Government partners implemented screening and treatment with visual inspection using acetic acid (VIA) and cryotherapy at demonstration sites in Peru, Uganda, and Vietnam. Evaluations were conducted in the three countries to explore the barriers and facilitating factors for the use of services and for incorporation of screen-and-treat programs using VIA and cryotherapy into routine services. Results showed that use of VIA and cryotherapy in these settings is a feasible approach to providing cervical cancer prevention services.
Keywords: Cervical cancer, Cancer screening, Program evaluation, Community outreach, Visual inspection with acetic acid, Perceptions
Abstract
Cervical cancer is preventable but continues to cause the deaths of more than 270,000 women worldwide each year, most of them in developing countries where programs to detect and treat precancerous lesions are not affordable or available. Studies have demonstrated that screening by visual inspection of the cervix using acetic acid (VIA) is a simple, affordable, and sensitive test that can identify precancerous changes of the cervix so that treatment such as cryotherapy can be provided. Government partners implemented screening and treatment using VIA and cryotherapy at demonstration sites in Peru, Uganda, and Vietnam. Evaluations were conducted in the three countries to explore the barriers and facilitating factors for the use of services and for incorporation of screen-and-treat programs using VIA and cryotherapy into routine services. Results showed that use of VIA and cryotherapy in these settings is a feasible approach to providing cervical cancer prevention services. Activities that can help ensure successful programs include mobilizing and educating communities, organizing services to meet women's schedules and needs, and strengthening systems to track clients for follow-up. Sustainability also depends on having an adequate number of trained providers and reducing staff turnover. Although some challenges were found across all sites, others varied from country to country, suggesting that careful assessments before beginning new secondary prevention programs will optimize the probability of success.
Implications for Practice:
Government-run cervical cancer screening programs in Peru, Uganda, and Vietnam showed that using visual inspection with acetic acid (VIA) and cryotherapy in these settings is a feasible approach to providing cervical cancer prevention services. Activities that can help to ensure success include mobilizing and educating communities, organizing services to meet women's schedules and needs, and strengthening systems to track clients for follow-up. Sustainability also depends on having an adequate number of trained providers and reducing staff turnover. While some challenges were found across all three countries, others varied, suggesting that careful assessments before beginning new secondary prevention programs will optimize the probability of success.
Introduction
Cervical cancer is preventable but continues to cause the deaths of more than 270,000 women worldwide each year [1]. More than 85 percent of these deaths occur in developing countries, where programs to detect precancerous lesions and provide timely treatment are not available or are beyond the means of most women [1, 2].
With human papillomavirus vaccines now available and approved in many countries, governments have begun to consider vaccination (primary prevention) as an important component of cervical cancer prevention strategies; however, current vaccines cannot protect against all cervical cancers, so vaccinated girls will need to access screening services at older ages. Moreover, because vaccination does not protect women already exposed to the viruses, screening and treatment services (secondary prevention) are essential for older, sexually active women.
The Papanicolaou (Pap) test has been in use for many years in high-resource countries, decreasing the number of deaths from cervical cancer dramatically over the past 50 years. This test has not been deployed successfully in developing countries because it requires trained physicians and laboratory technicians, adequate infrastructure to support processing and diagnosis, and multiple visits by women to health facilities. Women in high-resource areas who are diagnosed with cervical precancer can be treated with sophisticated methods such as the loop electrosurgical excision procedure (LEEP), laser conization, or cold knife conization. Again, these methods have limited use in low-resource settings because of equipment cost, lack of trained providers, and inadequate infrastructure.
In the past decade, visual inspection of the cervix using acetic acid (VIA) has been shown to be simpler and more affordable than Pap testing and to have at least equal sensitivity for identifying precancerous changes of the cervix. VIA does not require a physician and can be performed by a trained health care worker. Several large clinical trials evaluated VIA's ability to detect high-grade disease and found its performance to be similar to or better than Pap testing; a recent pooled estimate reports the sensitivity of VIA as 80% [3], although a more realistic estimate in practice may be 50% [4]. Most VIA-positive women can be treated with cryotherapy, a relatively simple freezing technique, to destroy precancerous lesions [4].
