The authors report an experience using biologic agents as second-line treatment for advanced thyroid cancer and show that patients derived a modest additional benefit in comparison to the front-line treatment. The findings are relevant for the clinical management of patients and for future studies of second-line targeted therapy of thyroid cancer.
Keywords: Thyroid cancer, Biologic agents, Second-line
Learning Objectives
List the therapeutic opportunities with tyrosine kinase inhibitors for advanced thyroid cancer, especially in the frontline setting.
Describe and discuss the current knowledge and experience with salvage therapy with biologic agents for thyroid cancer.
Abstract
Introduction.
Targeted biologic agents showed clinically meaningful efficacy as front-line therapy for advanced radioiodine-refractory and medullary thyroid cancer. The clinical benefit of these agents beyond the front line has yet to be established.
Methods.
We assessed the clinical benefit of targeted agents in patients with advanced differentiated and medullary thyroid cancer treated at a single academic cancer center. We determined efficacy and compared front-line and second-line benefit using biochemical and anatomic response, time to treatment failure, and progression-free survival (PFS). Statistical differences were assessed by t test and chi-square test. Survival curves were generated by the Kaplan-Meier method. Differences in survival were assessed using the log-rank test, and a p value <.05 was considered significant.
Results.
We identified 39 patients with advanced differentiated and medullary thyroid cancer treated with targeted biologic agents. Median age was 56.3 years. Overall, 25 men and 14 women participated. Histology showed 23% medullary and 77% differentiated cancer. Nineteen patients progressed on front-line therapy and subsequently received second-line therapy. Targeted agents conferred clinically meaningful benefit in the second-line setting in terms of biochemical response (13.3%), clinical benefit (83.3%), median time to treatment failure (4.0 months; 95% confidence interval: 2.6–8.2), and median PFS (4.6 months; 95% confidence interval: 3.2–8.2). Second-line benefit (median PFS) was more modest in comparison to the front-line setting in both genders (women: 3 months vs. 12.2 months; men: 6 months vs. 19.7 months), in differentiated cancers (4.1 months vs. 15.7 months), and with vascular targeting agents (4.4 months vs. 20.1 months).
Conclusion.
Patients with advanced thyroid cancer derived meaningful clinical benefit from additional therapy with a biologic agent following disease progression on front-line targeted therapy.
Implications for Practice:
Significant benefit can be achieved in patients with iodine-refractory thyroid cancer treated with targeted agents in the first-line setting. It is currently unknown whether additional benefit would be obtained with the use of different biologic agents to treat patients after failing first-line therapy. We report our experience using biologic agents as second-line treatment for advanced thyroid cancer and show that patients derived additional benefit, albeit modest, in comparison to the front-line treatment. Our findings are relevant for the clinical management of patients and for future studies of second-line targeted therapy of thyroid cancer.
Introduction
Thyroid cancer is the most common endocrine malignancy in the United States, and its incidence continues to rise worldwide [1, 2]. The majority of cases of thyroid cancer are either follicular cell derived (papillary/follicular) or medullary. Despite the excellent initial outcome of surgery for the vast majority of patients, disease recurrence is frequently observed in a large proportion of patients [3, 4]. Therapeutic options are currently limited for patients with advanced differentiated thyroid cancer or medullary thyroid cancer that is not amenable to radioiodine therapy, surgical resection, or local therapy with external-beam radiation.
A better understanding of the role of altered signaling pathways in cancer development and progression has led to the evaluation and subsequent approval of biologically targeted therapies in various cancers such as breast cancer, renal cell cancer, lung cancer, and melanoma [5–8]. Multiple targeted kinase inhibitors and antiangiogenic agents including vandetanib, sorafenib, sunitinib, motesanib, cabozantinib, levantinib, and pazopanib have also been evaluated in small prospective phase II studies as first-line therapy for patients with metastatic thyroid cancer, with promising results [9–15]. These initial results have led to confirmatory phase III trials of these agents as well as widespread off-label use outside the clinical trial setting [16, 17]. Despite these promising results, the majority of patients progress within 12 months of therapy.
Based on the experiences with other cancer types where targeted agents have become standard treatment options, thyroid cancer patients who progress on front-line kinase inhibitor therapy are also likely to be treated with other biologic agents following progression. However, published reports of clinical studies or robust experiences from routine clinical care supporting such an approach in thyroid cancer patients are currently lacking. Consequently, we conducted a systematic analysis of our institutional experience with the second-line use of biologic-agent therapy for advanced thyroid cancer in order to provide a useful benchmark to guide future prospective trials in this setting.
Materials and Methods
Enrollment
This retrospective study was approved by the Emory University institutional review board to review clinical records from patients with advanced thyroid cancer (medullary and differentiated tumors) treated at the Winship Cancer Institute of Emory University between 2006 and 2013. The primary study population consisted of advanced thyroid cancer patients treated with at least one line of targeted biologic agent during the period under review. Relevant clinical data including age, gender, ethnicity, date of diagnosis, type and duration of treatment, and treatment outcome were obtained from the electronic medical records.
Treatment Efficacy
Benefit from therapy was assessed in terms of anatomic response, biochemical response, and duration of benefit. A 50% or greater reduction in the level of standard tumor markers (thyroglobulin for differentiated cancers and calcitonin for medullary thyroid) compared with baseline measurement at the time of treatment initiation was considered as biochemical response. Best objective tumor response while on targeted agent therapy was determined by cross-sectional imaging obtained as part of the standard of care for the patients. Response assessment was categorized according to Response Evaluation Criteria In Solid Tumors version 1.1 in patients with appropriate anatomic lesions as complete response, partial response, stable disease, and progressive disease, as well as clinical benefit (complete response plus partial response plus stable disease).
