Abstract
Purpose:
To compare the outcomes of combination systemic and intravitreal antiviral therapy vs systemic antiviral therapy alone for treating acute retinal necrosis syndrome (ARN). We hypothesize that combination therapy might result in superior visual acuity (VA) and retinal detachment (RD) outcomes vs traditional systemic antiviral therapy alone.
Methods:
A retrospective, interventional, comparative single-center study of patients with ARN. We reviewed demographic data, herpesvirus diagnoses, polymerase chain reaction (PCR) results, VA, RD, and the use of systemic and intravitreal antiviral therapy. Outcome measures included VA improvement by 2 or more lines, severe visual loss, VA ≤20/200, and RD.
Results:
We studied 29 eyes of 24 patients, treated from 1987 through 2009. Mean age was 42.6 years and mean follow-up was 44.0 months. Twelve patients (14 eyes) were treated with combined systemic and intravitreal antiviral therapy and 12 patients (15 eyes) with systemic therapy alone. Kaplan-Meier survival analysis revealed that patients receiving combination intravitreal and systemic antiviral therapy were more likely to have VA improved by 2 lines or greater (P=.006). Patients receiving combination therapy also showed a decreased incidence of progression to severe visual loss (0.13/patient-years [PY]) compared to patients receiving systemic therapy alone (0.54/PY, P=.02) and had decreased incidence of RD (0.29/PY vs 0.74/PY, P=.03).
Conclusions:
Combination oral and intravitreal antiviral therapy may improve visual and functional outcomes in patients with ARN. Clinicians should consider prompt administration of combination systemic and intravitreal antiviral therapy as first-line treatment for patients with clinical features of ARN.
INTRODUCTION
The treatment of acute retinal necrosis (ARN) has evolved since Bird and Young1 coined the term in 1978 to describe 4 cases of bilateral necrotizing retinitis. Early functional and anatomic outcomes were almost universally poor given the limited understanding of the etiopathogenesis of ARN. Following the landmark discovery of the association of herpetic virus with ARN, systemic parenteral antivirals were administered with benefit in limited case series; however, retinal detachments (RDs), occlusive retinal vasculopathy, and optic neuropathy were still associated with a grim visual prognosis for many patients. More recently, newer oral antiviral medications capable of achieving vitreous concentrations high enough to rapidly inhibit herpetic viral replication have been utilized successfully in the treatment of ARN. Intravitreal delivery of antivirals (eg, foscarnet and ganciclovir) has also been described in limited case reports to effectively halt the destructive infectious and inflammatory sequelae of ARN. This thesis will describe 2 decades of ARN treatment at a university-based, tertiary referral center. Specifically, we address the role of combination systemic and intravitreal antiviral therapy for the treatment of ARN compared to traditional systemic antiviral therapy alone.
HISTORICAL PERSPECTIVE
The term bilateral acute retinal necrosis (BARN) was coined in the English literature by Young and Bird1 in 1978 to describe 4 cases of bilateral necrotizing retinitis. In this report, all 4 patients developed bilateral, confluent retinitis, with several eyes progressing to RD and phthisis despite corticosteroid and antibiotic therapy. Prior to this report, in 1971, Urayama and colleagues2 described 6 patients with similar disease characteristics occurring in a unilateral fashion. In their report, they used the term “Kirisawa-type uveitis” to describe a series of patients with unilateral acute uveitis with periarteritis, dense vitreous opacity, peripheral retinal exudates, RD, poor visual prognosis, a negative infectious workup, and ineffective medical treatment. Moreover, there were other reports of patients with clinical features resembling ARN in association with systemic herpes viral infections. These included reports by Brown and Mendis in 19733 and Cibis in 1975.4
In 1983, Hayasaka and colleagues5 recognized the striking similarity of reports in the Japanese literature to those in the English literature. They described the long-term (>10 years) follow-up of patients from the series originally reported by Urayama and colleagues. While the disease characteristics were virtually identical to those in Young and Bird’s series, the patients in Urayama’s series had not developed disease in the fellow eye, and the term acute retinal necrosis was recommended because the condition was not universally bilateral.
At the time of the report by Hayasaka and colleagues, 30 to 40 cases resembling ARN were reported in the Japanese and English literature. Reports of pathologic and electron microscopic findings of ARN from vitrectomy and enucleation specimens began to emerge. They showed chronic granulomatous inflammation,6 lymphocytic infiltrates in the vitreous,7 retinal vascular occlusions, extensive outer retinal atrophy, and retinal pigment epithelium degeneration.8 Although the precise etiology had not been identified, an infectious trigger with severe immune-mediated inflammation and vasculitis was suspected.
ETIOLOGY AND DIAGNOSTIC TESTING
Following these clinical and pathological reports, ARN was determined to be an infectious syndrome caused by members of the herpesvirus family.9 The causative organisms of ARN include varicella zoster virus (VZV), herpes simplex viruses (HSV-1 and HSV-2), and cytomegalovirus (CMV). Although VZV is the most common cause of ARN, HSV-1 and HSV-2 also may cause ARN. HSV-2 has been identified predominantly in younger patients with the ARN syndrome. Epstein-Barr virus has also been reported in association with ARN, albeit less frequently than VZV and HSVs.10–15
Culbertson and colleagues16 first demonstrated herpes group virus in all layers of diseased retina. Specifically, their histopathology and electron microscopic evaluation of a 67-year-old patient with ARN showed necrosis of retinal layers and eosinophilic nuclear inclusions in retinal cells consistent with herpesvirus. Varicella zoster virus was later identified from 2 blind enucleated eyes during the active phase of ARN.10 The virus was confirmed by electron microscopy and vitreous culture. Ocular and serum antibody and antigen testing have also been utilized for the diagnostic confirmation of herpetic virus,13,17,18 although recent reports favor the use of polymerase chain reaction (PCR) diagnostics owing to its rapidity and high sensitivity.10,13,19,20
Polymerase chain reaction is a highly sensitive and rapid method of detecting viral DNA from aqueous and vitreous humor samples from patients with suspected ARN,21,22 and its diagnostic utility has been increasingly recognized, particularly in diagnostic dilemmas. Indeed, the positive predictive value of PCR testing for infectious uveitis is nearly 99%, and its negative predictive value approaches 68%.22 Thus, PCR testing of the aqueous or vitreous humor may change a treatment decision and is valuable in the early diagnosis of ARN and selection of appropriate antiviral therapy.
DIAGNOSTIC CRITERIA
Diagnostic criteria for ARN were proposed by the American Uveitis Society (AUS) in 1994.23 Since then, no other criteria for diagnosis have been suggested, although the use of PCR for precise etiologic diagnosis has been reported with increasing frequency. According to the proposed diagnostic criteria, ARN is a syndrome characterized by full-thickness necrotizing retinitis, arteritis, and severe inflammation involving the anterior chamber and vitreous fluid. It was defined by the AUS by its clinical characteristics and disease course, regardless of the causative agent or patient immune status, but it is classically seen in immunocompetent hosts. Diagnostic criteria include (1) one or more discrete foci of peripheral retinal necrosis (located outside of the major temporal vascular arcades), (2) circumferential spread (if antiviral therapy has not been administered), (3) occlusive retinal vasculopathy, (4) a prominent vitreous or anterior chamber inflammation, and (5) rapid disease progression in the absence of therapy.
While these clinical features are central to the ARN syndrome, there is growing evidence that the different causative viral entities may present with varying degrees of severity (ie, greater severity in VZV when compared to HSV). Moreover, HSV-2 may have characteristic clinical findings that may differentiate HSV-2-ARN from HSV-1-ARN and VZV-ARN. Specifically, Tran and colleagues14 reported 12 eyes of 11 patients with HSV-2–related ARN syndrome. In their series, all patients were immunocompetent. Triggering events included neurosurgery, high-dose corticosteroids, and chorioretinal scars. To add further complexity to this diagnosis, Wensing and coworkers16 recently reported their experience with a cohort of patients with “non-ARN” ARN (ie, slowly progressive necrotic lesions or absence of necrotizing posterior uveitis altogether).
