observed in mice and humans. Indeed, the GRK1 and CMV promoters transduce a similar percentage of murine cones with the CMV reaching the highest expression levels. Consistent with this, the AAV2/8 vectors with either the CMV or the GRK1 promoter restore cone function in a mouse model of Leber congenital amaurosis type 1 (LCA1), supporting the use of AAV2/8 for gene therapy of LCA1 as well as of other retinal diseases requiring gene transfer to both PR types.
Introduction
Vectors based on the adeno-associated virus (AAV) are currently the most promising for gene therapy of inherited retinal diseases (IRDs), as they provide safe and long-lasting transgene expression after a single subretinal administration (Surace and Auricchio, 2008). AAV-mediated retinal gene transfer has been successfully applied to several animal models of IRDs (Stieger and Lorenz, 2010; Sundaram et al., 2012). More importantly, the safety and efficacy of subretinal administrations of AAV have been recently demonstrated in patients with Leber congenital amaurosis type 2 (LCA2) (Bainbridge et al., 2008; Cideciyan et al., 2008, 2009a,b, 2013; Hauswirth et al., 2008; Maguire et al., 2008, 2009; Simonelli et al., 2010; Ashtari et al., 2011; Testa et al., 2013). The ability of AAV to transduce retinal pigment epithelium (RPE), regardless of the serotype used (Vandenberghe and Auricchio, 2012), may partially explain these clinical successes as RPE65, the gene mutated in LCA2 patients, is indeed expressed in the RPE.
However, the majority of IRDs are caused by mutations in genes expressed in photoreceptors (PRs) rather than in RPE. Although in some genetic forms of blindness, such as retinitis pigmentosa, rods are preferentially affected, there are many IRDs that are caused by mutations in genes expressed in both rods and cones (Corbo et al., 2007; den Hollander et al., 2010), thus requiring transduction of both PR types simultaneously.
PR transduction appears more challenging than RPE transduction, likely because of the organization of PRs into tight rows (Clarke et al., 2000) and to the presence of both interphotoreceptor matrix and outer limiting membrane that may restrict access to PRs upon subretinal injections (Mieziewska, 1996; Omri et al., 2010). Among the naturally occurring serotypes previously tested in the retina, AAV2/5, AAV2/8, and AAV2/9 have been shown to be the most efficient for transduction of the outer nuclear layer (ONL) cells in mice (Allocca et al., 2007; Lebherz et al., 2008), pigs (Mussolino et al., 2011), and nonhuman primates (NHP) (Mancuso et al., 2009; Vandenberghe et al., 2011, 2013). These studies are based on assessment of AAV-mediated expression of the reporter gene enhanced green fluorescent protein (EGFP) in the ONL. Because cones represent 3–5% of all PRs in murine (Carter-Dawson and LaVail, 1979; Jeon et al., 1998) and primate (Ahnelt, 1998) retinas, it is clear that these serotypes efficiently transduce rod PRs; however, less understood is their capacity to transduce cones.
Among nonprimate mammals, the porcine eye shares many similarities with the human retina including anatomy and size, thus offering the possibility to test the feasibility of retinal surgery techniques such as subretinal injections, and high cone/rod ratio (1:7–8) throughout the retina with a large streak-like region across the retina, which is similar to the human fovea, where the cone/rod ratio reaches 1:3/1:5 (Hendrickson and Hicks, 2002). Thus, the cone-enriched porcine retina is particularly useful to assess cone transduction by viral vectors.
To identify the best combination of AAV serotype and regulatory elements to simultaneously transduce both rods and cones, we have compared AAV2/5, 2/8, and 2/9, which are known to efficiently transduce PRs, in both the pig and mouse retina. In addition, we evaluated AAV2/8 in combination with regulatory elements known to efficiently transduce rods. We selected both the ubiquitous cytomegalovirus (CMV) promoter, which is known to allow efficient RPE and PR transduction in different species (Bennett et al., 1999; Lai et al., 2004; Allocca et al., 2007; Mussolino et al., 2011), and the human rhodopsin kinase 1 (GRK1) promoter that drives PR-specific gene expression in both rods and cones (Young et al., 2003; Allocca et al., 2007; Khani et al., 2007; Pawlyk et al., 2010; Sun et al., 2010; Beltran et al., 2012; Boye et al., 2012; Petit et al., 2012). As an additional strategy to restrict transgene expression to PRs, we used the CMV promoter in combination with target sites of microRNA (miR) 204, which is expressed at higher levels in the RPE than in PRs (Karali et al., 2007). We have previously shown that the inclusion of mir204 target sites (miR204T) in the 3′ UTR of our transgene results in AAV2/5-CMV-mediated transgene expression restricted to murine and pig PRs (Karali et al., 2011).
In addition, we compared the ability of the various regulatory elements in combination with AAV2/8 to restore cone function in a murine model of IRD. To this end, we took advantage of a murine model of LCA1 owing to mutations in Gucy2e (Gucy2e−/− mice) and in which cone activity is absent from birth (Yang et al., 1999). Gucy2e encodes for the retinal guanylate cyclase 1 (RetGC1), a key protein involved in phototransduction. RetGC1 is responsible for the synthesis of the cyclic GMP, the second messenger for the phototransduction cascade, and its activity is regulated by Ca2+ (Garbers and Lowe, 1994). RetGC1 deficiency affects both cone and rod function in LCA1 patients, whereas only cone function is severely impaired in Gucy2e−/− mice, which is likely because of the redundancy provided in murine rods by the expression of Gucy2f (Baehr et al., 2007). Mutations in GUCY2D (the human ortholog of Gucy2e) account for around 20% of all LCA cases (Perrault et al., 2000; den Hollander et al., 2008). LCA1 patients maintain the retinal structure and retain all six retinal layers with the preserved PR inner/outer segment juncture (Pasadhika et al., 2010; Jacobson et al., 2013). The preservation of retinal structure in LCA1 patients suggests that they may be good candidates for gene therapy strategies. Thus, testing AAV2/8-mediated gene delivery in Gucy2e−/− mice not only provides a way to assess the ability of these vectors to restore cone function, but also provides a proof of concept of gene therapy for this highly debilitating condition.
Materials and Methods
Plasmid construction, AAV vector production, and purification
For the production of AAV encoding EGFP with different promoters, pAAV2.1-CMV-EGFP (Allocca et al., 2007), pAAV2.1-CMV-EGFP-4XmiR204T (Karali et al., 2011), and pAAV2.1-GRK1-EGFP (Allocca et al., 2007) plasmids were used for the production of AAV2/8 vectors. The GRK1 promoter was previously described (Young et al., 2003) and includes the −112 to +86 nucleotides from the promoter region of the human GRK1 gene (1810011–1810209 of GenBank ID: NW_004078078.1).
To generate the vectors expressing GUCY2D, the coding sequence of the human GUCY2D gene was amplified from Human ORFeome Collaboration Clone (Open Biosystem; Thermo Scientific, Waltham, MA) using the primers Gucy2dFORNotI (5′- ATAGCGGCCGCCATGACCGCCTGCG CCCG-3′) and Gucy2dREVBamHI (5′- ATAGGATCCTCAA GAGAACTGGCCCGGC-3′) and cloned into the plasmids with CMV and GRK1 elements, after digestion with restriction endonucleases NotI and BamHI.
AAV vectors were produced by triple transfection of HEK293 cells, purified by two rounds of CsCl2 ultracentrifugation, and titered (in GC/ml) using a real-time polymerase chain reaction-based TaqMan assay (Applied Biosystems, Foster City, CA) and a dot-blot analysis, as previously described (Allocca et al., 2007). AAV vector production was carried out by the Telethon Institute of Genetics and Medicine (TIGEM) AAV vector core.
Animal procedures and vector administration
Mice
All procedures on mice were performed in accordance with the institutional guidelines for animal research and with the Association for Research in Vision and Ophthalmology Statement for the Use of Animal in Ophthalmic and Vision Research.