Survival
The primary outcomes of interest included time to treatment failure (TTF), measured as the time interval from initiating a specific therapy until date of discontinuation for any reason, and progression-free survival (PFS), defined as the time interval from initiating treatment until objective disease progression or death. Differences in treatment efficacy were also assessed based on the known mechanism of action of the biologic agent, either as agents targeting tumor vasculature (antiangiogenic) or not targeting tumor vasculature (nonantiangiogenic). Similarly, the efficacy of single-agent biologic therapy was compared with that of combination therapy in both the front-line and second-line settings.
Statistical Analysis
The patients' categorical characteristics were summarized as frequency and proportions. Age was presented as mean and standard deviation. Chi-square test or Fisher's exact test was used to compare the categorical variables between patients in different treatment categories. McNemar's test was used to compare the response outcome between front-line and second-line treatment. The Kaplan-Meier method was used to generate survival curves and to estimate the survival rate. The log-rank test was used to compare survival between defined groups of interest.
The degree of benefit in the second-line setting was determined by comparing the primary survival outcomes of TTF and PFS associated with second-line therapy with the survival outcome with front-line therapy in the same group of patients with thyroid cancer. The impact of salient clinical factors, including gender, tumor histology (medullary vs. nonmedullary thyroid cancer), mechanism of drug action (antiangiogenesis vs. nonantiangiogenesis), and regimen (single agent vs. multiagent), was assessed by comparing front-line and second-line therapy using these factors as stratification factors. Separate subset analyses were conducted within the front-line and second-line patients to assess whether these factors (gender, histology, drug mechanism, and regimen) affect the efficacy of targeted agents. The significance p values were set at .05 for all comparisons without adjusting for multiple comparisons, given the exploratory nature of the subset analysis. The SAS statistical package version 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com) was used for data management and analyses.
Results
Demographics
We reviewed the clinical records of 62 patients and identified 39 eligible patients with stage IV thyroid cancer who received targeted biologic agents for progressive disease. There were 9 patients with medullary thyroid cancer and 30 patients with differentiated thyroid cancer in the treatment group, 25 males and 14 females, with a median age at diagnosis of 56.3 years (Table 1). Nineteen patients received second-line therapy after progressing on the initial biologic agent therapy.
Table 1.
Demographics and clinical characteristics
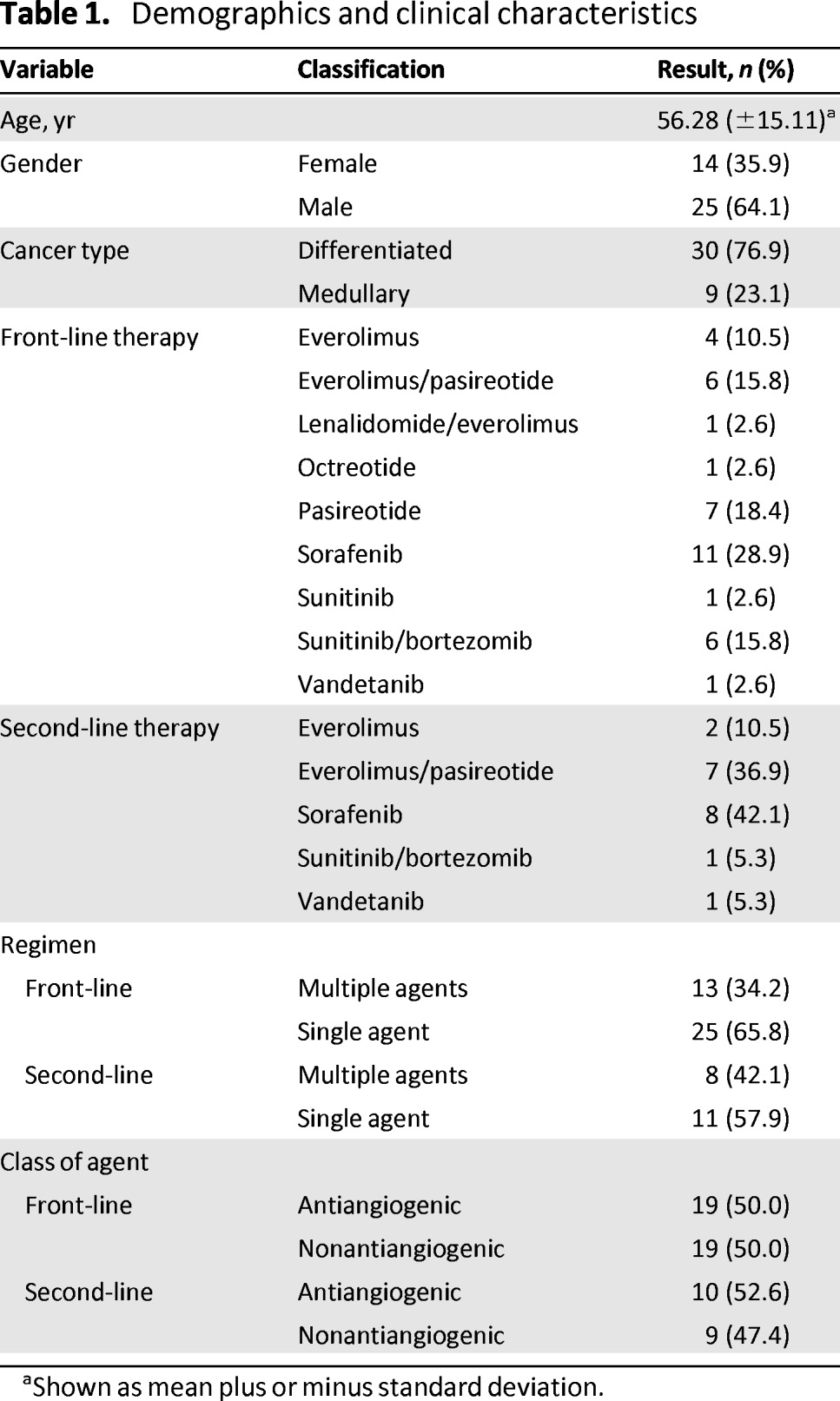
aShown as mean plus or minus standard deviation.