Taken together, this spectrum of clinical phenotypes, differential severity between viral organisms, and variations in host inflammatory response25 make the diagnosis and treatment of ARN challenging and worthy of further research. While a discussion of current diagnostic criteria, which may eventually include viral etiology, is beyond the scope of this work, inclusion of PCR diagnostic data to classify the patients studied (ie, VZV-ARN, HSV1/2-ARN) seems appropriate for current and future research, particularly in the assessment of clinical outcomes where the presenting clinical severity may be prognostic in the patient’s final visual outcome.
RATIONALE FOR SYSTEMIC ANTIVIRAL THERAPY
The treatment of ARN traditionally has included intravenous antiviral therapy with or without corticosteroids. Oral acyclovir following the intravenous treatment has been suggested to decrease the risk of fellow eye involvement.26,27 Blumenkranz and colleagues26 first reported their experience with parenteral acyclovir therapy (1500 mg/m2/day) for the treatment of ARN. Specifically, 13 eyes of 12 patients were treated for a mean of 10.9 days along with aspirin or warfarin. Oral prednisone was also administered in 75% of patients. While progression of the lesions was seen during the first 48 hours following initiation of therapy, regression of lesions was seen at 3.9 days following therapy and required 32.5 days for complete regression. In 11 of 13 eyes, RD was observed, but there were no treatment-related complications. Other reports of high-dose intravenous acyclovir followed, with lower rates of RD observed than in early case series.28,29
Palay and colleagues27 described a large series of 54 patients with ARN. Of these, 31 received acyclovir and 23 did not. Of the 31 patients treated with acyclovir, 27 fellow eyes (87%) remained disease-free with a median 12-month follow-up. In the untreated cohort, only 30% of fellow eyes remained disease-free through a median 11-month follow-up. Survival analysis revealed a significant difference in fellow-eye involvement favoring acyclovir vs observation (P=.0013), and at 2 years a minority of eyes (25%) developed disease in the treatment group, whereas over two-thirds in the untreated group developed fellow eye involvement (BARN).
While the use of oral acyclovir thus appears advantageous in the prevention of fellow eye involvement, its ability to treat active disease is less favorable. Specifically, while intravenous acyclovir administered at dosing of 1500 mg/m2 divided into 3 daily doses reaches the vitreous concentration required to inhibit HSV and VZV viral plaques by 50%, oral acyclovir fails to attain these levels. The newer oral antiviral agents, valacyclovir (Valtrex, GlaxoSmithKline, London, England) and famciclovir (Famvir, Novartis, Basel, Switzerland), which have demonstrated efficacy against the major causative viruses of ARN, have been introduced to the market more recently to address this need. Valacyclovir has demonstrated superior bioavailability compared to acyclovir (70% vs 21%).30 Valacyclovir, the prodrug of acyclovir, administered at a dose of 1000 mg every 8 hours 24 hours prior to pars plana vitrectomy (PPV) achieves vitreous concentrations exceeding the inhibitory concentration for the majority of VZV, HSV-1, and HSV-2 strains.31 Moreover, famciclovir, the oral prodrug of penciclovir, administered at a dose of 500 mg times 3 doses and then on the morning of PPV, also resulted in vitreous concentrations inhibiting the herpetic viruses.32
Indeed, the successful use of famciclovir for ARN was reported in 1 patient with ARN whose disease was refractory to intravenous and oral acyclovir. Oral famciclovir monotherapy resulted in the regression of retinitis in this patient following 1 month of therapy, similar to the original data on retinitis remission following intravenous acyclovir described by Blumenkranz and colleagues (32.5 days).26 A handful of case reports and small case series, which followed this original report, revealed 5 patients with ARN treated successfully with oral antiviral therapy alone.33–35 Subsequent larger case series of 4 and 8 patients by Emerson and colleagues36 and Aizman and coworkers,37 respectively, described the efficacy of oral antiviral monotherapy for the ARN syndrome. Following treatment either with oral valacyclovir or famciclovir, 3 of 4 patients (6 eyes) improved with 2 weeks to 1 month of therapy in the series reported by Emerson and colleagues; all 8 patients (10 eyes) had regression of retinitis after oral antiviral therapy in the report from Aizman and coworkers. At final follow-up in these 2 series, rates of RD were similar at 30% and 33%, respectively, with vision stabilization or improvement by 2 lines or greater in 80% to 100% of eyes. While these results were encouraging, with initial responses reported as early as 4 days following the initiation of therapy, other patients required up to 42 days to respond to treatment. One patient in the series by Aizman and coworkers also received intravitreal foscarnet, which was suggested as an adjunctive method of achieving immediate, high intravitreal drug levels.
RATIONALE FOR INTRAVITREAL ANTIVIRAL THERAPY
The primary goals of therapy for ARN include the eradication of disease activity in the involved eye and the reduction, elimination, or both, of ARN risk in the fellow eye. Rapid inhibition of active viral replication and its destructive sequelae is achieved by the introduction of high concentrations of antiviral medication to the vitreous and retina; the administration of concomitant systemic antiviral medication is strategic from a therapeutic standpoint in the involved eye and preventive for the fellow eye. Because ARN may progress rapidly over 24 hours, intravitreal antiviral medications have been considered primarily as an adjunctive measure in severe disease to achieve therapeutic concentrations.
The earliest report of intravitreal antiviral therapy was described by Peyman and colleagues in 1984,38 in which 2 patients with ARN were treated with vitrectomy and intravitreal infusion of acyclovir. Subsequent to their report, toxicity studies evaluated intravitreal ganciclovir and foscarnet, specifically in consideration for the treatment of HIV/AIDS-related cytomegalovirus retinitis and ARN.39 These toxicity studies suggested that intravitreally administered foscarnet in high levels ranging from 20 to 1000 μg/0.1 mL was nontoxic to the retina. Electroretinogram, ophthalmoscopy, and histologic studies of intravitreal foscarnet, with or without ganciclovir, showed no evidence of retinal toxicity with a terminal elimination half-life of 34 hours.40
Several recent series have reported encouraging results regarding intravitreal foscarnet therapy for ARN. Wong and colleagues41 recently provided evidence that intravitreal foscarnet in combination with systemic antiviral therapy may reduce the risk of RD in patients with ARN associated with VZV. Meghpara and coworkers42 found that intravitreal antiviral therapy for patients with ARN involving 25% to 50% of the retina stabilized or improved visual acuity (VA); however, no VA benefit was found in patients with severe disease involving greater than 50% of the retina. These data are tempered by another recent study by Tibbetts and coworkers43 comparing ARN outcomes in patients receiving intravenous acyclovir vs patients receiving newer antiviral agents, including intravitreal foscarnet in select cases. In this series, no difference was observed in VA and RD outcomes, although a minority of patients received intravitreal medications in their series. Thus, the question of whether intravitreal antiviral therapy is a primary or an adjunctive measure for the immediate treatment of ARN has not been adequately defined.44,45
THERAPEUTIC ADJUNCTS
A number of therapeutic adjuncts have been proposed in the literature and have included corticosteroids, anticoagulation, and laser barricade therapy. Aspirin and warfarin have been advocated to decrease the likelihood of occlusive events associated with vascular inflammation, although their benefit in preventing such events remains unproven.46 Early studies of platelet function in 7 patients with ARN showed hyperaggregation in 6 of 7 patients, and aspirin, 500 mg/day, was advocated. Laser barricade has also been described by some investigators to decrease the risk of RD47,48; however, other investigators have challenged the need for barricade laser with reports that RD risk does not appear to be decreased with laser photocoagulation.49 Early vitrectomy with acyclovir lavage has recently been proposed as a treatment option, although this treatment modality has not been widely studied.50,51
HYPOTHESIS
The present study examines the therapeutic efficacy of combination systemic and intravitreal antiviral foscarnet therapy as primary therapy for ARN compared to traditional systemic antiviral therapy alone. Combination antiviral therapy may lead to better visual outcomes and decrease the incidence of RD in affected eyes.