Gucy2e+/− heterozygote embryos were derived from a cryopreserved stock at The Jackson Laboratory (Bar Harbor, ME). Heterozygotes were mated to produce Gucy2e−/− and Gucy2e+/− as controls. Four-week-old C57BL/6 mice (Harlan, S. Pietro al Natisone, Italy) and 10-day-old Gucy2e−/− and their control Gucy2e+/− were anesthetized with an intraperitoneal injection of avertin (1.25% w/v of 2,2,2-tribromoethanol and 2.5% v/v of 2-methyl-2-Butanol; Sigma–Aldrich, St. Louis, MO) at 2 ml/100 g of body weight, and viral vectors were delivered via a transscleral–transchoroidal approach, as previously described (Allocca et al., 2007).
After injection, the extent of transduction was assessed by ophthalmoscopy at days 7 and 28 for the animals injected with the AAV2/8-EGFP. Eyes were harvested at day 28 after injection in the animals of the EGFP-expressing vectors. The Gucy2e−/− were mantained for analysis until 3 months postinjection.
Pigs
All the experiments involving pigs were conducted according to the rules approved by the Italian Ministry of Health. The Large White (LW) pigs (Sus scrofa) used in our study were registered as purebred in the LW Herd Book of the Italian National Pig Breeders' Association. Pigs were starved overnight leaving water ad libitum.
Surgical procedures in pigs
Before surgery, eyes were dilated with a topical application of 2.5% phenylephrine (Bausch & Lomb, London, United Kingdom). The surgical procedure consisted of a two-port sclerotomy: one for the light source and the other for the injection. The procedure started with a transconjunctival scleral tunnel incision via pars plana parallel to the corneoscleral limbus at 3.5 mm. The angle insertion of a 20- or 23-gauge stiletto blade (Alcon, Fort Worth, TX) was performed to facilitate the efficiency of self-sealing. The light fiber, attached to the vitrectomy unit (ACCURS vitrectomy machine; Alcon), and either a 38-gauge (Alcon) or extendible 41-gauge subretinal injection needles (DORC, Zuidland, The Netherlands) were inserted through the two conjunctival incisions and into the two scleral tunnels, respectively. The subretinal injection was performed with a 1 ml syringe connected to the subretinal needles, after illumination of the posterior pole with the light fiber and under direct observation with a stereoscopic microscope. At the end of surgery, paracentesis was performed removing 0.1 ml of aqueous humor using a 1 ml syringe with a 30-gauge needle in order to prevent a subsequent increase in intraocular pressure. Animals underwent handy slit lamp biomicroscopy and indirect ophthalmoscopy before and immediately after injection, within 3 days after surgery and on the day of sacrifice (i.e., 6 weeks after vector administration).
Anesthetic procedure for pigs
Pigs injected intramuscularly with azaperone (2 mg/kg of Stresnil; Janssen-Cilag SpA, Milan, Italy) were left undisturbed for 20 min and then received a second intramuscular injection of ketamine (20 mg/kg of Ketavet 100; Intervet Productions S.r.l., Segrate, Italy), medetomidine (30 μg/kg of Medetor; CP-Pharma Handelsgesellschaft mbH, D-Burgdorf), and atropine (0.04 mg/kg of Atropine sulfate; ATI S.r.l., Bologna, Italy).
An auricular vein was catheterized by the insertion of an over-the-needle catheter (Delta Ven 1; Delta Med S.r.l., Mantova, Italy) and pigs were maintained sedated by intermittent intravenous boluses of diazepam (0.5 mg/kg of Diazepam 0.5%; Intervet Productions S.r.l., Segrate, Italy) during the whole surgical procedure. Corneal analgesia was achieved using topical drops of oxibuprocaine 0.4% (Novesina, Novartis Farma, Italy).
Histological analysis
Mice were euthanized, and their eyeballs were then harvested and fixed overnight by immersion in 4% paraformaldehyde (PFA). Before harvesting the eyeballs, the temporal aspect of the sclerae was marked by cauterization, in order to orient the eyes with respect to the injection site at the moment of the inclusion. The eyeballs were cut so that the lens and vitreous could be removed while leaving the eyecup intact. Mice eyecups were infiltrated with 30% sucrose for cryopreservation and embedded in tissue-freezing medium (O.C.T. matrix; Kaltek, Padua, Italy). For each eye, 150–200 serial sections (10 μm thick) were cut along the horizontal plane and the sections were progressively distributed on 10 slides so that each slide contained 15 to 20 sections, each representative of the entire eye at different levels. The sections were stained with 4′,6′-diamidino-2-phenylindole (Vectashield; Vector Lab, Peterborough, United Kingdom) and EGFP was monitored with a Zeiss Axiocam (Carl Zeiss, Oberkochen, Germany) at different magnifications.
Pigs were sacrificed, and their eyeballs were harvested and fixed overnight by immersion in 4% PFA. The eyeballs were cut so that the lens and vitreous could be removed, leaving the eyecups in place. The eyecups were gradually dehydrated by progressively infiltrating them with 10%, 20%, and 30% sucrose. Tissue-freezing medium (O.C.T. matrix; Kaltek) embedding was performed. Before embedding, the swine eyecups were analyzed with a fluorescence stereomicroscope (Leica Microsystems GmbH, Wetzlar, Germany) in order to localize the transduced region whenever an EGFP-encoding vector was administered.
For each eye, 200–300 serial sections (12 μm thick) were cut along the horizontal meridian and the sections were progressively distributed on glass slides so that each slide contained 6–10 sections. Section staining and image acquisition was performed as described for mice.
Cone and GUCY2D immunofluorescence staining
Frozen retinal sections were washed once with PBS and then fixed for 10 min in 4% PFA. Sections were then permeabilized for 1 hr in PBS containing 0.1% Triton X-100. Blocking solution containing 10% normal goat serum (Sigma–Aldrich) was applied for 1 hr. Primary antibodies were diluted in PBS and incubated overnight at 4°C. The secondary antibody (Alexa Fluor 594, antirabbit, 1:1,000; Molecular Probes, Invitrogen, Carlsbad, CA) was incubated for 45 min. The affinity primary rabbit antibodies used were antimouse cone arrestin 4 (“Luminaire juniors”—mCAR, 1:1,000; Millipore, Watford, United Kingdom), antihuman CAR (Li et al., 2002) (“Luminaire founders”—hCAR, 1:10,000; kindly provided by Dr. Cheryl M. Craft, Doheny Eye Institute, Los Angeles, CA), and anti-GC1 (Santa Cruz Biotechnology, Santa Cruz, CA). Vectashield (Vector Lab) was used to visualize nuclei. Sections were photographed using either a Zeiss Axioplan microscope (Carl Zeiss) or a Zeiss Laser Confocal Microscope System (Carl Zeiss).
For the cone counts, both mouse and pig sections (from n=5 murine and n=3 pig eyes/group) stained with the anti-CAR antibodies were analyzed at 63× magnification using a Zeiss Laser Confocal Microscope System (Carl Zeiss). For each eye, six different z-stacks from six different transduced regions were taken. For each z-stack, images from single plans were used to count CAR+/EGFP+ cells. In doing this, we carefully moved along the Z-axis to distinguish one cell from another and thus to avoid to count twice the same cell.