Therapy
Patients were treated with targeted biologic agents either as off-label therapy or through participation in ongoing clinical trials evaluating the safety and/or efficacy of these agents. Sorafenib was the most frequently administered treatment in both the front-line and second-line settings. A two-drug combination regimen was administered to 34% of the patients treated in the front-line setting and to 42% of the patients in the second-line setting [18, 19]. Agents targeting angiogenesis were administered to approximately 50% of the patient population in both the front-line and second-line settings. Full details of patient demographics, tumor characteristics, and treatment are presented in Table 1.
Biochemical and Anatomic Response
There was no significant difference in biochemical or anatomic response outcome when comparing front-line and second-line treatment, with a trend in favor of the front-line setting (biochemical response rate: 15 [41.7%] vs. 2 [13.3%]; p = .625; clinical benefit rate: 30 [81.1%] vs. 15 [83.3%]; p = .337) (Tables 2 and 3).
Table 2.
Anatomic and biochemical response with front-line and second-line therapy
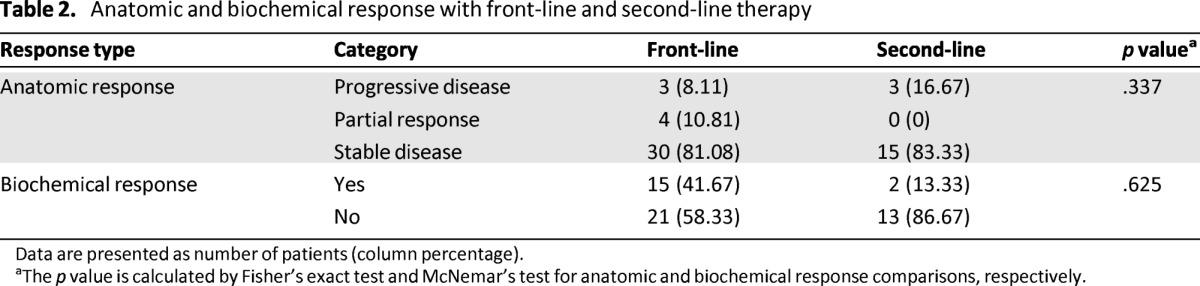
Data are presented as number of patients (column percentage).
aThe p value is calculated by Fisher's exact test and McNemar's test for anatomic and biochemical response comparisons, respectively.
Table 3.
Subset characteristics of front-line and second-line biochemical response
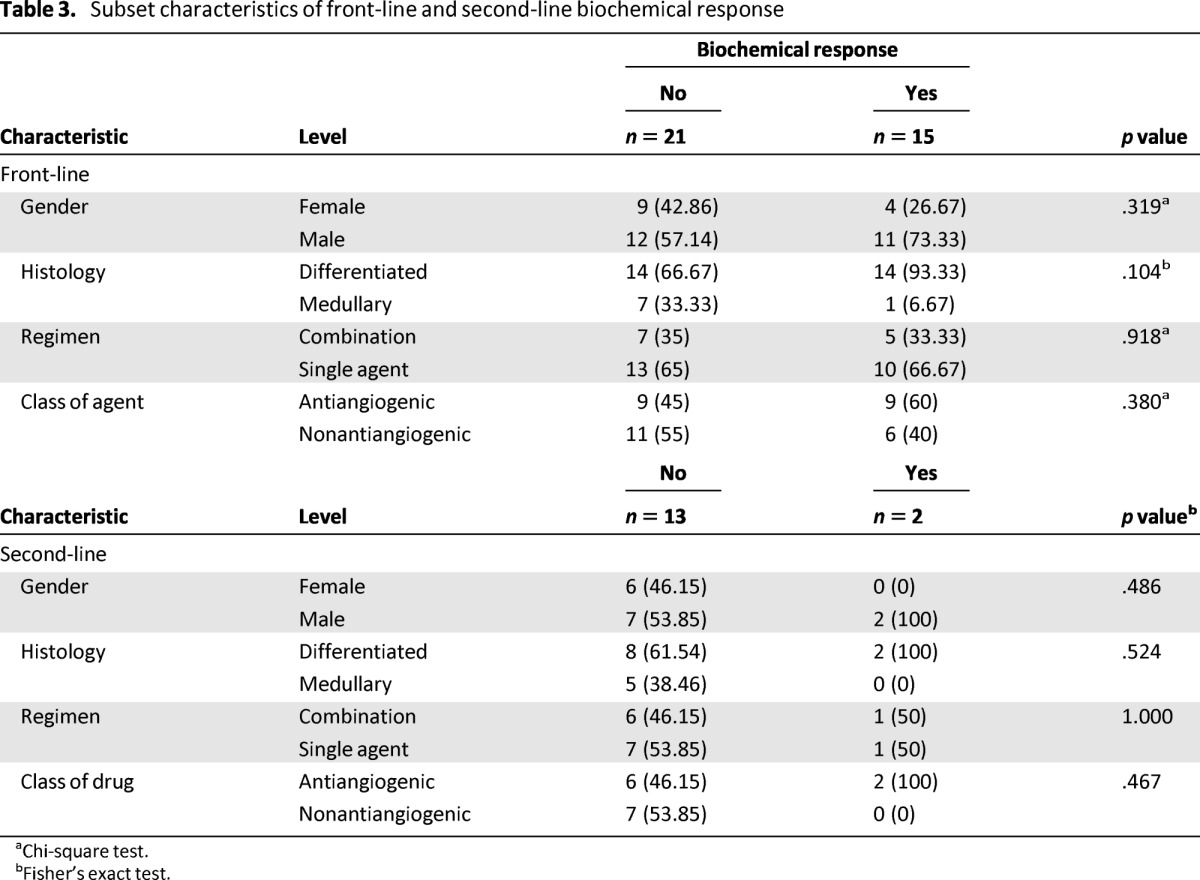
aChi-square test.