METHODS
Patients were identified via a database search of patients with ARN evaluated from 1987 to 2009 at the Casey Eye Institute, Oregon Health & Science University (OHSU) Retina and Uveitis Clinics in Portland, Oregon. The OHSU institutional review board approved this retrospective review of patients diagnosed with ARN (protocol identification number 4989).
MEDICAL RECORD REVIEW
Patients were diagnosed with ARN based on characteristic clinical features, response to antiviral therapy, and PCR diagnostics when available. Medical records of patients diagnosed with ARN were reviewed for ophthalmic evaluations (baseline and final follow-up) and included Snellen VA, slit-lamp examination, and dilated funduscopic examination. Demographic data (age, gender), laterality, associated systemic herpes virus diagnoses, and duration of symptoms prior to presentation were collected. Qualitative PCR studies were also reviewed for etiologic viral organisms. The occurrence of a RD during the follow-up period and the presence or absence of phthisis were also reviewed.
Systemic antiviral medications (intravenous or oral acyclovir, valacyclovir, or famciclovir) with or without intravitreal foscarnet injections (2.4 mg/0.1 mL) were recorded. Patients in the systemic antiviral therapy group received intravenous acyclovir, 10 mg/kg per dose 3 times daily for 2 weeks, followed by acyclovir, 800 mg 5 times daily, or valacyclovir, 1000 mg 3 times daily. Patients who were treated with combination therapy routinely received either intravenous acyclovir, 10 mg/kg per dose 3 times daily, or oral valacyclovir, 1000 mg 3 times daily, at the discretion of the treating physician, in addition to serial foscarnet injections (2.4 mg/0.1 mL) every 3 to 4 days until disease quiescence was achieved. Following consultation with an infectious disease specialist, 3 patients received valacyclovir, 2000 mg 3 times daily, as an induction regimen rather than intravenous acyclovir. Famciclovir,500 mg given 3 times daily, was an alternative therapy if renal dysfunction precluded the administration of acyclovir. The precise systemic medication regimens for each of the treatment groups are detailed below.
Patients were excluded from the medical record review if the ARN diagnosis was inconclusive or if fewer than 6 months of follow-up were available. If the patient was monocular on presentation to the Casey Eye Institute and had lost vision in the fellow eye for an unknown reason or due to ARN prior to our evaluation, the fellow eye was not included in the data analysis (ie, only the actively infected eye was included in the analysis even though there was a remote or possible history of ARN in the other eye).
ARN CLASSIFICATION
Patients with ARN were classified into presumptive, probable, and definite ARN categories based on the following criteria. Patients with presumptive ARN met ophthalmic diagnostic criteria defined by the AUS. Probable ARN included patients meeting the AUS ophthalmic criteria for ARN plus a history of systemic herpes infection, and definite ARN included those patients with AUS ophthalmic diagnostic criteria plus PCR confirmation of herpesvirus DNA from aqueous or vitreous fluid.
BASELINE DATA, VISUAL ACUITY, AND RETINAL DETACHMENT OUTCOMES ASSESSMENT
Descriptive and inferential statistical analyses were performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington), GraphPad Prism (GraphPad Software, La Jolla, California), and PASW software (IBM Corporation, Armonk, New York). For visual acuities poorer than 20/400, the following logMAR conversions were used: counting fingers=1.6, hand motions=2.0, light perception=2.5, and no light perception=3.0 (Yeh S, et al, IOVS 2009;50:ARVO E-Abstract 2406).36,43 An unpaired Student t test was used to determine if differences existed between the combination and systemic alone groups for patient age at presentation, duration of follow-up, the duration of symptoms prior to evaluation, and baseline VA. Paired Student t tests were used to assess for changes in logMAR VA at 6 months. An interval comparison of 6 months was used for logMAR VA because of variable follow-up. Two-year incidence rates of VA gain of 2 lines or greater, severe visual loss to 20/200 or poorer, and RD were reported by dividing the events by the total duration of risk in patient-years (PYs).37 For VA and RD outcomes in patients with bilateral ARN, the better-seeing eye at presentation prior to intervention was used for all analyses. Two-sample test of proportions was used to compare outcome variables between groups.
Kaplan-Meier survival analyses with log-rank tests were used to assess VA and RD outcomes stratified by treatment group. For VA analysis, a 2-line or greater VA improvement was considered a success. Progression to severe visual loss (ie, visual loss to 20/200 or poorer) was considered a failure. For eyes that never improved to better than 20/200, a failure time of 0.5 months was used to include these data in the survival analysis. For RD analysis, the end point was defined as the development of RD. Twenty-four months was chosen as the final common end point for all analyses, since the mean follow-up of the combination therapy group most closely approximated 24 months (ie, 26 months).
Cox proportional hazards regression analysis was used to assess factors potentially related to the major negative outcomes, including progression to RD and progression to severe VA impairment of 20/200 or poorer. Moreover, a positive outcome of a 2-line VA improvement was also assessed using a Cox regression model. Individual factors were assessed using univariate Cox regression analysis and were entered in the multivariate analysis in a forward stepwise manner if the P value was less than 0.2. Factors occurring infrequently (n <5) were excluded from the univariate analysis. Variables assessed included age at presentation, sex, antiviral delivery route (ie, parenteral, oral, intravitreal, or a combination), symptom duration, laser barricade, corticosteroid use (ie, periocular or oral), presence of HIV or immunosuppressed status, and initial logMAR VA. An alpha of 0.05 was considered statistically significant for all analyses.
RESULTS
We identified 29 eyes of 24 patients with ARN for analysis. Demographic information for patients treated is summarized in Table 1.
TABLE 1.
BASELINE CHARACTERISTICS, RESULTS OF POLYMERASE CHAIN REACTION TESTING, AND ASSOCIATED HERPES VIRUS DIAGNOSES OF 24 PATIENTS WITH ACUTE RETINAL NECROSIS
| VARIABLE | NUMBER |
|---|---|
| Total patients (eyes) | 24 (29) |
| Male | 10 (42%) |
| Female | 14 (58%) |
| Treatment group | |
| Combination (systemic + intravitreal) | 12 (14 eyes) |
| Systemic alone | 12 (15 eyes) |
| PCR testing* | |
| Total eyes tested | 14 |
| Total eyes positive | 11 (79%) |
| VZV | 2 (18%) |
| HSV-1 | 5 (45%) |
| HSV-2 | 3 (27%) |
| CMV | 1 (9%) |
| Associated herpes virus diagnoses or exposure† | |
| Total patients | 11‡ |
| Congenital HSV with seizures/developmental delay | 2 |
| Neonatal HSV infection | 2 |
| Herpes zoster ophthalmicus | 2 |
| HSV stromal keratitis | 2 |
| HSV meningitis or encephalitis | 2 |
| Genital herpes | 1 |
| Shingles | 1 |
| VZV stromal keratitisT | 1 |
| VZV attenuated vaccine | 1 |
CMV, cytomegalovirus; HSV, herpes simplex virus; PCR, polymerase chain reaction; VZV, varicella zoster virus.
PCR testing performed on 14 patients, 11 of whom were receiving combination systemic and intravitreal antiviral therapy. Aqueous fluid was obtained from 12 eyes and vitreous fluid from 2 eyes.
Although the 11 patients had a history of non-ARN herpesvirus diagnoses, 3 patients had more than 1 associated diagnosis (eg, HSV keratitis and congenital HSV).
Three patients presented with more than 1 systemic herpetic disease finding; 14 systemic findings are documented in 11 patients.
DEMOGRAPHIC INFORMATION, BASELINE CHARACTERISTICS, AND ARN CLASSIFICATION
Of 24 patients, 14 (58%) were women and 10 were men. Fourteen eyes of 12 patients were treated with combination systemic and intravitreal antiviral therapy, and 15 eyes of 12 patients were treated with systemic antiviral therapy alone. Mean patient age, plus or minus standard deviation (SD), was 47.8 ± 22.8 years in the combination therapy group and 37.3 ± 23.5 in group treated with systemic therapy alone (P=.29). The results of PCR diagnostic testing of ocular fluid are summarized in Table 1. Eleven patients had a documented history of associated systemic or non-ARN ocular herpes virus diagnoses (Table 1).