Most studies of secondary prevention in low- and middle-income countries have focused on the accuracy of VIA [5–11], although a few have explored feasibility and acceptability [12–14]. In the present study, government partners collaborated with PATH, an international nongovernmental organization, in Peru, Uganda, and Vietnam, to implement demonstration projects for screening and treatment of precancerous lesions. In our screen-and-treat approach, women were screened with VIA and treated with cryotherapy on the same day or shortly afterward, if treatment was indicated. When cryotherapy was not considered appropriate for the type of lesion, women were referred to the regional or national level for LEEP or another treatment. These demonstration sites were designed to serve as models for cervical cancer prevention that could be scaled up by governments. As a part of the projects, we conducted a process evaluation in each country to explore the barriers and facilitating factors for the use of services and for incorporation of screen-and-treat programs using VIA and cryotherapy into routine services.
Methods
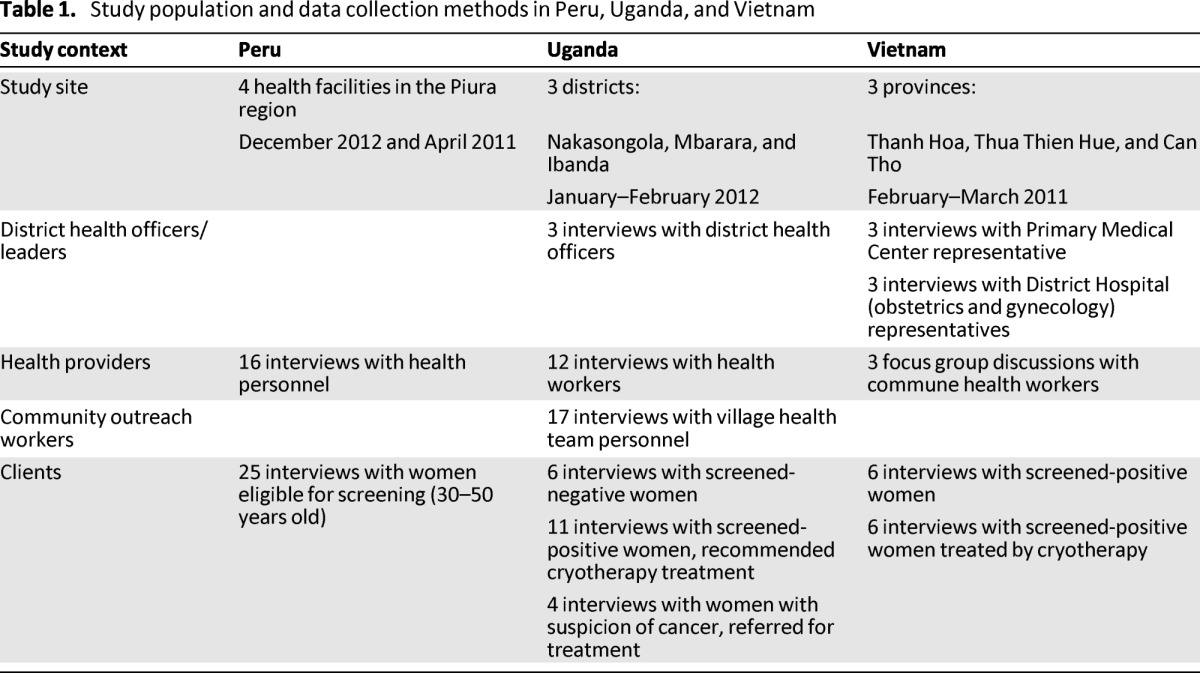
The process evaluation was carried out by conducting in-depth interviews (IDIs) and focus group discussions (FGDs) at selected demonstration project sites in each of the three countries, which included rural and urban populations. Table 1 presents the study populations, which consisted of clients who accessed screening and treatment and health workers who provided services. In Peru, women were age eligible but not all had yet participated in screening. In Uganda and Peru, evaluation was by IDI, whereas in Vietnam, data were collected by IDIs with clients and district-level health professionals and FGDs with commune health workers. Interviews were translated into English, and for all countries, an iterative, theme-based, manual content analysis was carried out to summarize the results.