bFisher's exact test.
Overall Efficacy Assessment
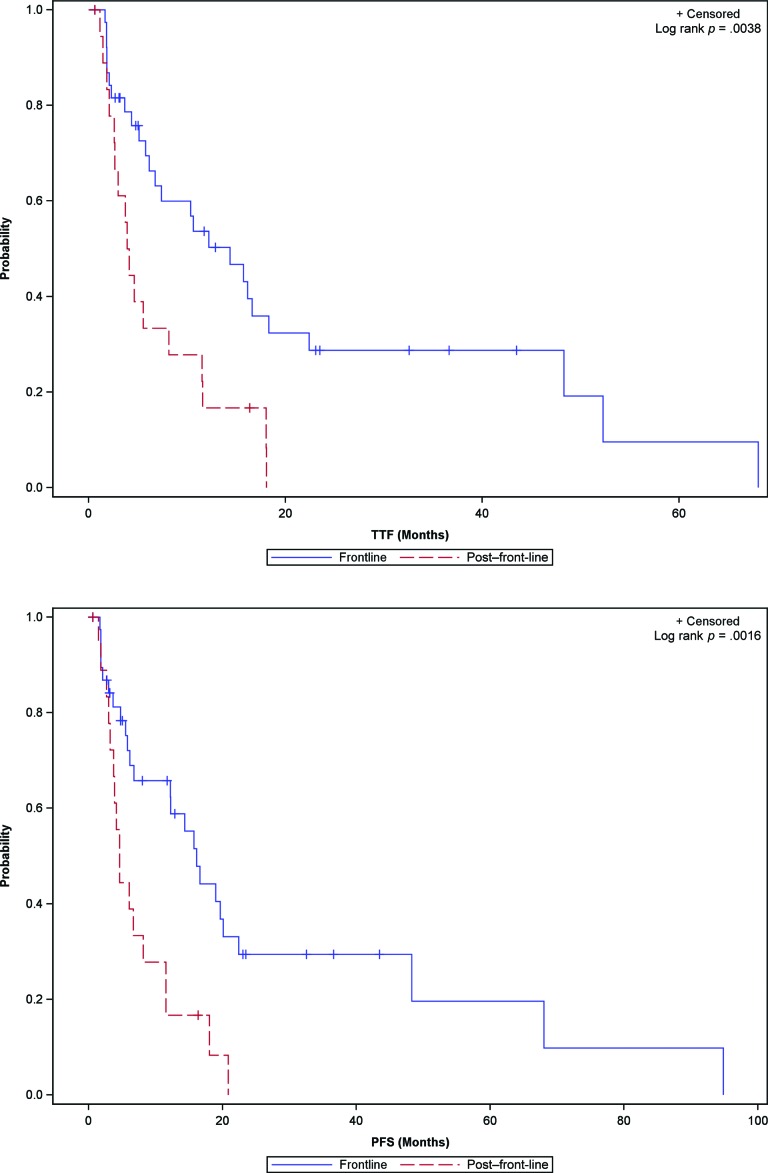
The overall efficacy of second-line targeted therapy was more modest in comparison to the efficacy observed in the front-line setting: Median PFS comparing front-line and second-line therapy was 16.2 months (95% confidence interval [CI]: 6.8–22.4) versus 4.6 months (95% CI: 3.2–8.2), with a p value of .0016 (Figs. 1 and 2).