The mean initial logMAR VA ± SD in the combination therapy group was 1.01 ± 0.61 (Snellen VA∼20/200) and 0.68 ± 0.74 (Snellen VA∼20/100) in the systemic therapy group (P=.20). The mean follow-up time ± SD was 26.6 ± 16.6 months in the combination therapy group and 64.1 ± 54.9 months in the systemic therapy group (P=.02). The increased follow-up time in the systemic therapy group was largely of a historic nature, as intravitreal antiviral therapy was utilized at this care center from 2006 through 2009, during which time all patients received combination therapy. From 1987 through 2005, patients at the center received systemic antiviral therapy alone. To account for variable follow-up, outcome measures were calculated using Kaplan-Meier estimates of outcomes, and incidence rates were assessed.
PCR DIAGNOSTICS, ETIOLOGY DIAGNOSES, AND SPECIFIC ANTIVIRAL THERAPIES
PCR diagnostic testing of ocular fluid was conducted in 14 patients, 11 of whom were in the combination therapy group. Aqueous fluid was obtained from 12 patients and vitreous fluid from 2 patients. Of the 11 positive results of PCR diagnostic testing in patients receiving combination therapy, 2 patients tested positive for VZV, 5 tested positive for HSV-1, 3 tested positive for HSV-2, and 1 tested positive for CMV. Table 1 provides further detail regarding PCR diagnostic testing.
Based on the classification of presumptive, probable, and definite ARN, there were 12 cases of definite ARN, 7 cases of probable ARN, and 5 cases of presumptive ARN. The majority of cases of definite ARN, as documented by positive PCR testing of ocular fluid in the presence of clinical features of ARN, were found in the combination therapy group. Specifically, 11 of 12 patients in this group had PCR confirmation of herpesvirus DNA. In the systemic antiviral group, there was 1 positive PCR test and 2 negative tests. The difference in PCR testing was largely of historic nature, as PCR testing was not widely available during the time when patients with ARN received systemic antiviral therapy alone. Patients were deemed to have probable ARN if there were clinical features of ARN in addition to a suggestive clinical or medical history of ocular or systemic herpesvirus infection. Examples of these findings, outlined in Table 1, include congenital HSV with developmental delay, seizures, or both; herpes zoster ophthalmicus; HSV or VZV stromal keratitis; HSV meningitis or encephalitis; or history of VZV vaccination. These findings were identified in 11 patients, and there were some patients with clinical features, systemic herpesvirus infection, and PCR confirmation of ARN following ocular fluid sampling. Five patients, all in the group treated with systemic antiviral therapy alone, were diagnosed with presumptive ARN based on meeting AUS criteria for a clinical diagnosis of ARN.
Patients in the group receiving combination systemic and intravitreal antiviral therapy received intravenous acyclovir or oral antiviral medications with intravitreal foscarnet (Table 2). The median number of injections was 3 (range, 1–7). In the systemic antiviral therapy group, 8 of 12 patients (67%) received intravenous acyclovir induction dosing followed by oral acyclovir or valacyclovir. Four patients in this group received oral antiviral monotherapy (Table 2).
TABLE 2.
SYSTEMIC ANTIVIRAL THERAPIES WITH OR WITHOUT CORTICOSTEROID IN 24 PATIENTS WITH ACUTE RETINAL NECROSIS TREATED WITH COMBINATION SYSTEMIC AND INTRAVITREAL ANTIVIRAL THERAPY VS SYSTEMIC ANTIVIRAL THERAPY ALONE
| TREATMENT GROUP AND MEDICATION(S) ADMINISTERED | NUMBER OF PATIENTS (%) |
|---|---|
| Combination systemic and intravitreal (foscarnet) | |
| Total | 12 (100) |
| Intravenous acyclovir | 2 |
| Valacyclovir* | 7 |
| Oral acyclovir | 1 |
| Famciclovir | 1 |
| Valganciclovir | 1 |
| Prednisone | 8 |
| Systemic antiviral alone | |
| Total | 12 (100) |
| Intravenous acyclovir | 8 |
| Oral acyclovir | 3 |
| Famciclovir | 1 |
| Prednisone | 4 |
Five patients received 1 gm 3 times daily (TID), and 3 patients received 2 gm TID, per infectious disease specialist recommendation.
VISUAL ACUITY OUTCOMES
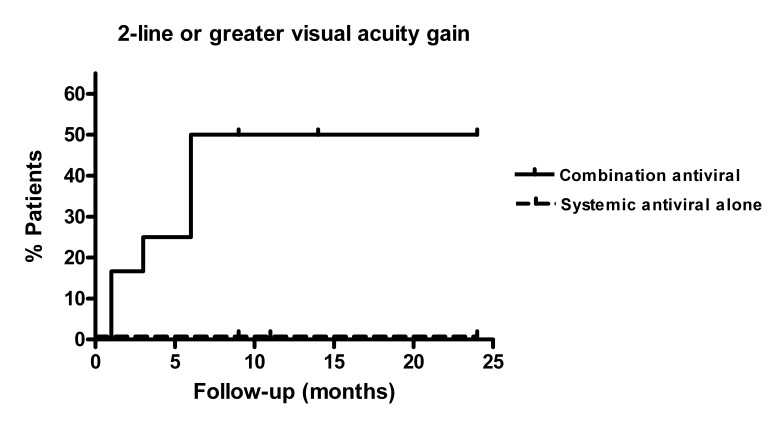
We used Kaplan-Meier analysis to analyze the time to improvement of patient VA by 2 or more lines during follow-up. Patients treated with combination systemic and intravitreal antiviral therapies were more likely to have 2 lines or more of VA improvement compared to patients treated with systemic antivirals alone (P=.0056, Figure 1). The incidence rate of a 2-line or greater visual improvement was 0.51 per PY in the combination therapy group compared to 0 per PY in the group treated with systemic therapy alone (P=.0004).
FIGURE 1.
Kaplan-Meier survival analysis of improvement in visual acuity by 2 or more Snellen chart lines in 24 patients treated with combination systemic and intravitreal antiviral therapy vs systemic antiviral therapy for acute retinal necrosis syndrome.
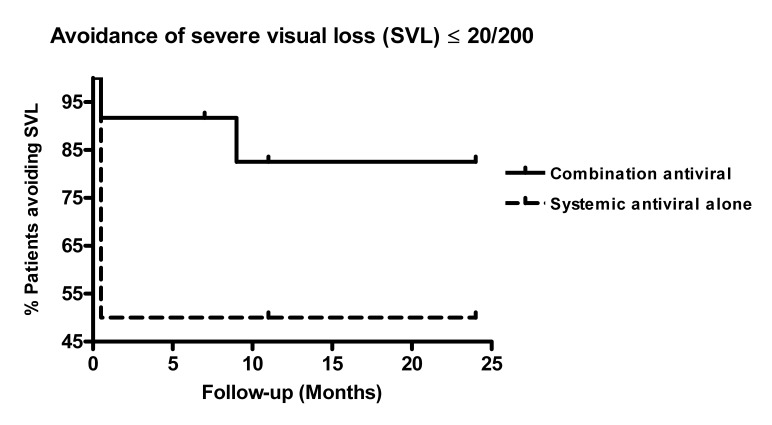
There was a trend toward avoidance of severe visual loss to 20/200 or poorer in patients treated with a combination of systemic and intravitreal antiviral therapy (P=.086, hazard ratio [HR] 0.31, 95% confidence interval [CI] 0.05–1.07, Figure 2). Severe visual loss occurred with an incidence rate of 0.13/PY in the combination therapy group and 0.54/PY in the systemic therapy alone group (P=.022).
FIGURE 2.
Kaplan-Meier survival analysis of avoidance of severe visual loss, defined as loss of vision to 20/200 or poorer, in 24 patients treated with combination systemic and intravitreal antiviral therapy vs systemic antiviral therapy for acute retinal necrosis syndrome.