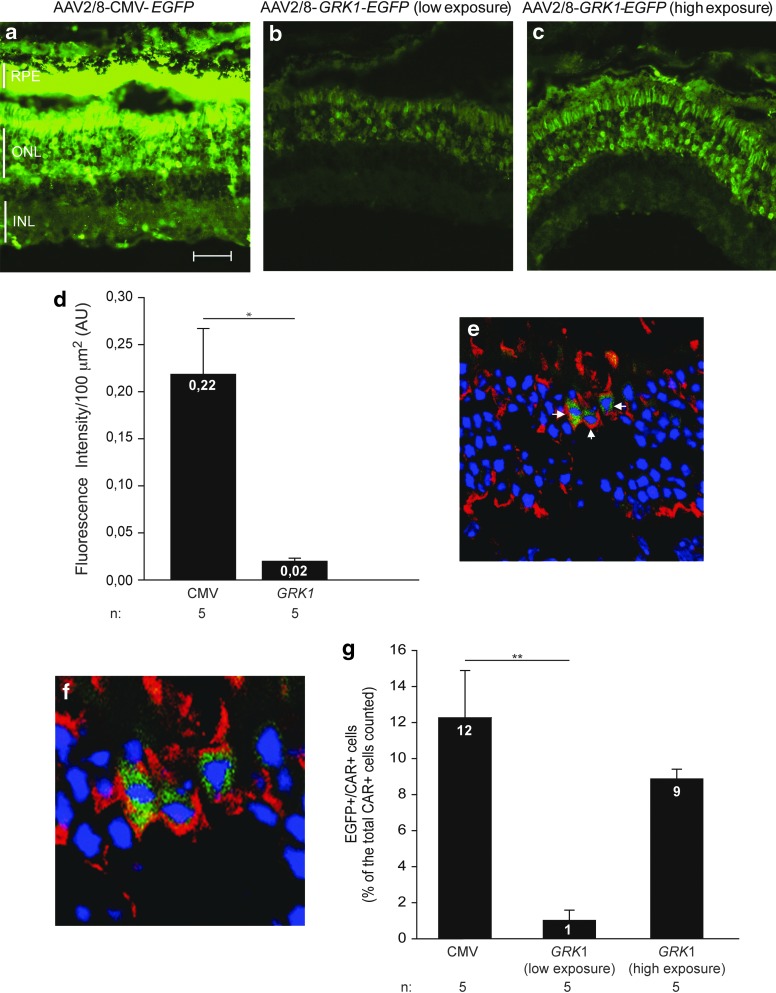
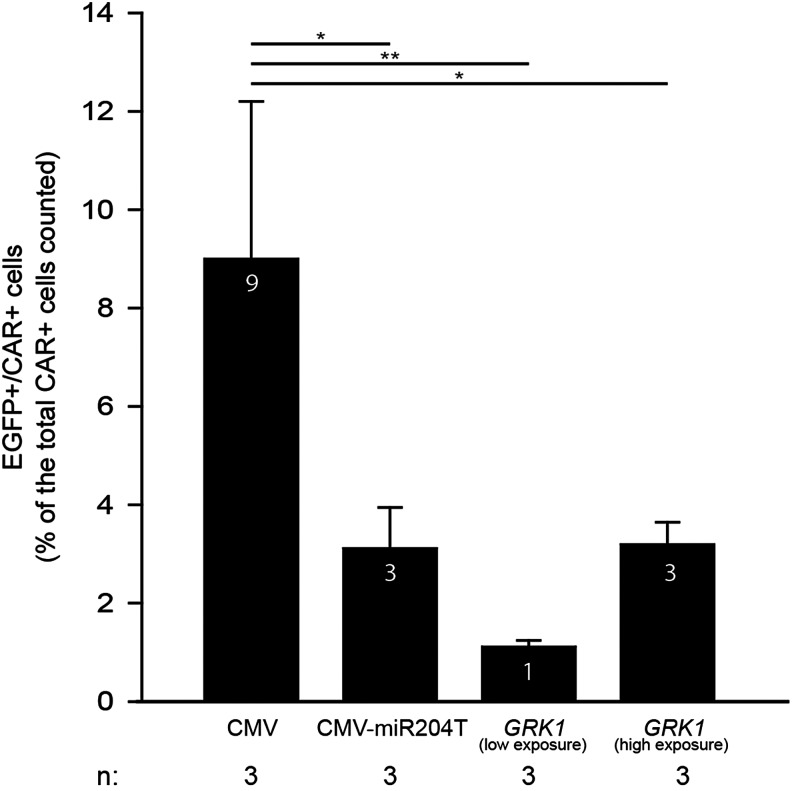
For each retina we counted the CAR-positive (CAR+)/EGFP-positive (EGFP+) cells on a total of 150–200 CAR+ cells. We then calculated the average number of CAR+/EGFP+ cells of the three eyes of each experimental group. In Fig. 5c we have used high exposure times to appreciate EGFP transduction mediated by the GRK1 promoter. To rule out any influence by autofluorescence, the cryosections were viewed using the same conditions under a nonrelevant filter (rhodamine for EGFP and FITC for red fluorescence).
FIG. 5.
AAV2/8-mediated retinal transduction in mouse retina. Four-week-old C57BL/6 mice were subretinally injected with 1.7×109GC/eye each of either AAV2/8-CMV-EGFP (a) or AAV2/8-GRK1-EGFP (b and c, at low and high exposure times, respectively). Retinal cryosections were obtained 4 weeks after injection, and EGFP was analyzed using fluorescence microscopy. (d) Fluorescence intensity in the PR layer was quantified for each group of animals; scale bar=40 μm. **p≤0.01; Student's t-test. (e) Retinal cryosections were immunostained using an anti-LUMIj-mCAR antibody (red) to label cones. Representative confocal microscopy image from a single plan (magnification 63×) of a retina injected with AAV2/8-GRK1-EGFP: EGFP+ cones are indicated by white arrows. (f) Higher magnification of the transduced cones. (g) Quantification of cone transduction efficiency of AAV2/8-CMV-EGFP and AAV2/8-GRK1-EGFP in mouse retina. The histograms represent the percentage of EGFP+/CAR+ cells counted on a total of 150–200 CAR+ cells. For the eyes injected with AAV2/8-GRK1-EGFP, the quantification of cone transduction was done at both low and high exposure times. **p≤0.01, one-way ANOVA.
EGFP quantitation
Fluorescence intensity in PRs was rigorously and reproducibly quantified in an unbiased manner as follows. Individual color channel images were taken using the Leica confocal microscope (Leica Microsystems GmbH). TIFF images were gray-scaled with image analysis software (LAS AF lite; Leica Microsystems GmbH). Six images of each eye were analyzed by a masked observer. A large region of either the pig retinas (size: area of 27,676.4±733 μm2 over a length of 416.2±6 μm) or murine retinas (size: area of 30,334.4±1,562 μm2 over a length of 463.6±17 μm) that contains PRs with the exclusion of their outer segment tips to avoid the RPE strong fluorescence was selectively outlined in every image, and the total fluorescence for the enclosed area was calculated in an unbiased manner using the image analysis software. The fluorescence in PRs was then averaged from six images collected from separate retinal sections from each eye.
Electroretinography
For electroretinogram (ERG) analysis, Gucy2e−/− mice and Gucy2e+/− controls were dark-adapted for 180 min. They were anesthetized with an intraperitoneal injection of Avertin (1.25% wt/vol of 2,2,2-tribromoethanol and 2.5% vol/vol of 2-methyl-2-butanol; Sigma–Aldrich) at 2 ml/100 g of body weight and positioned in a stereotaxic apparatus under dim red light. Their pupils were dilated with a drop of 1% tropicamide (Alcon) and their body temperature was maintained at 37.5°C. For 6 Hz flicker, flashes of different light intensities ranging from 10−4 to 15 cd•s/m2 in steps of 0.6 logarithmic units at 6 Hz frequency (Seeliger et al., 2011) were generated by a Ganzfeld stimulator (CSO, Florence, Italy). The electrophysiological signals were recorded through gold-plate electrodes inserted under the lower eyelids in contact with the cornea. The electrodes in each eye were referred to a needle electrode inserted subcutaneously at the level of the corresponding frontal region. The different electrodes were connected to a two-channel amplifier. Amplitudes of b-wave were plotted as a function of increasing light intensities. For paired-flash ERG the responses to two subsequent stimuli of 20 cd•s/m2 at interstimulus interval of 2 Hz (500 msec) were recorded in the presence of a scotopic conditions. For each group, the mean b-wave amplitude was plotted.