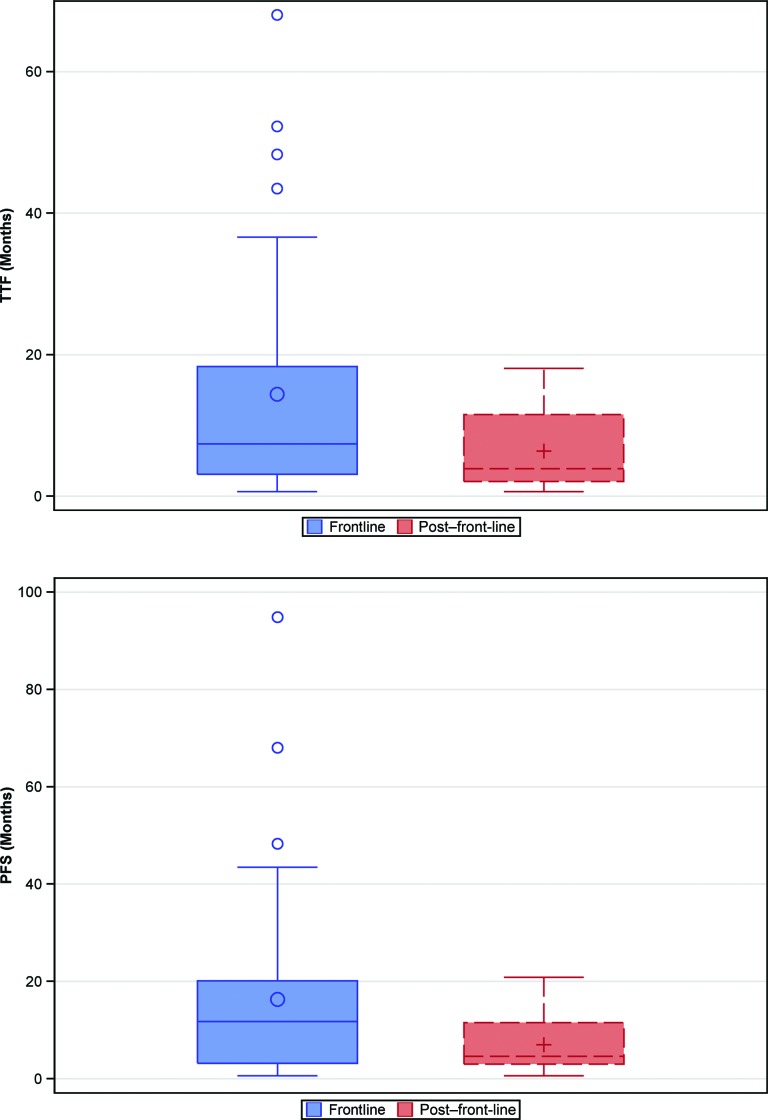
Figure 1.
Box plots showing the median value and interquartile range for TTF (top) and PFS (bottom) associated with biologic therapy of advanced thyroid cancer in the front-line and second-line settings.
Abbreviations: PFS, progression-free survival; TTF, time to treatment failure.
Figure 2.
TTF (top) and PFS (bottom) Kaplan-Meier survival curves for advanced thyroid cancer patients treated with biologic therapy in the front-line and second-line settings.
Abbreviations: PFS, progression-free survival; TTF, time to treatment failure.
Efficacy by Gender
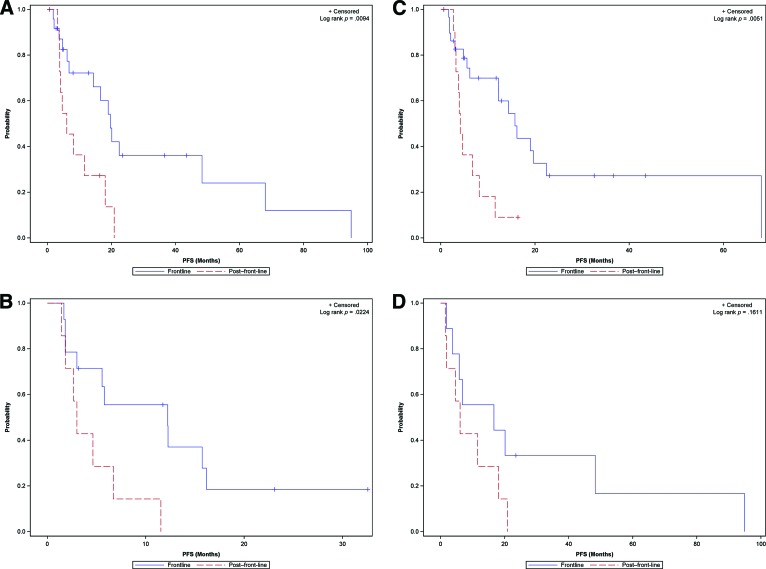
Front-line efficacy led to improved outcomes compared with second-line efficacy in both male and female patients: Median PFS was 19.7 months (95% CI: 6.8–48.3) versus 6 months (95% CI: 3.7–18) (p = .0094) in males and 12.2 months (95% CI: 1.8–16.2) versus 3 months (95% CI: 1.5–6.7) (p = .0224) in females (Fig. 3A).
Figure 3.
Gender-based comparison of front-line versus second-line efficacy of targeted agents in male (A) and female (B) patients. PFS comparison of front-line and second-line therapy in patients with differentiated (C) and medullary (D) thyroid cancer.
Abbreviation: PFS, progression-free survival.
Efficacy by Histology
There was a significant difference between front-line and second-line efficacy in differentiated cancers (median PFS: 15.7 months [95% CI: 6.1–22.4] vs. 4.1 [95% CI: 3–8.2]; p = .0051) and a similar but nonsignificant trend in medullary thyroid cancer patients (median PFS: 16.6 months [95% CI: 1.8–48.3] vs. 6 months [95% CI: 1.5–18]; p = .1611) (Fig. 3B).