A Cox proportional hazards regression model analysis was used to assess the factors contributing to a 2-line VA gain and severe visual loss ≤20/200 (Table 3). In the univariate Cox regression analysis, both combination systemic and intravitreal antiviral therapy (HR 4.43, P=.03, 95% CI 1.2–16.6) and poorer initial VA (HR 3.13, P=.009, 95% CI 1.3–7.4) were associated with increased likelihood of a 2-line or greater VA gain. The multivariate Cox regression analysis (P=.005) showed an approximately threefold likelihood of a 2-line VA gain with combination antiviral therapy (HR 3.34, P=.07, 95% CI 0.91–12.7) and also showed that patients with poorer initial logMAR VA were more likely to derive a 2-line or greater VA benefit (HR 2.85, P=.03, 95% CI 1.1–7.2). Age, female sex, symptom duration, parenteral antiviral therapy, and oral or periocular corticosteroid administration were not associated with increased likelihood of a 2-line VA gain. Laser barricade photocoagulation, periocular corticosteroid use, and immunosuppressed status (eg, HIV-positive status) could not be specifically assessed in the Cox regression analysis because the small numbers of patients in these groups (n ≤2) precluded statistically meaningful analysis.
TABLE 3.
UNIVARIATE AND MULTIVARIATE COX REGRESSION ANALYSIS OF FACTORS CONTRIBUTING TO VISUAL ACUITY GAIN OR SEVERE VISUAL ACUITY LOSS IN PATIENTS WITH ACUTE RETINAL NECROSIS
|
TWO-LINE OR GREATER VISUAL ACUITY GAIN: Univariate Analysis
| |||
| Factor | Hazard Ratio (95% CI) | P value | |
| Combination systemic plus intravitreal antiviral | 4.43 (1.2–16.6) | .03* | |
| Symptom duration | 0.99 (0.99–1.0) | .82 | |
| Male | 0.82 (0.26–2.6) | .75 | |
| Parenteral antiviral | 0.66 (0.20–2.2) | .50 | |
| Prednisone | 0.86 (0.27–2.71) | .80 | |
| Initial logMAR VA | 3.13 (1.3–7.4) | .009* | |
| Age | 0.98 (0.95–1.01) | .13 | |
| Multivariate Analysis | |||
| Initial logMAR VA | 2.85 (1.1–7.2) | .03* | Overall Model Fit |
| Combination systemic plus intravitreal antiviral | 3.34 (0.91–12.7) | .07 | .005* |
|
SEVERE VA LOSS (20/200 OR POORER):Univariate analysis
| |||
| Factor | Hazard Ratio (95% CI) | P value | |
| Combination systemic and intravitreal antiviral | 0.29 (0.06–1.38) | .12 | |
| Symptom duration | 0.97 (0.90–1.04) | .35 | |
| Male | 0.60 (0.15–2.39) | .47 | |
| Oral antiviral | 0.18 (0.038–0.88) | .035* | |
| No systemic prednisone | 1.39 (0.34–5.53) | .64 | |
| Initial logMAR VA | 2.36 (0.96–5.8) | .062 | |
| Age | 1.01 (0.98–1.04) | .65 | |
| Multivariate analysis | |||
| Init logMAR VA | 2.11 (0.84–5.31) | .11 | Overall Model Fit |
| Combination systemic and intravitreal antiviral | 0.25 (0.05–2.2) | .25 | .04* |
| Oral antiviral | 0.45 (0.06–3.3) | .44 | |
VA, visual acuity.
Statistically significant P <.05.
Severe VA loss to Snellen VA of 20/200 or poorer was also analyzed using a Cox regression model. Oral antiviral medication was associated with decreased risk of severe VA loss (HR 0.18; P=.04, 95% CI 0.038–0.88). In the univariate Cox regression analysis, poorer baseline logMAR VA trended toward a greater risk of severe VA loss (HR 2.36; P=.06, 95% CI 0.96–5.8) and combination systemic and intravitreal antiviral injection trended toward decreased risk of severe visual loss (HR 0.29, P=.12, 95% CI 0.06–1.38). In the multivariate Cox regression model, poorer baseline VA appeared to confer the greatest risk of severe VA loss (HR 2.11, P=0.11, 95% CI 0.841–5.31). Factors not associated with increased risk of severe VA loss included age, male sex, duration of symptoms, and oral prednisone use (P>.05 in both univariate and multivariate Cox regression analyses).
Because of variable follow-up, VA assessment was performed at an interval of 6 months. At 6 months, logMAR VA in patients treated with combination therapy had improved from 1.01 ± 0.61 (Snellen VA∼20/200) to 0.59 ± 0.51 (Snellen VA∼20/80, P=.048), whereas patients treated with systemic therapy alone worsened from 0.68 ± 0.74 (Snellen VA∼20/100) to 0.91 ± 1.08 (Snellen VA∼20/160, P=.12).
RETINAL DETACHMENT OUTCOMES
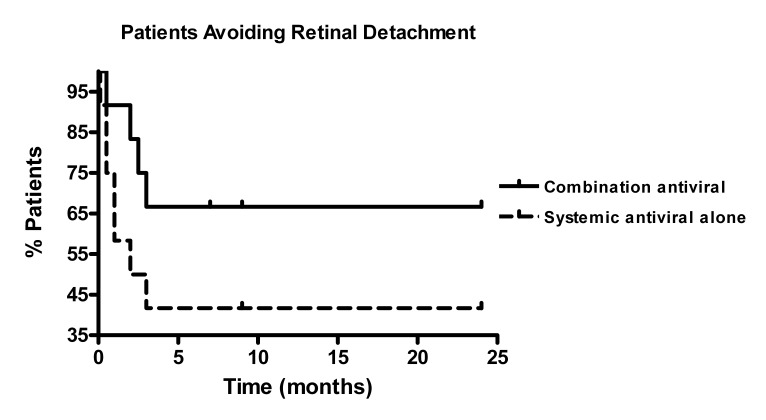
Of the 29 eyes from both cohorts, 13 eyes (45%) developed RD during follow-up. Four eyes detached in the combination group and 9 eyes detached in the systemic therapy group. Kaplan-Meier survival analysis revealed decreased hazard of RD in patients receiving combination therapy when compared to systemic antiviral therapy alone (Figure 3), although this was not significant from a statistical standpoint (P=.16, HR 0.44, 95% CI 0.12–1.43). However, the incidence of RD was significantly lower in the combination antiviral therapy group at 0.29 events per PY when compared to 0.74 events per PY in the systemic antiviral group (P=.03).
FIGURE 3.
Avoidance of retinal detachment in 24 patients treated with combination systemic and intravitreal antiviral therapy vs systemic antiviral therapy for acute retinal necrosis syndrome.
Involved eyes were also assessed for factors contributing to RD using a Cox regression model (Table 4). Patients who were not treated with prednisone showed a decreased risk of RD in the univariate Cox regression model (HR 0.22, P=.03, 95% CI 0.05–1.0). In the multivariate Cox regression model (P=.03), there was a trend toward decreased risk of RD in patients not receiving oral prednisone (HR 0.23, P=.06, 95% CI 0.05–1.0), and combination antiviral therapy was associated with decreased likelihood of RD (HR 0.43, P=.17, 95% CI 0.13–1.4), although this was not statistically significant. Risk factors that did not appear to influence RD risk included female sex, age, symptom duration, parenteral antiviral therapy, and initial logMAR VA (P>.05 for all factors).
TABLE 4.