Statistical analyses
We used the Student's t-test when two datasets were compared (Fig. 5d) and the one-way analysis of variance (ANOVA) when more than two datasets were compared (all other figures).
Results
AAV2/8 in combination with the CMV promoter provides the most efficient simultaneous rod and cone transduction in the pig retina
To identify the most effective AAV serotype to simultaneously transduce cone and rod PRs, we compared AAV2/5, 2/8, and 2/9, which are known to efficiently transduce PRs through subretinal administration in LW pigs that present a high cone:rod ratio (Hendrickson and Hicks, 2002). Thus, AAV2/5, 2/8, and 2/9 encoding for EGFP under the CMV promoter were subretinally injected in 11-week-old female LW pigs (1×1010 genome copies-GC/eye) as previously described (Mussolino et al., 2011). Four weeks after the injection, animals were sacrificed and eyes were harvested for histological analysis. Direct EGFP fluorescence was quantified on retinal cryosections using an unbiased method based on digital acquisition of fluorescence across a large transduced area of the pig retina (see Materials and Methods). As shown in Fig. 1, all the different serotypes tested efficiently transduce cells in the ONL (predominantly rods in this region, cone:rod=1:3) (Hendrickson and Hicks, 2002), with AAV2/8 being on average more efficient than the other two serotypes tested (Fig. 1d). Although the differences we observe between AAV2/8 and the other two serotypes are not statistically significant, presumably because of the number of eyes analyzed, which is limiting when using a large model, our results are in line with previous data: (1) in pigs, where we have shown that AAV2/8 outperforms AAV2/5 for PR transduction (Mussolino et al., 2011); (2) in NHP, where Vandenberghe et al. (2013) have shown that AAV2/9 outperforms AAV2/8 in cone but not in rod PR transduction.
FIG. 1.
Porcine retinal transduction after AAV subretinal delivery. LW pigs were subretinally injected with 1×1010 GC/eye of AAV2/5 (a), 2/8 (b), or 2/9-CMV-EGFP (c). Retinal cryosections were obtained 4 weeks after injection, and EGFP was analyzed using fluorescence microscopy. (d) Fluorescence intensity in the PR layer was quantified for each group of animals; p=0.3, one-way ANOVA. Scale bar=25 μm. AAV, adeno-associated virus; ANOVA, analysis of variance; AU, arbitrary units provided by the LAS AF lite software (see Materials and Methods); CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein; LW, Large White; n, number of eyes; ONL, outer nuclear layer; PR, photoreceptor; RPE, retinal pigment epithelium. Color images available online at www.liebertpub.com/hum
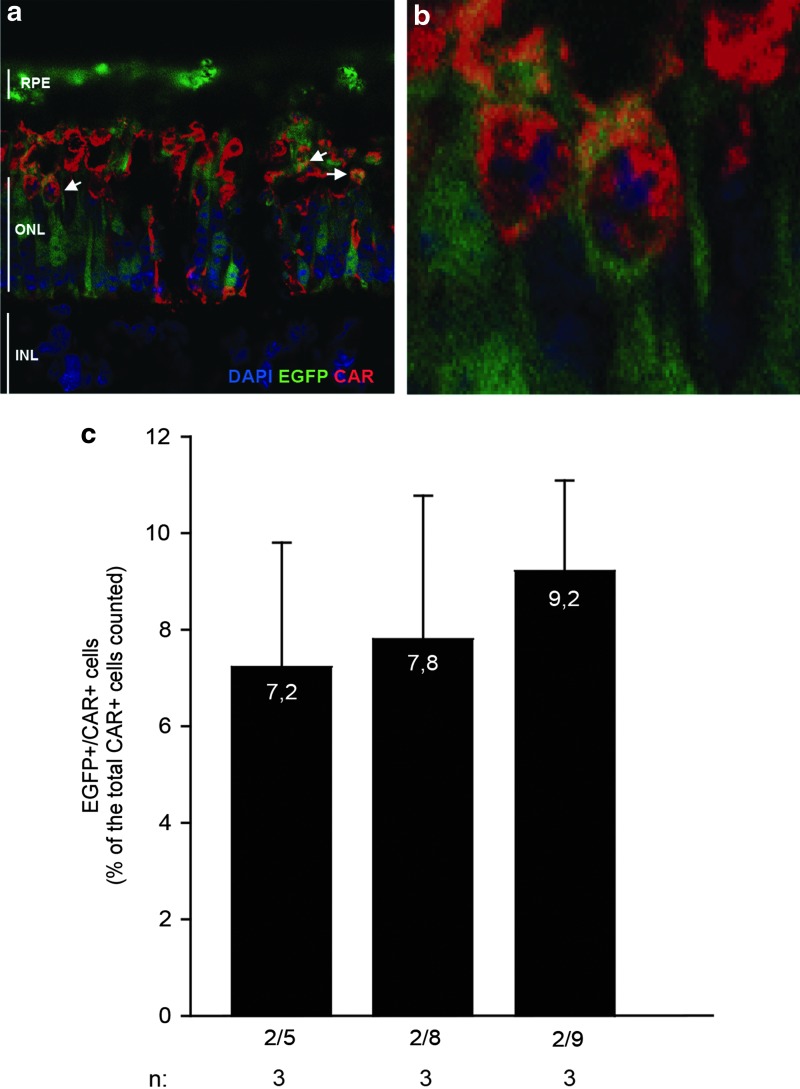
To evaluate the ability of the various AAV serotypes to transduce cones in addition to rods, we labeled the cone PRs using a rabbit polyclonal antibody raised against a carboxy-terminal peptide from the human cone Arrestin 4 protein (Luminaire founders) (LUMIf/hCAR) (Li et al., 2002) that also recognizes the porcine CAR and we analyzed them using confocal microscopy (Fig. 2). CAR-positive (CAR+)/EGFP-positive (EGFP+, white arrows in Fig. 2a, and an enlargement in Fig. 2b) cells are evident in sections from eyes injected with each of the various AAV serotypes. We then counted the double-labeled cells on a total of 150–200 CAR+ cells in the best transduced area of the pig retina; results of the quantifications are shown in Fig. 2c. All AAV serotypes showed a comparable efficiency in transducing porcine cones. Thus, we concluded that AAV2/8 provides the best combined rod and cone PR transduction in pigs, which are a relevant preclinical model and we carried out our next experiments using this serotype.
FIG. 2.
AAV-mediated cone transduction in pig retina. LW pigs were subretinally injected with AAV2/5, 2/8, and 2/9-CMV-EGFP (1×1010 GC/eye of each vector). (a) Retinal cryosections were obtained 4 weeks after injection, and anti-LUMIf-hCAR immunostaining (red) was performed to label cones; EGFP+ cones are indicated by white arrows. (b) Higher magnification of the transduced cones. The confocal microscopy images are representative of single plans of Z-stack taken at 63×magnification. (c) Quantification of cone transduction efficiency of AAV2/5, 2/8, and 2/9-CMV-EGFP. The histograms represent the percentage of EGFP+/CAR+ cells counted on a total of 150–200 CAR+ cells. p=0.63, one-way ANOVA. CAR, cone arrestin; DAPI, 4′,6′-diamidino-2-phenylindole; INL, inner nuclear layer.