Efficacy by Treatment Regimen
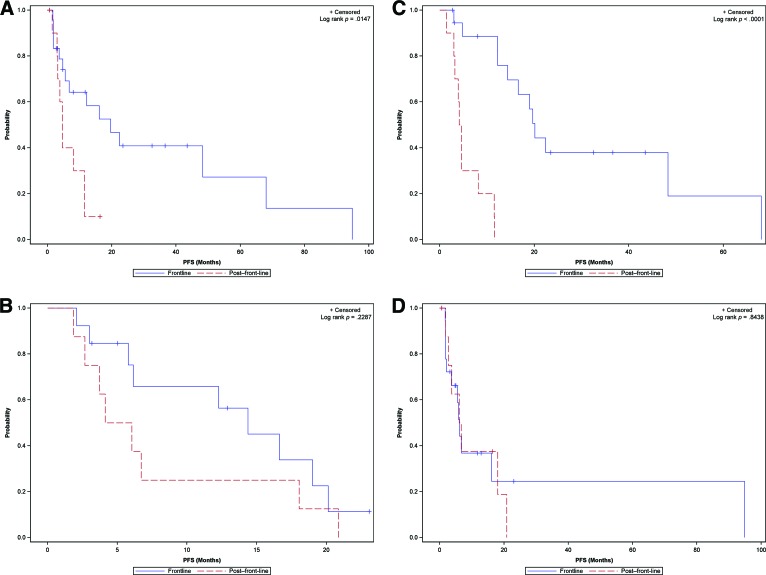
Both single-agent and two-drug combinations of targeted agents demonstrated superior efficacy, as measured by PFS in the front-line setting versus the second-line setting: Median PFS was 19.7 months (95% CI: 5.6–68) versus 4.6 months (95% CI: 1.5–11.5) (p = .0147) and 14.4 months (95% CI: 5.8–20.1) versus 5.1 months (95% CI: 1.9–18) (p = .2287) (Fig. 4A). There was no significant difference in the efficacy of two-drug combination regimens when compared with single-agent therapy both in the front-line setting (median PFS: 14.4 months [95% CI: 5.8–20.1] vs. 19.7 months [95% CI: 5.6–68]; p = .3213) and in the second-line setting (median PFS: 5.1 months [95% CI: 1.9–18] vs. 4.6 months [95% CI: 1.5–11.5]; p = .8020).
Figure 4.
Kaplan-Meier survival curves for PFS of showing comparison of front-line and second-line efficacy of biologic therapy in patients treated with a single-agent regimen (A) and a combination regimen (B). Kaplan-Meier survival curves of PFS comparing front-line and second-line efficacy of vascular-targeting (C) and non-vascular-targeting (D) agents.
Abbreviation: PFS, progression-free survival.
Efficacy by Mechanism of Drug Action
The efficacy of agents targeting angiogenesis was superior in the front-line setting compared with the second-line setting (median PFS: 20.1 months [95% CI: 12.3–68] vs. 4.4 months [95% CI: 1.5–8.2]; p < .0001), whereas nonangiogenesis-targeting agents showed comparable efficacy whether as front-line or second-line therapy (median PFS: 6.1 months [95% CI: 2.1–94.8] vs. 6.4 months [95% CI: 1.9–20.9]; p = .8438) (Fig. 4B).
Exploratory Analysis
In order to exclude potential misestimation of PFS because of varying schedules of restaging scan in up to 45% of the patients who were not treated as part of a clinical trial, we repeated the efficacy analysis using TTF as the endpoint of interest and obtained similar results (data not shown). Furthermore, subset analysis performed separately within the front-line and second-line patient populations did not show any significant difference in efficacy outcome based on tumor histology, gender, class of drug, or treatment regimen (Table 4).
Table 4.
Subset analysis within the front-line and the second-line patient populations
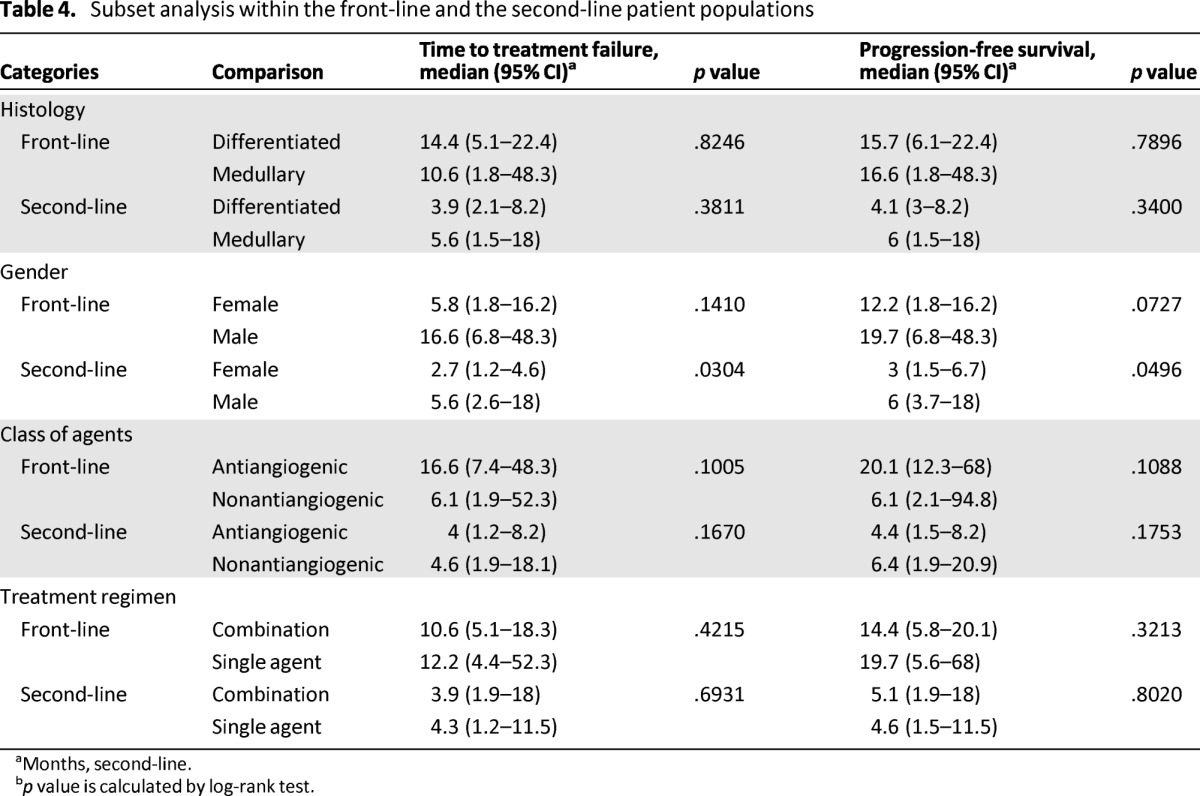
aMonths, second-line.
bp value is calculated by log-rank test.