UNIVARIATE AND MULTIVARIATE COX REGRESSION ANALYSIS OF FACTORS ASSOCIATED WITH RETINAL DETACHMENT IN 24 PATIENTS WITH ACUTE RETINAL NECROSIS
| COX REGRESSION ANALYSIS OF FACTORS ASSOCIATED WITH RETINAL DETACHMENT | |||
| Univariate analysis | |||
|
| |||
| Variable | Hazard Ratio (95% CI) | P value | |
| Combination systemic and intravitreal antiviral | 0.40 (0.12–1.30) | .13 | |
| Symptom duration | 0.97 (0.93–1.02) | .24 | |
| Male | 1.06 (0.36–3.2) | .91 | |
| Oral antiviral | 0.52 (0.18–1.5) | .24 | |
| No prednisone | 0.22 (0.05–1.0) | .03 | |
| Initial logMAR VA | 1.06 (0.47–2.4) | .90 | |
| Age | 0.99 (0.97–1.02) | .49 | |
| Multivariate analysis | |||
| No prednisone | 0.23 (0.05–1.0) | .06 | Overall Model Fit |
| Combination | 0.43 (0.13–1.4) | .17 | .03 |
Interestingly, all eyes that developed RD in this series were identified within the first 6 months of follow-up. By the 6-month time point, retinas in 4 of 14 eyes detached in the combination therapy group, and retinas in 9 of 15 eyes detached in the systemic therapy group. Of the 13 eyes with RDs, 12 underwent surgical repair. Seven eyes were macula-off preoperatively. Pars plana vitrectomy, endolaser (EL), and silicone oil (SO) tamponade were performed as the primary surgery in 6 patients, scleral buckle (SB) with cryotherapy in 1 patient, and combination PPV/SB/EL/SO in 5 eyes. Four retinas in 4 eyes remained attached after removal of SO. Three eyes developed recurrent detachments after their primary surgery, including the patient who underwent primary SB. The 2 patients who developed recurrent RD following PPV and removal of SO had detachments secondary to inferior proliferative vitreoretinopathy. These detachments were treated with subsequent PPV, SB, and membrane peel or retinectomy with SO reinstillation in 1 patient and C3F8 gas instillation in the other patient. Four eyes developed recurrent RD following SO removal and required SO reinstillation for long-term tamponade. One eye became blind and painful following PPV and SO and was eventually enucleated. The median number of surgical vitreoretinal procedures was 2 per eye (range, 1–3). All eyes were completely attached at final follow-up.
FELLOW EYE INVOLVEMENT AND PHTHISIS OUTCOMES
Two patients receiving combination therapy developed ARN in the initially unaffected eye at 16 and 27 months after diagnosis. In the systemic antiviral group, 3 of 6 patients with bilateral ARN presented with bilateral involvement, whereas the other 3 patients presented with unilateral eye involvement and had a remote history of ARN or visual loss of undetermined etiology in the opposite eye. No difference was observed in fellow eye involvement between the treatment groups (P=.22, 2-sample test of proportions).
Phthisis was observed in 3 of 29 eyes. All eyes observed to progress to phthisis were derived from the systemic therapy group (3 of 15 eyes), and no eyes in the combination therapy progressed to phthisis; however, no significant difference in phthisis was observed between groups (P=.08).
DISCUSSION
The traditional treatment regimen for ARN since the 1980s has been induction therapy with intravenous acyclovir followed by oral antiviral medications.27 In recent years, oral antiviral therapy with or without intravitreal foscarnet has emerged as a treatment option.31,42,43,49 In our 24-month analysis, patients treated with a combination of systemic and intravitreal antiviral therapy demonstrated an increased likelihood of 2-line or greater VA improvement compared to patients treated with conventional antiviral therapy alone.
Although the patients in the combination antiviral therapy group had poorer VA at presentation, they were also able to avoid severe visual loss of 20/200 or worse and demonstrated increased likelihood of a 2-line VA gain when compared to the patients in the group receiving systemic antiviral therapy alone. This group also demonstrated a decreased incidence of RD when compared to the systemic antiviral group. These results are consistent with previously reported short-term data showing improvement of VA in ARN patients following combination therapy as well as the trend toward decreased risk of RD (Yeh S, et al, IOVS 2009;50:ARVO E-Abstract 2406).
The results presented herein compare favorably to results reported by Wong and colleagues.41 They described the effect of intravitreal foscarnet and virus type (VZV vs HSV) on RD outcomes and found a non–statistically significant reduction in the rate of RD in VZV-ARN patients treated with foscarnet compared to eyes treated with systemic antiviral alone. Meghpara and coworkers42 reported a VA benefit of intravitreal antiviral in patients with ARN of moderate severity (ie, 25% to 50% of retina involved) with diminished benefit in severe ARN. These reports, combined with our series of patients, support further consideration of combination antiviral therapy for ARN.
In a recent study, Tibbetts and coworkers43 compared ARN outcomes in patients treated with intravenous acyclovir alone vs patients treated with newer antiviral agents, including 8 patients treated with intravitreal injections of ganciclovir or foscarnet, and found no difference in outcomes. They also found no variables predictive of retinal detachment, including intravitreal injections or intravenous treatment. However, a minority of the patients in their series received intravitreal injections, making a direct comparison between their series and our results difficult. In keeping with their recommendations on VA and RD outcomes reporting, we utilized survival analysis with development of RD and progression to severe visual loss as end points with a final common end point of 24 months given the discrepancy between follow-up times between the cohorts examined in this series. Furthermore, we also assessed a third treatment success end point of VA improvement by 2 lines or greater, which may be another useful outcome measure in future ARN studies. This benchmark has previously been discussed by Aizman and colleagues37 in their noncomparative, retrospective interventional series describing the treatment efficacy of oral antiviral monotherapy for ARN.
The incidence rate of RD in our series was significantly lower in patients receiving combination therapy compared to systemic antiviral therapy alone (ie, 0.29/PY vs 0.74/PY). A trend toward decreased RD over time was observed in our survival analysis, although we acknowledge that Kaplan-Meier survival analyses are most appropriate in prospective, randomized clinical trials, and our findings are thus tempered by the limited numbers of patients in this series. Overall VA improved following RD repair. However, there was variability in visual outcomes, most likely related to the variable extent and severity of disease presentation as well as differences in secondary visually significant sequelae such as cataract and epiretinal membrane formation. At final follow-up, successful retinal attachment was achieved in all patients; however, several patients required multiple surgeries. Overall, 42% of operated eyes developed significant visual impairment of 20/200 or poorer, whereas 58% developed moderate visual impairment ranging from 20/50 to 20/150 despite successful RD repair. Interestingly, there was a trend toward an association of oral prednisone with RD in the Cox regression model. This is most likely due to selection bias, in that patients with worse inflammation may be at increased likelihood of an RD in eyes with ARN and are more likely to receive corticosteroids due to the increased inflammation. The RD rate of 29% in patients treated with combination therapy and overall rate of 45% in this series is comparable to prior series with RD rates ranging from 17% to 85%.26,36,37,48,49,52,53
Limitations of this study include the retrospective design with variable follow-up, differences in systemic treatment, and the limited numbers of patients in both groups. The limited follow-up could decrease the proportion of patients who develop RD; however, 96% of RDs have been reported to occur within 5 months from symptom onset, and in our series, all of the RDs were recognized within 6 months.52 Another potential limitation in comparing the 2 treatment groups relates to the availability of diagnostic testing, including PCR, for patients diagnosed in the late 1980s and early 1990s. The ARN syndrome is more widely recognized today than in the late 1980s, when the precise etiologies were under investigation, and it is possible that delayed referral and disease severity on presentation, which was not specifically evaluated in this study, could have influenced the outcomes as well. We acknowledge that the lack of recognition of the ARN syndrome, limited availability of molecular diagnostics, and delays in referral are limitations of this study, as these factors could have led to more extensive disease and a more guarded visual prognosis. To account for the possibility that patients presented with more severe disease at presentation, other markers, including duration of symptoms prior to diagnosis and initial VA, were analyzed using the Cox regression model, but these are unlikely to account for all residual confounding variables that were not specifically assessed and which could have also influenced our findings.
One final consideration regarding the presenting VA is that the baseline VA appeared slightly worse in the combination therapy group, possibly biasing the likelihood of visual gain. However, there was no statistically significant difference between VA at baseline when comparing treatment groups. Moreover, patients treated with combination therapy also benefited from a decreased incidence rate of severe visual loss despite slightly reduced baseline VA, and experienced an increased incidence rate of 2 lines or greater visual gain when compared to systemic antiviral therapy alone.