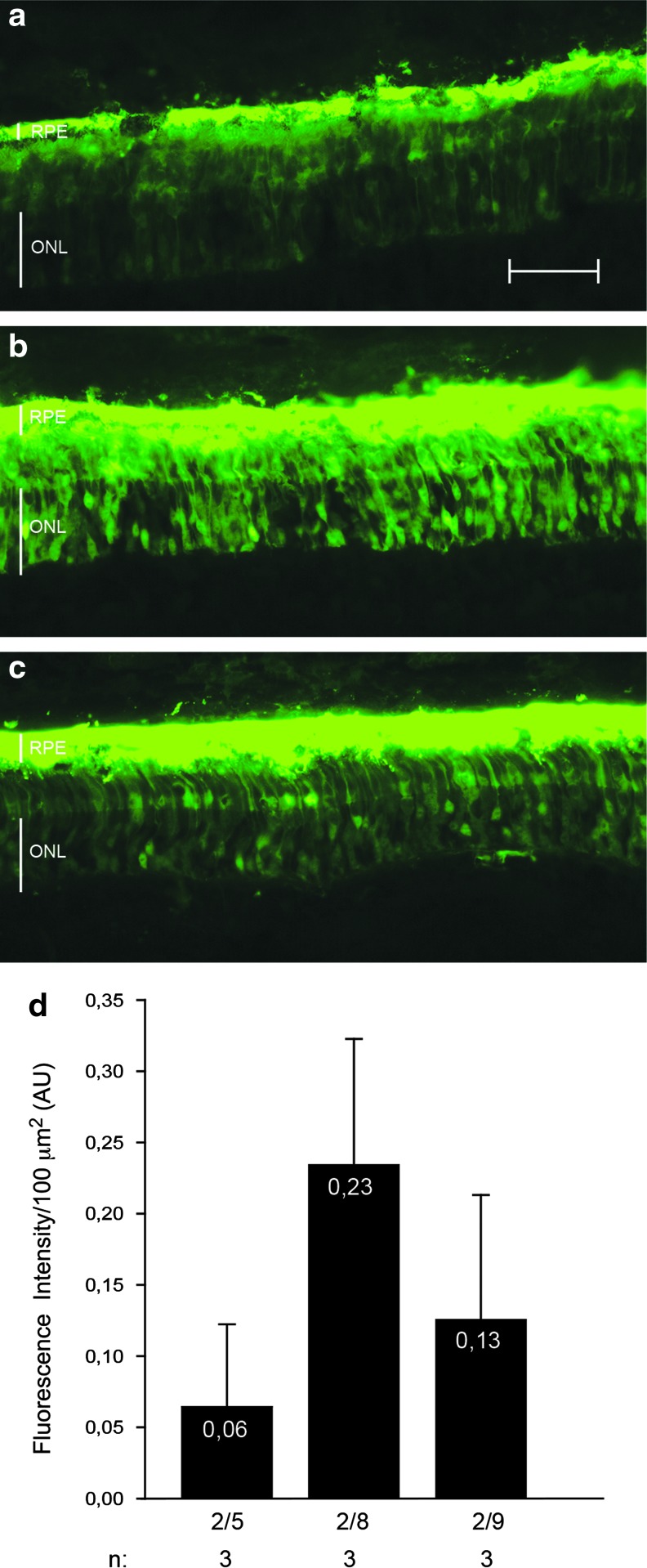
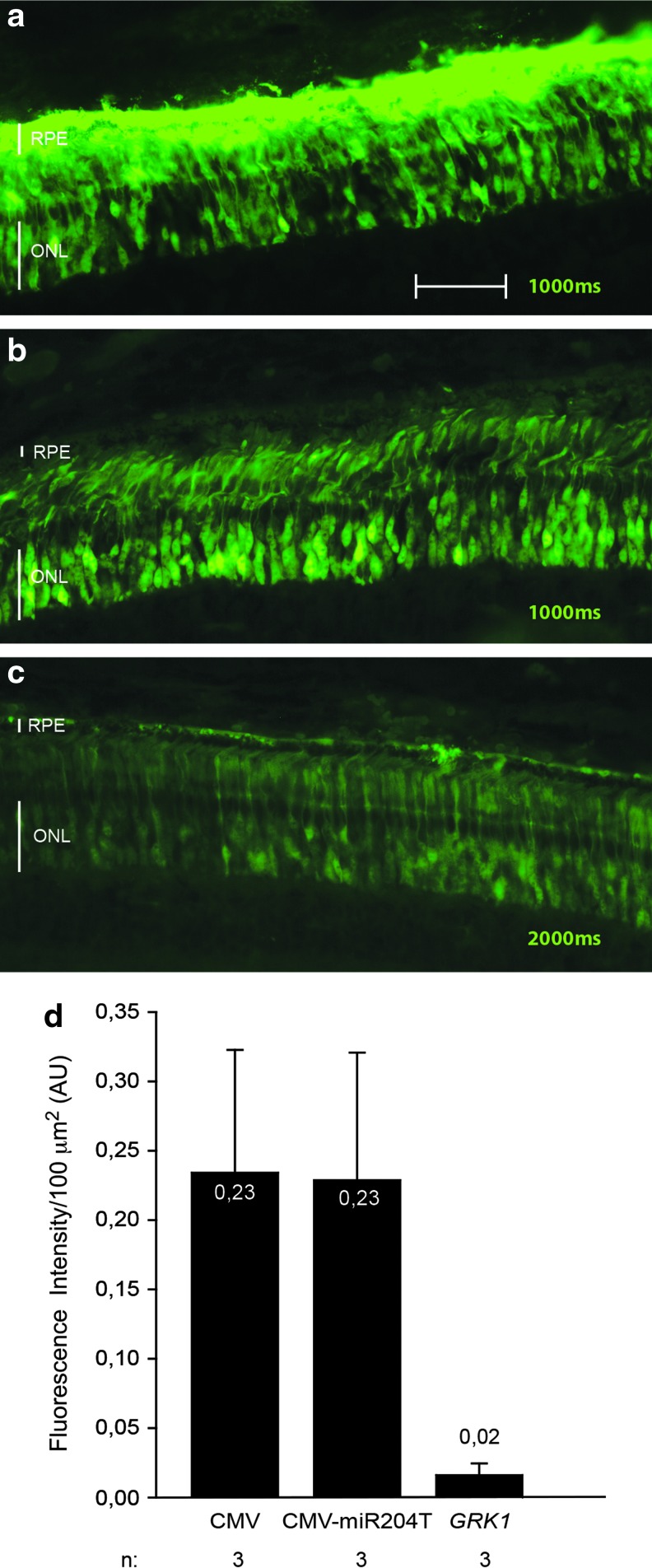
We then tested the rod and cone PR transduction of AAV2/8 in combination with (1) the ubiquitous CMV promoter; (2) the PR-specific GRK1 promoter; and (3) the CMV promoter in combination with miR204T, which we had previously demonstrated to restrict AAV-mediated transgene expression to the ONL in mice and pigs (Karali et al., 2011). We therefore subretinally injected the various AAV2/8 vectors encoding for EGFP (1×1010 GC/eye of each vector) in 11-week-old female LW pigs (n=3 eyes/vector). Eyes were harvested 4 weeks after the injection for histological analysis. Figure 3 shows that the CMV promoter with and without miR204T is the most efficient for PR transduction, considering that the representative image of the retina injected with AAV2/8-GRK1-EGFP (Fig. 3c) was taken at double of the exposure time (2,000 msec) used for the other retinas. Quantification of green fluorescent signal in the PR layer is shown in Fig. 3d.
FIG. 3.
AAV2/8-mediated retinal transduction in pig retina. LW pigs were subretinally injected with AAV2/8-CMV-EGFP (a), AAV2/8-CMV-EGFP-miR204T (b), and AAV2/8-GRK1-EGFP (c, 1×1010 GC/eye of each vector). Retinal cryosections were obtained 4 weeks after injection, and EGFP was analyzed using fluorescence microscopy. The exposure time is the same for the eyes injected with AAV2/8-CMV-EGFP and AAV2/8-CMV-EGFP-miR204T, while for AAV2/8-GRK1-EGFP the representative image was taken at an exposure time that was double the other vectors. (d) Fluorescence intensity in the PR layer was quantified for each group of animals at the same exposure time; p=0.13, one-way ANOVA. Scale bar=25 μm. Color images available online at www.liebertpub.com/hum
To assess the cone transduction efficiency, we labeled the sections with the anti-hCAR antibody and analyzed them using confocal microscopy (as previously described for Fig. 2). By counting CAR+/EGFP+ cells, we demonstrated that the CMV promoter resulted in the highest pig cone transduction levels and that the inclusion of miR204T markedly reduced transgene expression in cones (Fig. 4). Since GRK1-mediated EGFP expression was markedly lower than that obtained with CMV, we were forced to use longer exposure times than with CMV to count CAR+/EGFP+ cells in retinas transduced with the AAV2/8-GRK1-EGFP vectors (Fig. 4). These were significantly lower than those obtained with the CMV promoter and were similar to those obtained with the CMV-miR204T construct, despite the longer exposure times (p≤0.05, ANOVA). Previous studies documented that the GRK1 promoter sequence we used is effective to drive rod and cone expression in both mice (Allocca et al., 2007; Khani et al., 2007) and NHPs (Boye et al., 2012), and we hypothesized that the low porcine cone transduction levels we observed in pigs could have been caused by interspecies differences. Interestingly, GRK1 is expressed in both rods and cones in mice and humans but not in pig and dog cones, where its cyclic GMP synthesis function is supplied by the GRK7 homolog (Weiss et al., 2001). Therefore, the murine retina may be more appropriate than the porcine retina to assess cone transduction mediated by the GRK1 promoter.