Discussion
Vandetanib and cabozantinib recently received regulatory approval for the front-line treatment of advanced medullary thyroid cancer based on efficacy data from randomized phase III studies [14, 20]. The first registration trial of sorafenib as front-line therapy for differentiated thyroid cancer has just concluded [17, 21]. Treatment with sorafenib delayed disease progression by more than 5 months in comparison to survival [21]. Although these therapeutic advances are very encouraging, virtually all patients treated with targeted agents eventually progress and require additional therapy. There is no published report to date of a prospective study designed to assess whether the use of targeted agents beyond the front-line setting is associated with meaningful clinical benefit. This retrospective study of patients with advanced thyroid cancer was conducted to bridge this knowledge gap and demonstrated that treatment with targeted agents beyond the front-line setting was associated with meaningful clinical benefit in a patient group for which therapeutic options are currently limited.
By comparing front-line efficacy and second-line efficacy in the same patient group treated in a single institution, we were able to reduce potential bias and confounding associated with retrospective observational studies and comparison across studies and patient populations. Similar to observations in the prospective phase II and registration phase III studies, objective anatomic responses were infrequent with the use of these agents; however, the majority of patients achieved durable control of disease. The duration of disease control observed with the front-line therapy in this observational study was comparable to results from prospective phase II studies [9–14]. As anticipated, the efficacy of targeted agents in the front-line setting was superior to the second-line setting. Nonetheless, the median PFS of 4.6 months achieved in the second-line setting by previously treated patients suggested that this approach may confer meaningful benefit to a significant number of patients.
The diminished second-line efficacy in comparison to the front-line setting is expected and is consistent with the experience of other cancer types for which therapeutic efficacy declines with an increasing number of prior lines of therapy [22–24]. A recent study reported comparable efficacy of second-line and first-line multiple kinase inhibitor therapy for differentiated thyroid cancer patients with a trend in favor of front-line therapy (median PFS: 6.7 month vs. 7.6 months; hazard ratio: 0.85 [95% CI: 0.45–1.61]; p = .6) [25]. Interestingly, we observed efficacy diminution with the vascular-targeting class of agents, whereas treatment with nonantiangiogenic agents achieved comparable efficacy in the front-line and second-line settings. This intriguing observation may inform the appropriate sequencing of targeted agents in thyroid cancer and other cancer patient populations as well as the design of prospective clinical trials to evaluate second-line therapy.
The observation of greater benefit of targeted agents in male patients versus female patients, in both the front-line and second-line settings, has not been previously reported with any of the agents evaluated in this study. Although gender-based differences in efficacy resulting from altered pharmacodynamics and/or toxicity-induced treatment discontinuation may play a role, it is noteworthy that the large majority of patients in this analysis were treated with sorafenib. Population-based pharmacokinetic studies did not show any gender-based differences in sorafenib handling [26]. Moreover, similar results were obtained using TTF or PFS endpoints as the basis for comparison and indicated that early treatment discontinuation because of increased toxicity was not responsible for this difference. Prospective assessment of this observation will be of interest in the future. We did not observe any significant difference in the clinical benefit of biologic agents based on tumor histology, consistent with other observational and prospective studies of biologic therapy in this patient population [11, 16].
Limitations of our study include the retrospective design and the relatively large number of targeted agents. Although a more homogenous population of patients treated with the same type of drug would have been more desirable, such a population of patients is very challenging to assemble at the present time because the choice of treatment agents is dictated by their availability as part of a clinical trial protocol or through a special access program. In addition, as a single-institution experience, the generalizability of these findings requires further replication and validation by other studies, especially prospective randomized studies. Moreover, we were not able to determine and control for the potential impact of driver mutations such as B-Raf or K-Ras, which have been shown to have prognostic implications in resected differentiated thyroid cancer [27–29]. Given the use of the same cohort of patients to assess efficacy in the front-line and second-line settings, any potential impact of molecular differences between patients is unlikely to have confounded our analysis in this regard. Overall, the opportunity to use the same set of patients to compare the benefit of front-line and second-line use of biologic agents is a major strength of our study that enabled us to limit the some of the known drawbacks of a retrospective observational study.
Conclusion
This retrospective single-institution study provides the first systematic evidence supporting the use of targeted biologic agents beyond the front-line setting in advanced thyroid cancer patients. We expect that findings from this study will facilitate and inform the design of prospective studies of targeted biologic agents in the second-line setting in patients with advanced thyroid cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We thank Anthea Hammond, Ph.D. for her editorial assistance with the manuscript. T.K.O. received research material and funding from Novartis Pharmaceuticals in support of an ongoing preclinical study and a phase II clinical trial of pasireotide and everolimus in advanced thyroid cancer. The study was supported in part by the following grant funding to the authors: NIH 1K23CA164015 (principal investigator [PI]: T.K.O.); P01 CA116676-S1 (PI: F.R.K.) and Georgia Cancer Coalition Distinguished Cancer Scholar Award (PI: T.K.O.).