Table 1.
Study population and data collection methods in Peru, Uganda, and Vietnam
The studies were approved by the ethics committee of PATH USA. Local country approvals were obtained in each country from the Instituto de Investigación Nutricional in Peru, the National HIV/AIDS Research Committee and Uganda National Council for Science and Technology in Uganda, and the Maternal and Child Health Department of the Ministry of Health in Vietnam. Verbal consent was obtained prior to interviews and discussions in all countries.
Results
Data analyses for the three countries revealed a number of common themes in the discussions with clients who received screen-and-treat services and health workers who provided services. Although the use of services by women was assessed mainly in the IDIs with clients, health workers also provided information that women conveyed to them. The data on sustainability of services were gathered from IDIs and FGDs with providers.
Factors Related to Use of Screen-and-Treat Programs: Client Perspectives
Locally Available Services
Convenient and local access to cervical cancer screening and precancer treatment options was a perceived benefit of the screen-and-treat program, especially in Uganda and Vietnam:
In the past, they used to hear over the radio that one would have to go to Mulago Hospital [in the capital city] … however, when they heard that the screening services had been brought closer to them, they liked it and took it up (village health team member, Nakasongola, Uganda).
Women in Uganda who had to travel long distances to the regional referral center for screening mentioned travel-related expenses as an obstacle and preferred that screening be offered at local health centers. In Vietnam, offering VIA screening at the commune health center was one of the advantages mentioned by women. Bringing cryotherapy services to the health facility where women were screened improved access to treatment.
Institutional Structure and Capacity
In Peru and Uganda, institutional factors such as long wait times and timing of services were mentioned as barriers to accessing VIA. In Peru, women described long lines to receive services and the inconvenience of having services offered only in the mornings. Ugandan women mentioned that in addition to long waiting times that interfered with their daily responsibilities, they were not able to afford related expenses, such as those for food and drink. Clients sometimes were turned away because of the large number of women attending the clinic and the limited staff available. Women in Peru also described motivating factors within the institutional structure, such as referral by a health care provider. In addition, VIA was seen as an advanced, modern test by Peruvian women because the results were available immediately, unlike Pap test results.
Trust and Safety Concerns
A lack of trust in quality of services emerged as a key challenge for implementing screen-and-treat programs in all three countries. In Peru and Uganda, some women stated a concern about whether the instruments were properly cleaned or whether they might spread infections or even cause cancer:
They said that the instruments they use have a lot of infections and cause many diseases. So when they tell some women that the instruments cause cancer, they refuse to come (screened-positive woman, Nakasongola, Uganda).
Awareness and Information Dissemination
Women in Vietnam and Uganda stressed the value of multiple communication channels. Community sensitization and direct counseling were cited as being important in Vietnam:
First, I learned from the local loudspeaker, second, I heard from the communication of population-family planning motivators and the Women's Union. In each meeting they talked about it (screened-positive woman, treated, Hoang Hoa, Vietnam).
In Uganda, a village health worker noted that acceptance of screening was facilitated by women who had sought services and then recommended them to other women or who knew someone who had suffered from cervical cancer: “When one woman comes for screening, she will tell another one and the other one will tell another one, so you find that it becomes easy for you” (village health team member, Ibanda, Uganda).
In Peru, advice from friends, the use of mass media, and facility-based education were mentioned: “I actually came to have a Pap, but I heard the talk about VIA, so VIA is better, isn't it” (screened woman, Piura, Peru).
Family and Community Support
Across all three countries, family support—especially husband support—facilitated screening and treatment. In addition to husband support, women screened in Peru and Vietnam mentioned the importance of family and friends in creating a supportive environment to access cervical cancer prevention services. Community gatherings in Uganda where political leaders encouraged women to seek cervical cancer screening services were also mentioned as a motivating factor.