FIG. 4.
Effect of different regulatory elements on AAV2/8-mediated cone transduction in pig retina. LW pigs were subretinally injected with AAV2/8-CMV-EGFP, AAV2/8-CMV-EGFP-miR204T, and AAV2/8-GRK1-EGFP (1×1010 GC/eye of each vector). Retinal cryosections were obtained 4 weeks after injection, and anti-LUMIf-hCAR immunostaining was performed to label cones (see Fig. 2a and b). Quantification of cone-transduction efficiency is shown. The histograms represent the percentage of EGFP+/CAR+ cells counted on a total of 150–200 CAR+ cells. For the eyes injected with AAV2/8-GRK1-EGFP, the quantification of cone transduction was done at both low and high exposure times. *p≤0.05; **p≤0.01, one-way ANOVA.
AAV2/8 in combination with the CMV and GRK1 promoters transduces efficiently murine rods and cones resulting in murine LCA1 cone rescue
The results we obtained in pigs support the use of AAV2/8 in combination with CMV, but not CMV-mir204T, for rod and cone transduction. Given the endogenous GRK1 expression in mice but not in pig cones, we went on to compare the efficacy of AAV2/8-CMV to that of AAV2/8-GRK1 in a mouse model of LCA1, in which cone function had been impaired since birth. We did not include AAV2/5 and AAV2/9 in our murine experiments based on our previous data in pigs and also since we have previously shown that AAV2/8 outperforms AAV2/5 in murine PR transduction, while AAV2/9 provides similar levels of PR transduction to AAV2/8 (Allocca et al., 2007). We initially compared the overall PR and cone transduction in the wild-type murine retina using the reporter EGFP gene. Thus, we subretinally injected 1.7×109 GC/eye of either AAV2/8-CMV- or GRK1-EGFP vectors in 4-week-old C57/BL6 mice. Four weeks after the injection, we analyzed the corresponding retinal cryosections under a fluorescence microscope (Fig. 5a and b). Quantification of fluorescence intensity showed that the CMV provided statistically higher levels of murine PR transduction than GRK1 (p≤0.01, Fig. 5d). When we overexposed the retinal sections transduced with the vector containing the GRK1 promoter (Fig. 5c), we observed widespread PR transduction proving that this promoter element is indeed active in murine PRs. We then stained the retinal cryosections with a rabbit polyclonal carboxy-terminal peptide antibody that is species specific for mouse cone Arrestin 4 (Luminaire junior- [LUMIj]-mCAR) to identify EGFP+ murine cone PRs (Fig. 5e, white arrows, and enlargement in Fig. 5f). To quantify cone transduction levels, we counted the number of CAR+/EGFP+ cells on a total of 150–200 CAR+ cells in different regions of the transduced area using confocal microscopy and found that the CMV promoter induced higher cone transduction levels than GRK1 (Fig. 5g; p≤0.01, ANOVA). Since the GRK1 promoter showed extended, although weaker, transduction of the murine ONL than the CMV (Fig. 5b and c), we evaluated the number of EGFP+ cones in GRK1-transduced retinas at longer exposure times and found that, under these conditions, the number of GRK1-transduced cones was similar to those transduced by CMV (Fig. 5g). Thus, the GRK1 promoter induced weaker levels of transgene expression per cone than the CMV promoter; however, the number of transduced cones was similar.
We then compared the ability of the two promoter elements in combination with AAV2/8 to rescue cone function in the Gucy2e−/− mouse model of LCA1. AAV2/8 vectors with the CMV and GRK1 promoters and encoding the human GUCY2D coding sequence were subretinally injected (1.5×109 GC/eye) in Gucy2e−/− mice at postnatal day 10 (P10). Contralateral eyes were either left uninjected or were injected with the same dose of AAV2/8-CMV or GRK1-EGFP vectors as control. Three months after subretinal injection of AAV vectors in Gucy2e−/− mice, we performed immunofluorescence analysis of retinal cryosections to show that GUCY2D expression was properly localized in mouse PRs (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/hum).
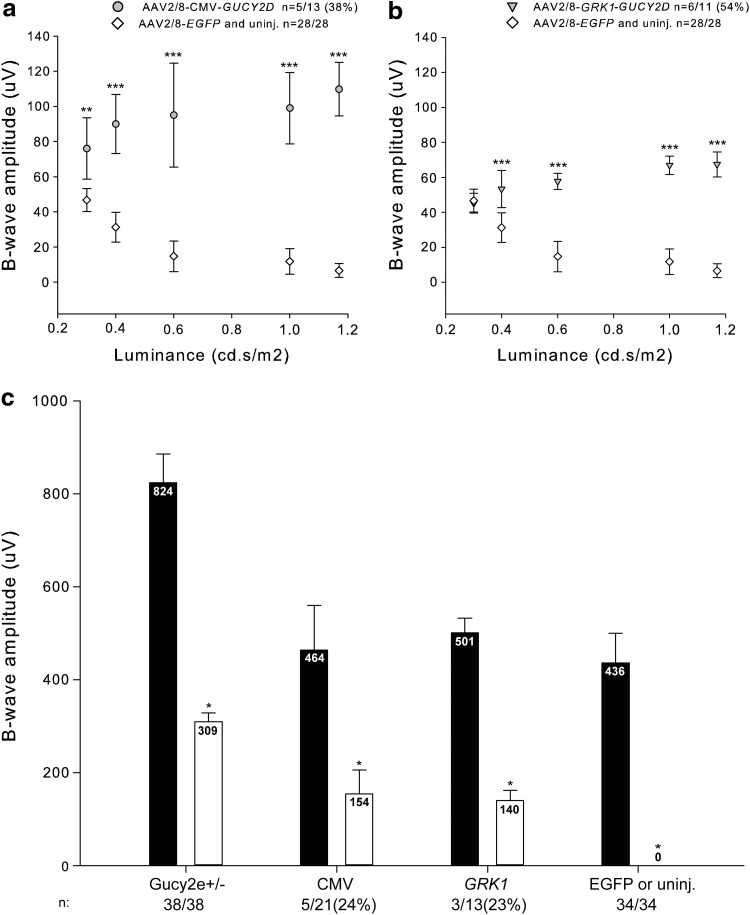
Cone function was analyzed 1 month postinjection. The 6 Hz scotopic flicker test (6 Hz flicker) was used to distinguish rod from cone responses. In this test, a 6 Hz stimulus is presented with increasing light intensity. At higher light intensities (0.2–1.2 cd•s/m2), the electrical response is predominantly derived from cones (Seeliger et al., 2011). The results from the 6 Hz flicker 1 month postinjection showed that Gucy2e−/− cone function was significantly improved using both the CMV and GRK1 promoters (Fig. 6a and b). The average amplitude at higher light intensities was higher with the CMV promoter, while the percentage of retinas showing improved cone function was higher with the GRK1 (54%) than with the CMV (38%) promoter. Using a binomial test, the statistical analysis of the results confirmed the significance of the improvement obtained with both the CMV and the GRK1 promoters (p≤0.001, ANOVA). Although significant, the improvement in the 6 Hz flicker achieved in the AAV2/8-GUCY2D-treated retinas was partial compared with Gucy2e+/− mice (Supplementary Fig. S2).