This work was presented in part at the 82nd annual meeting of the American Thyroid Association, September 19–22, 2012, in Quebec City, Canada.
Author Contributions
Conception/Design: Taofeek K. Owonikoko, Rajasree P. Chowdry
Provision of study material or patients: Taofeek K. Owonikoko
Collection and/or assembly of data: Taofeek K. Owonikoko, Rajasree P. Chowdry, Zhengjia Chen, Sungjin Kim
Data analysis and interpretation: Taofeek K. Owonikoko, Rajasree P. Chowdry, Zhengjia Chen, Sungjin Kim, Nabil F. Saba, Dong M. Shin, Fadlo R. Khuri
Manuscript writing: Taofeek K. Owonikoko, Rajasree P. Chowdry, Zhengjia Chen, Sungjin Kim, Nabil F. Saba, Dong M. Shin, Fadlo R. Khuri
Final approval of manuscript: Taofeek K. Owonikoko, Rajasree P. Chowdry, Zhengjia Chen, Sungjin Kim, Nabil F. Saba, Dong M. Shin, Fadlo R. Khuri
Disclosures
Taofeek K. Owonikoko: Novartis Oncology, Bayer/Onyx Pharmaceuticals (RF). The other authors indicated no financial relationships.
Section editors: Herbert Chen: None; Stan Sidhu: None
Reviewer “A”: None
Reviewer “B”: Novartis (RF)
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the united states, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Palme CE, Waseem Z, Raza SN, et al. Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:819–824. doi: 10.1001/archotol.130.7.819. [DOI] [PubMed] [Google Scholar]
- 4.Tsang RW, Brierley JD, Simpson WJ, et al. The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer. 1998;82:375–388. [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: A retrospective analysis. Lancet Oncol. 2011;12:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 9.Bible KC, Suman VJ, Molina JR, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: Results of a phase 2 consortium study. Lancet Oncol. 2010;11:962–972. doi: 10.1016/S1470-2045(10)70203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells SA, Jr., Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28:767–772. doi: 10.1200/JCO.2009.23.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman SI, Wirth LJ, Droz JP, et al. Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J Med. 2008;359:31–42. doi: 10.1056/NEJMoa075853. [DOI] [PubMed] [Google Scholar]
- 14.Wells SA, Jr., Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurzrock R, Sherman SI, Ball DW, et al. Activity of XLl184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29:2660–2666. doi: 10.1200/JCO.2010.32.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabanillas ME, Hu MI, Durand JB, et al. Challenges associated with tyrosine kinase inhibitor therapy for metastatic thyroid cancer. J Thyroid Res. 2011:985780. doi: 10.4061/2011/985780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brose MS, Nutting CM, Sherman SI, et al. Rationale and design of decision: A double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer. 2011;11:349. doi: 10.1186/1471-2407-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvey RD, Kauh JS, Ramalingam SS, et al. Combination therapy with sunitinib and bortezomib in adult patients with radioiodine refractory thyroid cancer. J Clin Oncol. 2010;28:5589a. [Google Scholar]
- 19.Owonikoko TK, Harvey RD, Kauh JS, et al. A phase I study of the safety and pharmacodynamic effects of everolimus in combination with lenalidomide in patients with advanced solid malignancies. J Clin Oncol. 2012;30:2576a. [Google Scholar]
- 20.Thornton K, Kim G, Maher VE, et al. Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary Clin Cancer Res. 2012;18:3722–3730. doi: 10.1158/1078-0432.CCR-12-0411. [DOI] [PubMed] [Google Scholar]
- 21.Brose MS, Nutting C, Jarzab B, et al. Sorafenib in locally advanced or metastatic patients with radioactive iodine-refractory differentiated thyroid cancer: The phase III decision trial. J Clin Oncol. 2013;31:4a. doi: 10.3978/j.issn.2304-3865.2014.01.02. [DOI] [PubMed] [Google Scholar]
- 22.Porta C, Procopio G, Carteni G, et al. Sequential use of sorafenib and sunitinib in advanced renal-cell carcinoma (RCC): An Italian multicentre retrospective analysis of 189 patient cases. BJU Int. 2011;108:E250–E257. doi: 10.1111/j.1464-410X.2011.10186.x. [DOI] [PubMed] [Google Scholar]
- 23.Dudek AZ, Zolnierek J, Dham A, et al. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer. 2009;115:61–67. doi: 10.1002/cncr.24009. [DOI] [PubMed] [Google Scholar]
- 24.Eichelberg C, Heuer R, Chun FK, et al. Sequential use of the tyrosine kinase inhibitors sorafenib and sunitinib in metastatic renal cell carcinoma: A retrospective outcome analysis. Eur Urol. 2008;54:1373–1378. doi: 10.1016/j.eururo.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 25.de la Fouchardiere C, Massicotte M-H, Borget I, et al. Sequential TKI treatments for iodine-refractory differentiated thyroid carcinomas. J Clin Oncol. 2013;31:6092. [Google Scholar]
- 26.Jain L, Woo S, Gardner ER, et al. Population pharmacokinetic analysis of sorafenib in patients with solid tumours. Br J Clin Pharmacol. 2011;72:294–305. doi: 10.1111/j.1365-2125.2011.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lupi C, Giannini R, Ugolini C, et al. Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2007;92:4085–4090. doi: 10.1210/jc.2007-1179. [DOI] [PubMed] [Google Scholar]
- 28.Elisei R, Ugolini C, Viola D, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 29.Kim TY, Kim WB, Rhee YS, et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2006;65:364–368. doi: 10.1111/j.1365-2265.2006.02605.x. [DOI] [PubMed] [Google Scholar]