Abstract
Background.
Anti-angiogenic treatment with targeted agents is effective in advanced hepatocellular carcinoma (HCC). This trial evaluated the safety and efficacy of metronomic capecitabine in patients with HCC.
Methods.
This single-institution phase II trial included 59 previously untreated patients with advanced HCC and 31 patients resistant to or intolerant of sorafenib. The treatment schedule was capecitabine 500 mg twice daily until progression of disease, unacceptable toxicity level, or withdrawal of informed consent. Progression-free survival (PFS) was chosen as the primary endpoint.
Results.
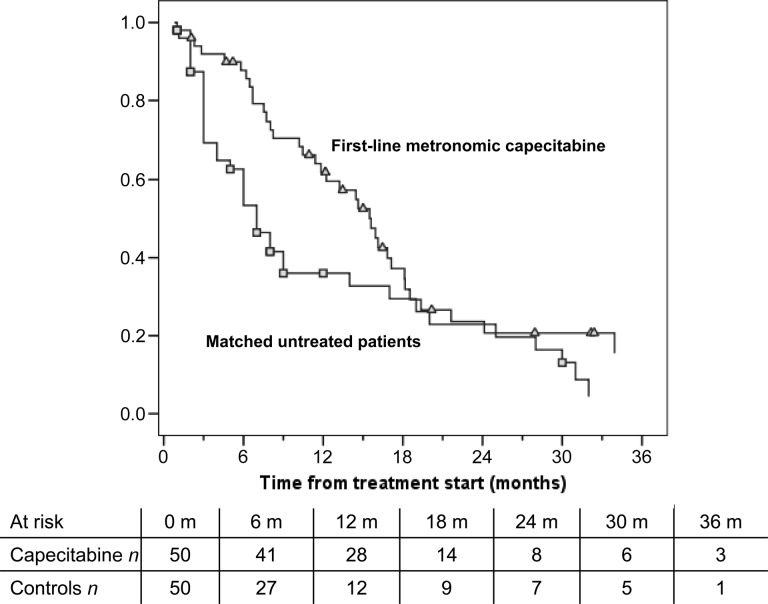
A total of 59 previously untreated and 31 previously treated patients with HCC were enrolled. The first cohort achieved a median PFS of 6.03 months and an overall survival (OS) of 14.47 months. Two patients achieved a complete response, 1 patient achieved partial response, and in 30 patients, stable disease was the best outcome. The second cohort achieved a median PFS of 3.27 months and a median OS of 9.77 months. No complete or partial responses were observed, but 10 patients had stable disease. An unscheduled comparison of the first cohort of patients with 3,027 untreated patients with HCC from the Italian Liver Cancer (ITA.LI.CA) database was performed. One-to-one matching according to demographic/etiologic/oncologic features was possible for 50 patients. The median OS for these 50 capecitabine-treated patients was 15.6 months, compared with a median OS of 8.0 months for the matched untreated patients (p = .043).
Conclusion.
Metronomic capecitabine is well tolerated by patients with advanced HCC and appears to have activity both in treatment-naive patients and in those previously treated with sorafenib.
Author Summary
Discussion
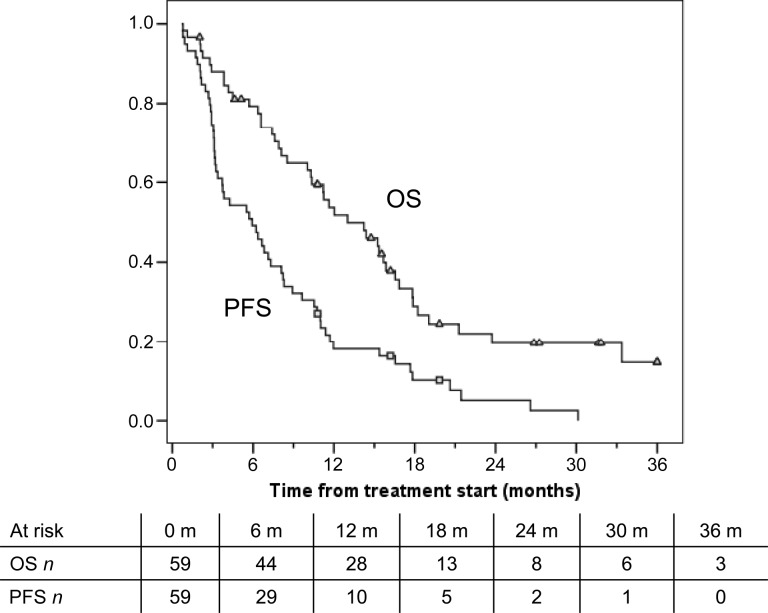
In patients treated with first-line metronomic capecitabine, the median progression-free survival, which was our primary outcome measure, was >6 months, much longer than the 2.5 months reported for historical control patients. A median overall survival OS of 14.5 months (Fig. 1) is also encouraging, and interestingly, was not different for the 26 patients classified Barcelona-Clinic Liver Cancer (BCLC)-B and 33 patients classified BCLC-C. Median survival time of previously untreated patients was nearly double that of patients undergoing palliation, who were individually matched from the ITA.LI.CA database (15.6 vs. 8.0 months, respectively) (Fig. 2), providing further support for the usefulness of capecitabine. This comparison has several biases, and therefore the results should be considered with caution. Caution should also be applied when comparing our survival data with the lower survival rates reported by the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) and the Asia-Pacific trials because of the different characteristics of the study populations. Results of the SHARP and Asia-Pacific trials were not available when our trial was designed. Those trials demonstrated a survival advantage for sorafenib, now considered standard treatment for advanced hepatocellular carcinoma HCC. However, the absolute advantage is still unsatisfactory (2.8 months among Western patients and 2.3 months among Asian patients) and is achieved at the expense of frequent toxicity. In fact, as many as 38% of patients discontinue treatment because of adverse events, and approximately 26% of patients require a dose reduction. In addition to the better disease control and survival rates, metronomic capecitabine also has a rather low toxicity profile. In fact, no treatment-related deaths were observed, and no patient withdrew from treatment because of adverse events. Most adverse effects were mild or moderate and were manageable with supportive care or a brief drug-free period. Although the toxicity rate among the first- and second-line cohorts seems similar, the efficacy was remarkably lower in the second-line setting. However, a median OS of approximately 10 months obtained in the second-line setting is an interesting result. In conclusion, metronomic capecitabine is safe and is associated with promising results in both treatment-naive and pretreated patients with advanced HCC who have preserved liver function.
Figure 1.
Kaplan-Meier plot of PFS and OS for the first-line treatment cohort. The squares and triangles signify the censored patients in a group (squares) and in the other group (triangles) on the different curves.
Abbreviations: n, number of patients; OS, overall survival; PFS, progression-free survival.
Figure 2.
Kaplan-Meier plot of overall survival for 50 patients in the first-line treatment cohort and individually matched untreated patients from the Italian Liver Cancer (ITA.LI.CA) database. The squares and triangles signify the censored patients in a group (squares) and in the other group (triangles) on the different curves.
Abbreviation: n, number of patients.
Supplementary Material
Footnotes
Access the full results at: Brandi-13-93.theoncologist.com
Agenzia Italiana del Farmaco Identifier: EudraCT 2008-002299-92 Sponsor(s): Azienda Ospedaliera di Bologna Policlinico Sant'Orsola Malpighi (54/2008/U/Sper)
Principal Investigator: Giovanni Brandi IRB Approved: Yes
Disclosures
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.