Despite these caveats, our findings highlight the potential for significant VA gain in patients treated with combination antiviral therapy and avoidance of visual loss in ARN, a condition typically associated with significant morbidity and severe visual loss. These findings are encouraging and differ from a recent report that newer antiviral therapies and treatment algorithms do not appear to alter patient outcomes.43 In addition, the trend toward avoidance of RD suggests that patients treated aggressively with combination systemic and local therapy may benefit from decreased need for vitreoretinal surgery when compared to patients treated with systemic antiviral medication alone. Moreover, patients treated with oral and intravitreal antiviral did not fare worse, as detectable within the limits of this study, at long-term (24-month) follow-up when compared to patients treated with immediate hospitalization for parenteral antiviral therapy.
CONCLUSION
The treatment of patients with combination systemic and intravitreal antiviral is potentially promising for the acute management of ARN with a greater likelihood of VA gain over time and a trend toward decreased risk of RD when compared to patients receiving conventional systemic antiviral therapy alone. We hypothesize that high concentrations of locally administered antiviral medication effectively halt active viral replication, preventing their local destructive sequelae and improving outcome measures. In patients in whom this treatment algorithm is utilized, consideration may be given to the outpatient management of ARN. Nonetheless, judicious monitoring of ARN patients remains essential to evaluate patients for disease resolution and secondary complications including RD, which remains a major contributor to visual morbidity.
Acknowledgments
Funding/Support: This study was supported in part by unrestricted grants to Casey Eye Institute, Oregon Health & Science University (OHSU), and Emory Eye Center, Emory University School of Medicine from Research to Prevent Blindness, New York, New York.
Financial Disclosures: None of the authors have any commercial interests in any product discussed in this manuscript. Dr Flaxel has disclosed the following relationships: National Eye Institute, National Institutes of Health (research funding). Dr Yeh has disclosed the following financial relationships: Abbott Laboratories (research grant), Novartis (research grant), EyeGate Pharma (research grant), National Eye Institute, National Institutes of Health (research grant), and Knights Templar Foundation (research grant). Dr Lauer has disclosed the following relationships: National Eye Institute, National Institutes of Health (research grant), and Oxford Biomedica (UK) Ltd (research grant).
Author Contributions: Design of the study (C.J.F., S.Y., A.K.L.); conduct of the study (C.J.F., S.Y., A.K.L.); data collection (C.J.F., S.Y., A.K.L.); data management (C.J.F., S.Y.); data analysis (C.J.F., S.Y.); data interpretation (C.J.F., S.Y., A.K.L.); funding (C.J.F., S.Y., A.K.L.); manuscript preparation (C.J.F., S.Y.); critical revision and approval of final manuscript (C.J.F., S.Y., A.K.L.). Dr Yeh was a vitreoretinal fellow at the Casey Eye Institute, OHSU, during data collection, management, and analysis and presented a portion of this data at the 2009 Retina Congress in New York, New York. He is currently an attending physician at the Emory Eye Center.
Other Acknowledgments: We graciously thank Beau Bruce, MD, of Emory Eye Center, Emory University School of Medicine, Atlanta, Georgia, and Eric B. Suhler, MD, MPH, Casey Eye Institute, Oregon Health & Science University, and the Department of Ophthalmology, Portland Veterans Affairs Medical Center, Portland, Oregon, for statistical consultation. This research was presented in part as a paper at the 2009 Retina Congress in New York, New York.
REFERENCES
- 1.Young NJ, Bird AC. Bilateral acute retinal necrosis. Br J Ophthalmol. 1978;62(9):581–590. doi: 10.1136/bjo.62.9.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urayama A, Yamada N, Sasaki T. Unilateral acute uveitis with retinal periarteritis and detachment. Jpn J Clin Ophthalmol. 1971;25:607. [Google Scholar]
- 3.Brown RM, Mendis U. Retinal arteritis complicating herpes zoster ophthalmicus. Br J Ophthalmol. 1973;57(5):344–346. doi: 10.1136/bjo.57.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cibis GW. Neonatal herpes simplex retinitis. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;196(1):39–47. doi: 10.1007/BF00410025. [DOI] [PubMed] [Google Scholar]
- 5.Hayasaka S, Asano T, Yabata K, Ide A. Acute retinal necrosis. Br J Ophthalmol. 1983;67(7):455–460. doi: 10.1136/bjo.67.7.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sternberg P, Jr, Han DP, Yeo JH, et al. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology. 1988;95(10):1389–1393. doi: 10.1016/s0161-6420(88)32999-4. [DOI] [PubMed] [Google Scholar]
- 7.Topilow HW, Nussbaum JJ, Freeman HM, Dickersin GR, Szyfelbein W. Bilateral acute retinal necrosis. Clinical and ultrastructural study. Arch Ophthalmol. 1982;100(12):1901–1908. doi: 10.1001/archopht.1982.01030040881002. [DOI] [PubMed] [Google Scholar]
- 8.Saari KM, Böke W, Manthey KF, et al. Bilateral acute retinal necrosis. Am J Ophthalmol. 1982;93(4):403–411. doi: 10.1016/0002-9394(82)90128-3. [DOI] [PubMed] [Google Scholar]
- 9.Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35(5):327–343. doi: 10.1016/0039-6257(91)90183-g. [DOI] [PubMed] [Google Scholar]
- 10.Culbertson WW, Blumenkranz MS, Pepose JS, Stewart JA, Curtin VT. Varicella zoster virus is a cause of the acute retinal necrosis syndrome. Ophthalmology. 1986;93(5):559–569. doi: 10.1016/s0161-6420(86)33701-1. [DOI] [PubMed] [Google Scholar]
- 11.Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TP. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129(2):166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 12.Walters G, James TE. Viral causes of the acute retinal necrosis syndrome. Curr Opin Ophthalmol. 2001;12(3):191–195. doi: 10.1097/00055735-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Van Gelder RN, Willig JL, Holland GN, Kaplan HJ. Herpes simplex virus type 2 as a cause of acute retinal necrosis syndrome in young patients. Ophthalmology. 2001;108(5):869–876. doi: 10.1016/s0161-6420(01)00556-5. [DOI] [PubMed] [Google Scholar]
- 14.Tran TH, Rozenberg F, Cassoux N, Rao NA, LeHoang P, Bodaghi B. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol. 2003;87(1):79–83. doi: 10.1136/bjo.87.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knox CM, Chandler D, Short GA, Margolis TP. Polymerase chain reaction–based assays of vitreous samples for the diagnosis of viral retinitis. Use in diagnostic dilemmas. Ophthalmology. 1998;105(1):37–44. doi: 10.1016/s0161-6420(98)71127-2. [DOI] [PubMed] [Google Scholar]
- 16.Culbertson WW, Blumenkranz MS, Haines H, Gass DM, Mitchell KB, Norton EW. The acute retinal necrosis syndrome. Part 2: Histopathology and etiology. Ophthalmology. 1982;89(12):1317–1325. doi: 10.1016/s0161-6420(82)34638-2. [DOI] [PubMed] [Google Scholar]
- 17.Soushi S, Ozawa H, Matsuhashi M, Shimazaki J, Saga U, Kurata T. Demonstration of varicella-zoster virus antigens in the vitreous aspirates of patients with acute retinal necrosis syndrome. Ophthalmology. 1988;95(10):1394–1398. doi: 10.1016/s0161-6420(88)33012-5. [DOI] [PubMed] [Google Scholar]
- 18.Itoh N, Matsumura N, Ogi A, et al. High prevalence of herpes simplex virus type 2 in acute retinal necrosis syndrome associated with herpes simplex virus in Japan. Am J Ophthalmol. 2000;129(3):404–405. doi: 10.1016/s0002-9394(99)00391-8. [DOI] [PubMed] [Google Scholar]