Perception of Health and Health-Seeking Behaviors
Concerns about health motivated women to seek cervical cancer screening in Peru and Uganda. Women in both of these countries described seeking screening because they experienced discomfort, pain, or discharge:
Client (screened-negative woman, Mbarara, Uganda): I had much pain. It hurt a lot and I wondered whether I had the disease that they were talking about. I then decided to come and get examined so that I would know if I had it or not.
Interviewer: Would you have come if you did not have pain?
Client: No, I would not.
In Peru, women mentioned that they sought cervical cancer screening to prevent cervical cancer or to be healthy: “The doctors say to go and do a check-up before there is cervical cancer. And when one goes to the doctor and knows, one can be saved faster” (age-eligible woman, Piura, Peru).
In Uganda, some women noted a fear of cervical cancer death as a reason to be screened. Clients in both Peru and Uganda have a fear of leaving their children without a mother:
All I want is to be treated so I can get better. That is all I want. I want to be treated so that I can see my children grow up to study to higher levels because when I am sick, everything gets to a standstill (screened-positive woman, treated, Mbarara, Uganda).
In Vietnam, clients believed that women were not screened because they did not perceive themselves to be at risk for developing cervical cancer. When there is not a perceived risk, they felt women would not make an effort to get screened.
Attitudes Toward Cervical Cancer Screening Procedures
Although general attitudes toward cervical cancer screening were not mentioned as a barrier to programs in Vietnam, clients from Uganda and Peru believed fear, shame, or embarrassment were barriers to screening. A number of women interviewed in Peru believed that fear of pain prevented women from being tested, although some thought that VIA was less painful than a Pap smear. Clients in Peru and Uganda also mentioned “the fear of learning the result” or “fear of positive diagnosis.” Shame or embarrassment about having a pelvic exam emerged as a barrier to VIA screening in Peru and Uganda:
They will see my private parts. It is okay for them to see my private parts when giving birth because I am in pain at that time, but how can I walk there now and expose myself for them to see (village health team member, Ibanda, Uganda).
In Uganda, myths about the screening and treatment process were perceived as a barrier to accessing services. In addition to mentioning that women believed the examination to be extremely painful, women were fearful that the equipment would “pull out the uterus,” affect the ability to perform sexually, or cause other harm to reproductive organs.
Factors Related to Sustainability of Screen-and-Treat Programs: Provider Perspectives
Characteristics of VIA
Providers at all sites noted some characteristics of the VIA test that made it particularly appropriate for use in their settings. In Peru, providers said that VIA was quick, taking a maximum of 15–20 minutes for the entire procedure. In Vietnam and Uganda, providers commented on the ease of use, the ability for VIA to be administered by nonphysician providers, and the potential for treatment on the same day as screening. A doctor in Vietnam noted how this compared favorably with personal experiences of Pap test screening:
The midwife in the communal level could also provide the result after 1 minute, while the national program for cancer prevention using Pap smear was implemented in September last year, but so far there is no test result sent back to the clients (doctor, Huong Thuy, Vietnam; referencing a wait of at least 5 months for the Pap test results).
The subjective nature of interpreting VIA tests and the lack of trust in the results of some gynecologists and others in the medical community who did not have training in VIA was experienced as a barrier in Vietnam and Peru:
That's what happened to me with this woman I'm telling you about. She's been to the gynecologist and he's told her no, that they have to have a good eye to do that [VIA], and the other patient as well, a patient who had already told me ‘Miss, the fact is the gynecologist told me I'm fine, I've already had the Papanicolaou done’ … the fact is that they [gynecologists] give them the idea that maybe I'm lying to them (health worker, Piura, Peru).
VIA Supplies and Equipment
Providers in all countries indicated they had sufficient space and equipment necessary for implementing VIA screening. In Peru, providers described having to change their initial setup of performing screening in available consulting rooms to a designated space prepared for the examination (with specula, a privacy screen, and a lamp) to provide a better environment for both clients and providers.
Maintaining an adequate supply of materials, including acetic acid, was a barrier to the implementation of VIA at all three sites. In Vietnam, providers described how the supply of acetic acid was not enough for conducting large-scale VIA screening.