FIG. 6.
Retinal function assessment in Gucy2e−/− mice after AAV2/8-mediated gene transfer. Gucy2e−/− mice were treated at P10 with 1.5×109 GC/eye of either AAV2/8-GUCY2D in one eye or AAV2/8-EGFP (or untreated) in the contralateral eye. Six-hertz flicker results of Gucy2e−/− animals treated with either AAV2/8-CMV-GUCY2D (a) or AAV2/8-GRK1-GUCY2D (b) were analyzed 1 month postinjection. (c) Retinal function was also assessed by paired flash ERG. The histograms represent the average amplitudes of the responses to either the first (black bar) or the second (white bar) flash. n is the ratio between the number of represented eyes and the total number of injected eyes. The numbers of represented eyes in the CMV and GRK1 groups are those in which improvement of cone activity was evident, which is additionally indicated as % of total in parentheses. *p≤0.05; **p≤0.01; ***p≤0.001, one-way ANOVA.
The paired-flash ERG was also used to distinguish cone from rod responses. This procedure was based on the method of Birch et al. (1995) with minor modifications. Cone responses were measured by a probe flash that follows an initial adapting flash. The interval between two flashes is very short (500 msec), and the rod system is unable to generate a response to the second flash (probe), which will therefore be mainly cone driven (Fig. 6c). While the ERG response to the probe flash was absent in untreated Gucy2e−/− mice, this was improved 1 month postinjection of AAV2/8-GUCY2D (Fig. 6c). The paired-flash ERG results confirmed the AAV2/8-mediated improvement in cone function observed with the 6 Hz flicker, and that the CMV and the GRK1 promoters performed similarly well.
Discussion
In this study we compared various AAV serotypes and promoter elements for their ability to efficiently transduce both cones and rods in mice and pigs, which are a relevant preclinical large model. We have selected AAV2/5, 2/8, and 2/9, which we and others have demonstrated to efficiently transduce the ONL, including cones of different species (Allocca et al., 2007; Lebherz et al., 2008; Mancuso et al., 2009; Mussolino et al., 2011; Vandenberghe et al., 2011, 2013). A recent study suggests that AAV2/8 is best suited for the transduction of rod PRs in NHP retina and subsequently for those diseases that involve mutations in genes expressed only in rods (Vandenberghe et al., 2013). This is in line with our previous observation that AAV2/8 outperforms AAV2/5 for murine (Allocca et al., 2007) and porcine (Mussolino et al., 2011) PR transduction. However, AAV2/9 seems to be the most efficient for cone transduction (Vandenberghe et al., 2013). The data we present here show that AAV2/8 transduces pig cones to levels that are similar to AAV2/9, and the ONL (mainly rods) to levels that are on average, although not statistically significant, higher than AAV2/9.
The levels of pig PR transduction we report here after AAV2/8 subretinal injection are on average higher than those after AAV2/5 administration, similarly to what we have reported in Mussolino et al. (2011). However, the difference in AAV2/8- versus AAV2/5-mediated porcine PR transduction we observe here appears less marked than in Mussolino et al. (2011). This may be because of the different (1) time of harvesting (4 weeks here vs. 6 weeks there); (2) method used for quantification (histological fluorescence quantification here vs. Western blot there); and (3) sampling (fluorescence restricted to the PR layer with exclusion of their outer segments tip adjacent to the RPE here vs. whole neural retina lysates there).
Among the various regulatory elements we have tested, the CMV promoter shows the highest levels of rod and cone transduction. However, when we combine this with miR204T, though we de-target transgene expression from RPE cells, we also reduce cone transduction levels in pigs. It is possible that this is caused by higher levels of endogenous mir204 expression in pig cones than rods.
We show that the levels of murine cone transduction provided by the GRK1 promoter element are lower than those achieved by CMV, although the number of cells transduced by both elements is similar. Still, the levels of cone rescue obtained with the GRK1 promoter are similar to those achieved with CMV, which suggests that replacing low levels of guanylate cyclase may provide a therapeutic benefit.
Boye and her collaborators (Boye et al., 2010, 2011) with either AAV2/5 or AAV2/8(Y733F) and Mihelec et al. (2011) with AAV2/8 have both reported more consistent and long-term restoration of cone function in Gucy2e−/− mice than we have observed. This may be because of differences in (1) the promoter elements used [the chicken beta actin promoter in Boye et al. (2010) and a longer human GRK1 promoter element than what we have used in both Boye et al. (2010) and Mihelec et al. (2011)]; (2) the electroretinographic assays used; and (3) the extent of retinal transduction, which is reported to be >60% in Boye et al. (2010, 2011) and is around 30% in our case. Similarly, Mihelec et al. (2011) performed double subretinal administrations, which probably allowed a wider retinal transduction than the single injection we performed.
Our results in mice and pigs support the use of AAV2/8 in combination with the GRK1 and CMV promoters for gene transfer to both rods and cones for treatment of patients affected by LCA1 or other IRDs caused by genes expressed in both PR types. Two studies in LCA1 patients (age range 6 months to 53 years) have reported preservation of retinal structures despite markedly impaired visual functions (Pasadhika et al., 2010; Jacobson et al., 2013), which deems LCA1 a disease amenable to gene therapy.
Supplementary Material
Acknowledgments
We thank Annamaria Carissimo and Luisa Cutillo (Bioinformatics Core, TIGEM, Naples, Italy) for their help with statistical analyses, the TIGEM AAV Vector Core for AAV vector production, and Ellen Abrams and Graciana Diez-Roux (Scientific Office, TIGEM, Naples, Italy) for the critical reading of this article. This work was supported by the European Research Council/ERC Grant agreement no. 282085 “RetGeneTx”; the European Community's Seventh Framework Programme (FP7/2007–2013) under Grant agreement no. 242013 “Treatrush”; the NIH (Grant R24 RY019861-01A); and the Italian Telethon Foundation (Grant TGM11MT1). C.M.C., who is the Mary D. Allen Chair in Vision Research (Doheny Eye Institute, DEI), acknowledges the following fundings: NIH Grant EY015851 (C.M.C.), Research to Prevent Blindness (DEI), and National Eye Institute Core Grant EY03040 (DEI).
Author Disclosure Statement
No competing financial interests exist.
