Abstract
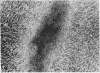
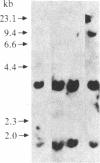
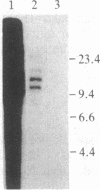
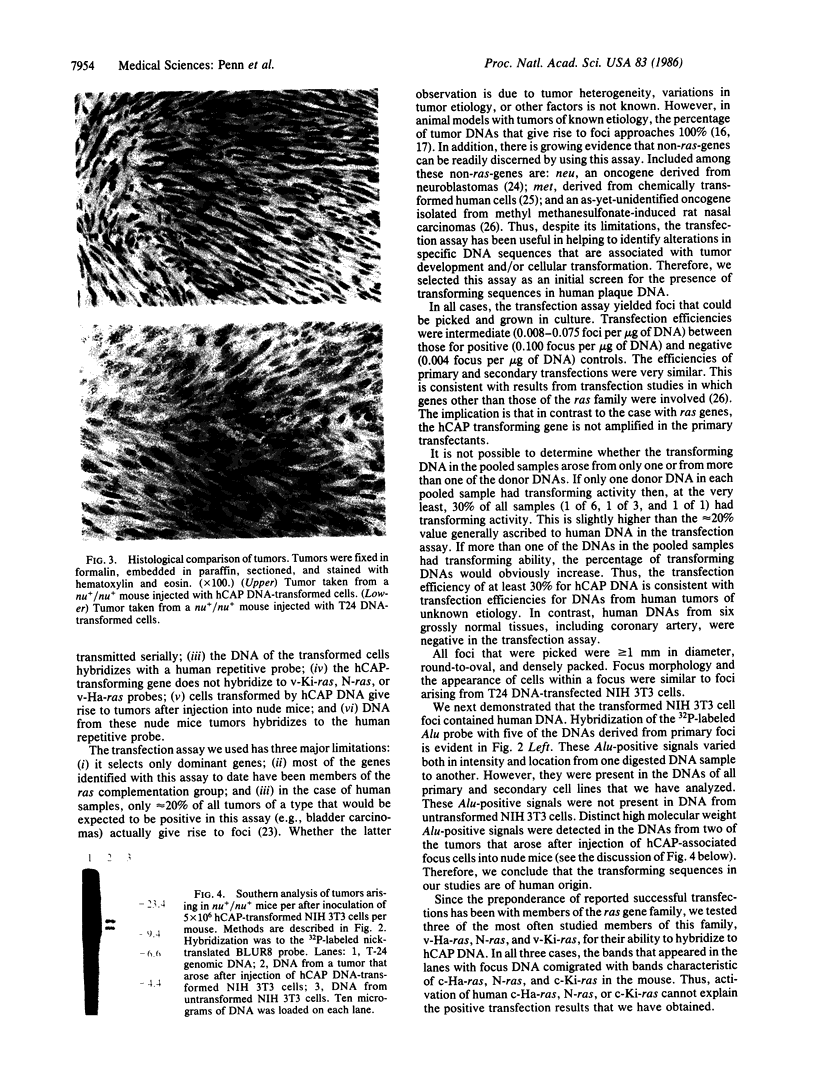
The monoclonal hypothesis equates atherosclerotic plaques with benign smooth muscle cell tumors and proposes that plaques can arise via mutational or viral events. Here, we provide direct evidence that molecular events, heretofore associated only with tumor cells, are common to plaque cells as well. Three distinct groups of human coronary artery plaque (hCAP) DNA samples transfected into NIH 3T3 cells gave rise to transformed foci. DNA samples from a panel of normal noncancerous human tissues, including coronary artery, were negative in the assay. Southern-blotted focus DNA yielded positive signals when hybridized to the 32P-labeled nick-translated repetitive human "Alu" DNA sequence. The DNA from cloned foci was used successfully in a second round of transfection. Focus DNA hybridized to nick-translated v-Ki-ras, v-Ha-ras, or N-ras probes failed to detect human fragments of these genes. Primary focus cells from each of five clones elicited tumors after injection into nude mice (6/42). Several distinct high molecular weight (greater than 6.6 kilobases) bands were detected after BamHI-digested tumor DNA was hybridized to Alu. Preliminary characterization of these hCAP DNA-associated tumors indicates that they are similar to the fibrosarcomas that arise after injection of ras-transformed cells into nude mice. We propose that transforming genes in plaque cells behave in a manner analogous to the way in which oncogenes behave in cancer cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Vanderlaan M., Burns F. J., Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977 Jul;37(7 Pt 1):2232–2235. [PubMed] [Google Scholar]
- BERENBLUM I., SHUBIK P. The persistence of latent tumour cells induced in the mouse's skin by a single application of 9:10-dimethyl-1:2-benzanthracene. Br J Cancer. 1949 Sep;3(3):384–386. doi: 10.1038/bjc.1949.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict W. F., Considine N., Nebert D. W. Genetic differences in aryl hydrocarbon hydroxylase induction and benzo(a)pyrene-produced tumorigenesis in the mouse. Mol Pharmacol. 1973 Mar;9(2):266–277. [PubMed] [Google Scholar]
- Bond J. A., Kocan R. M., Benditt E. P., Juchau M. R. Metabolism of benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in cultured human fetal aortic smooth muscle cells. Life Sci. 1979 Jul 30;25(5):425–430. doi: 10.1016/0024-3205(79)90574-5. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cooper C. S., Park M., Blair D. G., Tainsky M. A., Huebner K., Croce C. M., Vande Woude G. F. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984 Sep 6;311(5981):29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Parada L. F., Weinberg R. A. Specific growth response of ras-transformed embryo fibroblasts to tumour promoters. Nature. 1985 Dec 5;318(6045):472–475. doi: 10.1038/318472a0. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garte S. J., Hood A. T., Hochwalt A. E., D'Eustachio P., Snyder C. A., Segal A., Albert R. E. Carcinogen specificity in the activation of transforming genes by direct-acting alkylating agents. Carcinogenesis. 1985 Dec;6(12):1709–1712. doi: 10.1093/carcin/6.12.1709. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Yim S. O., Stehlin J. S., Williams L. J., Jr Development of invasive tumors in the "nude" mouse after injection of cultured human melanoma cells. J Natl Cancer Inst. 1972 May;48(5):1531–1533. [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Benditt E. P., Juchau M. R. Focal smooth muscle proliferation in the aortic intima produced by an initiation-promotion sequence. Proc Natl Acad Sci U S A. 1985 May;82(10):3450–3454. doi: 10.1073/pnas.82.10.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Yang H. Y., Benditt E. P., Juchau M. R. Carcinogenesis and atherogenesis: differences in monooxygenase inducibility and bioactivation of benzo[a]pyrene in aortic and hepatic tissues of atherosclerosis-susceptible versus resistant pigeons. Carcinogenesis. 1983;4(6):647–652. doi: 10.1093/carcin/4.6.647. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Penn A., Batastini G., Soloman J., Burns F., Albert R. Dose-dependent size increases of aortic lesions following chronic exposure to 7,12-dimethylbenz(a)anthracene. Cancer Res. 1981 Feb;41(2):588–592. [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Lauver A. V., Long L. K., Aaronson S. A., Barbacid M. Oncogenes in solid human tumours. Nature. 1982 Dec 9;300(5892):539–542. doi: 10.1038/300539a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shih C., Padhy L. C., Murray M., Weinberg R. A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981 Mar 19;290(5803):261–264. doi: 10.1038/290261a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. J., Bradley S. M., Bargmann C. I., Weinberg R. A., Papageorge A. G., Scolnick E. M., Dhar R., Lowy D. R., Chang E. H. Mechanism of activation of a human oncogene. Nature. 1982 Nov 11;300(5888):143–149. doi: 10.1038/300143a0. [DOI] [PubMed] [Google Scholar]
- Taparowsky E., Suard Y., Fasano O., Shimizu K., Goldfarb M., Wigler M. Activation of the T24 bladder carcinoma transforming gene is linked to a single amino acid change. Nature. 1982 Dec 23;300(5894):762–765. doi: 10.1038/300762a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]