Cryotherapy
In Peru and Vietnam, providers noted that having the equipment necessary for implementing cryotherapy allowed them to link screening and treatment, and they saw that as an advantage because it reduced loss to follow-up. This was particularly true when the cryotherapy machine was brought to the commune level in one province in Vietnam and reduced loss to follow-up. In Peru, having cryotherapy at the site was considered to save money for women who, as a result, did not need to travel to the capitol city for treatment. In Peru, permitting only doctors to perform cryotherapy was seen as a limitation:
[Cryotherapy is] done by the general doctor, and we have a little bit of a problem right now because he has another job and the days he can come here have been cut. But he was the one who was trained together with the two professional midwives and we can't do cryotherapy, just the doctor (health worker, Piura, Peru).
At all three sites, challenges were associated with ensuring an uninterrupted supply of the gas used for cryotherapy. Health workers in Uganda described long delays in obtaining gas once the initial tank ran out in all three districts. A provider in Peru explained: “It's been pretty difficult, because for example the last tank ran out in December and it was only recently in the first half of February that the tank was delivered to us” (health worker, Piura, Peru).
In Uganda, one cryotherapy machine stopped working and could not be repaired by the local technician: “We had a cryotherapy machine, however it is now down…. We took it to the technician who is working on medical equipment and he has tried several times and he has failed” (health worker, Mbarara, Uganda).
Staff Training and Turnover
Training on VIA and cryotherapy was well received at all three sites. In Peru, providers appreciated having a training that included enough time to practice, which they saw as vital for sharpening the clinical eye and providing confidence to carry out the procedures. Staff trained to perform VIA in Uganda reported feeling confident in their ability to provide VIA testing: “Yes, we are competent and not only competent but I will say we are proficient” (health worker, Nakasongola, Uganda).
At all three sites, a core barrier to the sustainability of VIA screening was that limited staff were expected to provide multiple services and were stretched thin. Providers in Peru and Uganda also described burnout on the part of providers who were overwhelmed by their workloads:
The only problem here is that there is only one nurse and she has a lot of other work to do…. She works on antenatal mothers and on women for cervical cancer. There are times you find her in the HIV clinic all alone. The work is too much for her so some people go back without receiving services (village health team member, Ibanda, Uganda).
In Uganda and Peru, high staff turnover meant that many providers trained in VIA were no longer working in clinics where VIA was implemented. Despite broader systems issues, including these staffing limitations in Uganda, providers described being very motivated to screen women using VIA:
It is our duty to treat those mothers. We know it is not very easy to leave your home when you do not have any complaint to come and screen…. If you send that lady away, she will not come back or if she comes back the next day, she will have a cancer (health worker, Mbarara, Uganda).
Referral
At all sites, no standardized referral or follow-up system was in place to ensure that VIA-positive women received appropriate treatment. In Peru, the follow-up method consisted of telephone calls, but this did not guarantee the consistent return of patients positively diagnosed by VIA. In Vietnam, it was noted that other infections frequently prevented immediate treatment, and this led to loss to follow-up. In Uganda, cost impeded follow-up phone calls, although very motivated staff sometimes used their own money to make calls:
Sometimes you end up going in your pocket when you realize that a woman who has not yet got treatment is not coming back…. We are not helped in that, in communication between workers in the clinic and those women that are worked on or those who are referred (health worker, Mbarara, Uganda).
Information, Education, Communication, and Outreach
In Vietnam and Uganda, providers discussed how outreach about VIA was successfully integrated into other outreach activities, such as reproductive health talks at women's meetings in Vietnam and village health team messaging to communities in Uganda. The focus of screening outreach in both Vietnam and Peru was at the facility level, and broader community-based outreach was not implemented. In both countries, providers saw potential for using mass media to promote the new services for cervical cancer prevention.
In Uganda, although outreach activities were seen as important, they were limited by resources (e.g., funding for travel costs and protective gear during harsh weather) for sensitization and mobilization by village health workers:
Imagine having neither raincoat nor gum boots during the rainy season and the rain starts falling while you are moving out there…. By the time you get to the people, you are drenched wet or you get to them late, all because you do not have these things. That too is a challenge (village health team member, Nakasongola, Uganda).