- 19.de Boer JH, Luyendijk L, Rothova A, et al. Detection of intraocular antibody production to herpesviruses in acute retinal necrosis syndrome. Am J Ophthalmol. 1994;117(2):201–210. doi: 10.1016/s0002-9394(14)73077-6. [DOI] [PubMed] [Google Scholar]
- 20.Abe T, Tsuchida K, Tamai M. A comparative study of the polymerase chain reaction and local antibody production in acute retinal necrosis syndrome and cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1996;234(7):419–424. doi: 10.1007/BF02539407. [DOI] [PubMed] [Google Scholar]
- 21.Van Gelder RN. CME review: polymerase chain reaction diagnostics for posterior segment disease. Retina. 2003;23(4):445–452. doi: 10.1097/00006982-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Harper TW, Miller D, Schiffman JC, Davis JL. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009;147(1):140–147.e2. doi: 10.1016/j.ajo.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117(5):663–667. doi: 10.1016/s0002-9394(14)70075-3. [DOI] [PubMed] [Google Scholar]
- 24.Wensing B, de Groot-Mijnes JD, Rothova A. Necrotizing and nonnecrotizing variants of herpetic uveitis with posterior segment involvement. Arch Ophthalmol. 2011;129(4):403–408. doi: 10.1001/archophthalmol.2010.313. [DOI] [PubMed] [Google Scholar]
- 25.Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies. A spectrum of herpes virus–induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5(4):259–265. doi: 10.3109/09273949709085066. [DOI] [PubMed] [Google Scholar]
- 26.Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93(3):296–300. doi: 10.1016/s0161-6420(86)33740-0. [DOI] [PubMed] [Google Scholar]
- 27.Palay DA, Sternberg P, Jr, Davis J, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112(3):250–255. doi: 10.1016/s0002-9394(14)76725-x. [DOI] [PubMed] [Google Scholar]
- 28.Crapotta JA, Freeman WR, Feldman RM, et al. Visual outcome in acute retinal necrosis. Retina. 1993;13(3):208–213. doi: 10.1097/00006982-199313030-00004. [DOI] [PubMed] [Google Scholar]
- 29.Hirst LW, Beyer TL, Waters D, Fleischman J. Successful management of acute retinal necrosis with intravenous acyclovir. Ann Ophthalmol. 1987;19(12):445–448. [PubMed] [Google Scholar]
- 30.Steingrimsdottir H, Gruber A, Palm C, Grimfors G, Kalin M, Eksborg S. Bioavailability of aciclovir after oral administration of aciclovir and its prodrug valaciclovir to patients with leukopenia after chemotherapy. Antimicrob Agents Chemother. 2000;44(1):207–209. doi: 10.1128/aac.44.1.207-209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huynh TH, Johnson MW, Comer GM, Fish DN. Vitreous penetration of orally administered valacyclovir. Am J Ophthalmol. 2008;145(4):682–686. doi: 10.1016/j.ajo.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Chong DY, Johnson MW, Huynh TH, Hall EF, Comer GM, Fish DN. Vitreous penetration of orally administered famciclovir. Am J Ophthalmol. 2009;148(1):38–42.e1. doi: 10.1016/j.ajo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Savant V, Saeed T, Denniston A, Murray PI. Oral valganciclovir treatment of varicella zoster virus acute retinal necrosis. Eye. 2004;18(5):544–555. doi: 10.1038/sj.eye.6700703. [DOI] [PubMed] [Google Scholar]
- 34.Guex-Crosier Y, Meylan PR. High dosage of oral valaciclovir as an alternative treatment of varicella zoster acute retinal necrosis syndrome. Eye. 2006;20(2):247. doi: 10.1038/sj.eye.6701821. [DOI] [PubMed] [Google Scholar]
- 35.Aslanides IM, De Souza S, Wong DT, et al. Oral valacyclovir in the treatment of acute retinal necrosis syndrome. Retina. 2002;22(3):352–354. doi: 10.1097/00006982-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Emerson GG, Smith JR, Wilson DJ, et al. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology. 2006;113(12):2259–2261. doi: 10.1016/j.ophtha.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 37.Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007;114(2):307–312. doi: 10.1016/j.ophtha.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 38.Peyman GA, Goldberg MF, Uninsky E, Tessler H, Pulido J, Hendricks R. Vitrectomy and intravitreal antiviral drug therapy in acute retinal necrosis syndrome. Report of two cases. Arch Ophthalmol. 1984;102(11):1618–1621. doi: 10.1001/archopht.1984.01040031308013. [DOI] [PubMed] [Google Scholar]
- 39.She SC, Peyman GA, Schulman JA. Toxicity of intravitreal injection of foscarnet in the rabbit eye. Int Ophthalmol. 1988;12(2):151–154. doi: 10.1007/BF00137142. [DOI] [PubMed] [Google Scholar]
- 40.Berthe P, Baudouin C, Garraffo R, Hofmann P, Taburet AM, Lapalus P. Toxicologic and pharmacokinetic analysis of intravitreal injections of foscarnet, either alone or in combination with ganciclovir. Invest Ophthalmol Vis Sci. 1994;35(3):1038–1045. [PubMed] [Google Scholar]
- 41.Wong R, Pavesio CE, Laidlaw DA, Williamson TH, Graham EM, Stanford MR. Acute retinal necrosis: the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology. 2010;117(3):556–560. doi: 10.1016/j.ophtha.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Meghpara B, Sulkowski G, Kesen MR, Tessler HH, Goldstein DA. Long-term follow-up of acute retinal necrosis. Retina. 2010;30(5):795–800. doi: 10.1097/IAE.0b013e3181c7013c. [DOI] [PubMed] [Google Scholar]
- 43.Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824. doi: 10.1016/j.ophtha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Luu KK, Scott IU, Chaudhry NA, Verm A, Davis JL. Intravitreal antiviral injections as adjunctive therapy in the management of immunocompetent patients with necrotizing herpetic retinopathy. Am J Ophthalmol. 2000;129(6):811–813. doi: 10.1016/s0002-9394(00)00462-1. [DOI] [PubMed] [Google Scholar]
- 45.Chau Tran TH, Cassoux N, Bodaghi B, Lehoang P. Successful treatment with combination of systemic antiviral drugs and intravitreal ganciclovir injections in the management of severe necrotizing herpetic retinitis. Ocul Immunol Inflamm. 2003;11(2):141–144. doi: 10.1076/ocii.11.2.141.15915. [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi T, Spencer DB, Mochizuki M. Therapy for acute retinal necrosis. Semin Ophthalmol. 2008;23(4):285–290. doi: 10.1080/08820530802111192. [DOI] [PubMed] [Google Scholar]
- 47.Han DP, Lewis H, Williams GA, Mieler WF, Abrams GW, Aaberg TM. Laser photocoagulation in the acute retinal necrosis syndrome. Arch Ophthalmol. 1987;105(8):1051–1054. doi: 10.1001/archopht.1987.01060080053027. [DOI] [PubMed] [Google Scholar]
- 48.Sternberg P, Jr, Han DP, Yeo JH, et al. Photocoagulation to prevent retinal detachment in acute retinal necrosis. Ophthalmology. 1988;95(10):1389–1393. doi: 10.1016/s0161-6420(88)32999-4. [DOI] [PubMed] [Google Scholar]
- 49.Sims JL, Yeoh J, Stawell RJ. Acute retinal necrosis: a case series with clinical features and treatment outcomes. Clin Experiment Ophthalmol. 2009;37(5):473–477. doi: 10.1111/j.1442-9071.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 50.Carney MD, Peyman GA, Goldberg MF, Packo K, Pulido J, Nicholson D. Acute retinal necrosis. Retina. 1986;6(2):85–94. doi: 10.1097/00006982-198600620-00004. [DOI] [PubMed] [Google Scholar]
- 51.Hillenkamp J, Nolle B, Bruns C, Rautenberg P, Fickenscher H, Roider J. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology. 2009;116(10):1971–1975.e2. doi: 10.1016/j.ophtha.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 52.Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114(4):756–762. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 53.Sternberg P, Jr, Knox DL, Finkelstein D, Green WR, Murphy RP, Patz A. Acute retinal necrosis syndrome. Retina. 1982;2(3):145–151. doi: 10.1097/00006982-198200230-00003. [DOI] [PubMed] [Google Scholar]