References
- Ahnelt P.K. (1998). The photoreceptor mosaic. Eye 12, 531–540 [DOI] [PubMed] [Google Scholar]
- Allocca M., Mussolino C., Garcia-Hoyos M., et al. (2007). Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J. Virol. 81, 11372–11380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M., Cyckowski L.L., Monroe J.F., et al. (2011). The human visual cortex responds to gene therapy-mediated recovery of retinal function. J. Clin. Invest. 121, 2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W., Karan S., Maeda T., et al. (2007). The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J. Biol. Chem. 282, 8837–8847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge J.W., Smith A.J., Barker S.S., et al. (2008). Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 358, 2231–2239 [DOI] [PubMed] [Google Scholar]
- Beltran W.A., Cideciyan A.V., Lewin A.S., et al. (2012). Gene therapy rescues photoreceptor blindness in dogs and paves the way for treating human X-linked retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 109, 2132–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J., Maguire A.M., Cideciyan A.V., et al. (1999). Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc. Natl. Acad. Sci. USA 96, 9920–9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch D.G., Hood D.C., Nusinowitz S., and Pepperberg D.R. (1995). Abnormal activation and inactivation mechanisms of rod transduction in patients with autosomal dominant retinitis pigmentosa and the pro-23-his mutation. Invest. Ophthalmol. Vis. Sci. 36, 1603–1614 [PubMed] [Google Scholar]
- Boye S.E., Boye S.L., Pang J., et al. (2010). Functional and behavioral restoration of vision by gene therapy in the guanylate cyclase-1 (GC1) knockout mouse. PloS One 5, e11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S.L., Conlon T., Erger K., et al. (2011). Long-term preservation of cone photoreceptors and restoration of cone function by gene therapy in the guanylate cyclase-1 knockout (GC1KO) mouse. Invest. Ophthalmol. Vis. Sci. 52, 7098–7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S.E., Alexander J.J., Boye S.L., et al. (2012). The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum. Gene Ther. 23, 1101–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson L.D., and Lavail M.M. (1979). Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 188, 245–262 [DOI] [PubMed] [Google Scholar]
- Cideciyan A.V., Aleman T.S., Boye S.L., et al. (2008). Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. USA 105, 15112–15117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V., Hauswirth W.W., Aleman T.S., et al. (2009a). Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum. Gene Ther. 20, 999.–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V., Hauswirth W.W., Aleman T.S., et al. (2009b). Vision 1 year after gene therapy for Leber's congenital amaurosis. N. Engl. J. Med. 361, 725.–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan A.V., Jacobson S.G., Beltran W.A., et al. (2013). Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl. Acad. Sci. USA 110, E517–E525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G., Heon E., and Mcinnes R.R. (2000). Recent advances in the molecular basis of inherited photoreceptor degeneration. Clin. Genet. 57, 313–329 [DOI] [PubMed] [Google Scholar]
- Corbo J.C., Myers C.A., Lawrence K.A., et al. (2007). A typology of photoreceptor gene expression patterns in the mouse. Proc. Natl. Acad. Sci. USA 104, 12069–12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hollander A.I., Roepman R., Koenekoop R.K., and Cremers F.P. (2008). Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 27, 391–419 [DOI] [PubMed] [Google Scholar]
- Den Hollander A.I., Black A., Bennett J., and Cremers F.P. (2010). Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J. Clin. Invest. 120, 3042–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbers D.L., and Lowe D.G. (1994). Guanylyl cyclase receptors. J. Biol. Chem. 269, 30741–30744 [PubMed] [Google Scholar]
- Hauswirth W.W., Aleman T.S., Kaushal S., et al. (2008). Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 19, 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson A., and Hicks D. (2002). Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp. Eye Res. 74, 435–444 [DOI] [PubMed] [Google Scholar]
- Jacobson S.G., Cideciyan A.V., Peshenko I.V., et al. (2013). Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants. Hum. Mol. Genet. 22, 168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon C.J., Strettoi E., and Masland R.H. (1998). The major cell populations of the mouse retina. J. Neurosci. 18, 8936–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karali M., Peluso I., Marigo V., and Banfi S. (2007). Identification and characterization of microRNAs expressed in the mouse eye. Invest. Ophthalmol. Vis. Sci. 48, 509–515 [DOI] [PubMed] [Google Scholar]
- Karali M., Manfredi A., Puppo A., et al. (2011). MicroRNA-restricted transgene expression in the retina. PloS One 6, e22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khani S.C., Pawlyk B.S., Bulgakov O.V., et al. (2007). AAV-mediated expression targeting of rod and cone photoreceptors with a human rhodopsin kinase promoter. Invest. Ophthalmol. Vis. Sci. 48, 3954–3961 [DOI] [PubMed] [Google Scholar]
- Lai C.M., Yu M.J., Brankov M., et al. (2004). Recombinant adeno-associated virus type 2-mediated gene delivery into the Rpe65−/− knockout mouse eye results in limited rescue. Genet. Vaccines Ther. 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebherz C., Maguire A., Tang W., et al. (2008). Novel AAV serotypes for improved ocular gene transfer. J. Gene Med. 10, 375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A., Zhu X., and Craft C.M. (2002). Retinoic acid upregulates cone arrestin expression in retinoblastoma cells through a Cis element in the distal promoter region. Invest. Ophthalmol. Vis. Sci. 43, 1375–1383 [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A., et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358, 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., High K.A., Auricchio A., et al. (2009). Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374, 1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso K., Hauswirth W.W., Li Q., et al. (2009). Gene therapy for red-green colour blindness in adult primates. Nature 461, 784–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieziewska K. (1996). The interphotoreceptor matrix, a space in sight. Microsc. Res. Tech. 35, 463–471 [DOI] [PubMed] [Google Scholar]
- Mihelec M., Pearson R.A., Robbie S.J., et al. (2011). Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum. Gene Ther. 22, 1179–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussolino C., Della Corte M., Rossi S., et al. (2011). AAV-mediated photoreceptor transduction of the pig cone-enriched retina. Gene Ther. 18, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omri S., Omri B., Savoldelli M., et al. (2010). The outer limiting membrane (OLM) revisited: clinical implications. Clin. Ophthalmol. 4, 183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasadhika S., Fishman G.A., Stone E.M., et al. (2010). Differential macular morphology in patients with RPE65-, CEP290-, GUCY2D-, and AIPL1-related Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 51, 2608–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlyk B.S., Bulgakov O.V., Liu X., et al. (2010). Replacement gene therapy with a human RPGRIP1 sequence slows photoreceptor degeneration in a murine model of Leber congenital amaurosis. Hum. Gene Ther. 21, 993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I., Rozet J.M., Gerber S., et al. (2000). Spectrum of retGC1 mutations in Leber's congenital amaurosis. Eur. J. Hum. Genet. 8, 578–582 [DOI] [PubMed] [Google Scholar]
- Petit L., Lheriteau E., Weber M., et al. (2012). Restoration of vision in the pde6beta-deficient dog, a large animal model of rod-cone dystrophy. Mol. Ther. 20, 2019–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger M.W., Brombas A., Weiler R., et al. (2011). Modulation of rod photoreceptor output by HCN1 channels is essential for regular mesopic cone vision. Nat. Commun. 2, 532. [DOI] [PubMed] [Google Scholar]
- Simonelli F., Maguire A.M., Testa F., et al. (2010). Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 18, 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K., and Lorenz B. (2010). Gene therapy for vision loss—recent developments. Discov. Med. 10, 425–433 [PubMed] [Google Scholar]
- Sun X., Pawlyk B., Xu X., et al. (2010). Gene therapy with a promoter targeting both rods and cones rescues retinal degeneration caused by AIPL1 mutations. Gene Ther. 17, 117–131 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sundaram V., Moore A.T., Ali R.R., and Bainbridge J.W. (2012). Retinal dystrophies and gene therapy. Eur. J. Pediatr. 171, 757–765 [DOI] [PubMed] [Google Scholar]
- Surace E.M., and Auricchio A. (2008). Versatility of AAV vectors for retinal gene transfer. Vis. Res. 48, 353–359 [DOI] [PubMed] [Google Scholar]
- Testa F., Maguire A.M., Rossi S., et al. (2013). Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology 120, 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H., and Auricchio A. (2012). Novel adeno-associated viral vectors for retinal gene therapy. Gene Ther. 19, 162–168 [DOI] [PubMed] [Google Scholar]
- Vandenberghe L.H., Bell P., Maguire A.M., et al. (2011). Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 3, 88ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe L.H., Bell P., Maguire A.M., et al. (2013). AAV9 targets cone photoreceptors in the nonhuman primate retina. PloS One 8, e53463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E.R., Ducceschi M.H., Horner T.J., et al. (2001). Species-specific differences in expression of G-protein-coupled receptor kinase (GRK) 7 and GRK1 in mammalian cone photoreceptor cells: implications for cone cell phototransduction. J. Neurosci. 21, 9175–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.B., Robinson S.W., Xiong W.H., et al. (1999). Disruption of a retinal guanylyl cyclase gene leads to cone-specific dystrophy and paradoxical rod behavior. J. Neurosci. 19, 5889–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.E., Vogt T., Gross K.W., and Khani S.C. (2003). A short, highly active photoreceptor-specific enhancer/promoter region upstream of the human rhodopsin kinase gene. Invest. Ophthalmol. Vis. Sci. 44, 4076–4085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.