In Uganda, there was concern on the part of health care providers that the outreach workers were not including the message about the appropriate age for screening: at least 25 years of age. In some cases, community sensitization worked so well that there was difficulty in meeting the demand, with more women coming to some screening sessions than providers could see:
The community sensitization worked well…. You would get about 150 women and yet it was two of us going at every visit. We would stop at about 70 women and then tell the others we would give them another date and yet we would not go back because we have to go to another subcounty (health worker, Nakasongola, Uganda).
Discussion
Outreach and Education
Although the intention of the programs we examined was to implement population-based screening programs targeting women in a specific age range, we found that information was not broadly disseminated; rather, most education was facility based, and most screening was opportunistic. Symptoms such as pain or discomfort were the main motivators for screening, and this finding is consistent with previous studies [15, 16]. This barrier to population-based screening is important because women with precancerous lesions are typically asymptomatic. Embarrassment or anxiety about having a pelvic examination was also seen as reducing attendance [15–17]. Our findings on the role of the male partner were similar to those from other studies in Africa and South and Central America noting that women may need approval from their husbands at various steps of the screen-and-treat process [18, 19].
Organization of Services
Organization of services was a central factor in both the acceptability of services to women and the feasibility of service sustainability from providers' perspectives. Important factors for clients included distance to the facility, length of wait times, and ability to schedule visits around work and family obligations. Other studies have shown that cost associated with travel is a barrier to women attending screening [20] and that short wait times and convenient scheduling are essential [21, 22].
From a provider perspective, primary problems were unreliable gas supply for cryotherapy and equipment breakdown. Cryotherapy has been recognized as the simplest and most feasible treatment option for use in low-resource settings [23], but difficulties in replenishing the gas supply emerged in all of the settings we evaluated. Maintaining an adequate supply of acetic acid (which is needed for VIA) also was a challenge. In addition, clinics struggled to keep VIA services staffed with trained providers because of high staff turnover.
Follow-up of Screened-Positive Women
Our study, like others [24], identified difficulties in tracking women with a positive screening result and ensuring that treatment was completed. An alternative to client tracking is offering screening and treatment in a single day. Although this approach has been highly successful in research settings [12, 25–27], it may not be practical to implement as a health service program. Our data suggest that during screening, other infections are often identified and need to be treated prior to cryotherapy for precancerous lesions. Consequently, lesions cannot always be treated the same day.
Although cryotherapy is more affordable than treatments such as LEEP or laser therapy, the costs of equipment and gas supplies and of ensuring that personnel have adequate training may be barriers. As noted previously, unreliable gas supply is a major barrier. Alternative treatment options that do not rely on consumables such as compressed gas could address this issue.
Conclusion
Programmatic Insights
Our experience highlighted lessons and suggestions for implementing and expanding cervical cancer programming.
Developing a community mobilization plan that targets eligible (age-appropriate) women, spouses, and the broader community and that includes messages about the lack of symptoms of precancerous lesions can improve awareness about cervical cancer screening.
Appropriate training will help health promoters deliver accurate information to their communities, including messages about the safety of screening and treatment.
Coordination between outreach workers and service providers will help ensure that women can travel to clinics at convenient times and be seen in a timely manner.
Health workers need sufficient training to become proficient and have confidence in their clinical skills, and successful programs must work to reduce staff turnover.
Well-defined procurement policies and better forecasting practices can help health systems provide a reliable flow of equipment and supplies.
When appropriate tracking is in place, outreach workers can make sure that screened-positive women get treatment. An alternative to tracking is offering screening and treatment in a single day for women who do not have infections that preclude immediate cryotherapy.
Establishing centralized treatment services in a catchment area may be more feasible than trying to offer treatment at many local facilities.
Our process evaluation of services at selected sites in three low- and middle-income countries showed that successful programs will need to address challenges in the areas of outreach and education, organization of services, and follow-up of screened-positive women. Challenges may vary from country to country, so careful assessments should be made before planning new secondary prevention programs.
Acknowledgments
P.P. and J.L.W. contributed equally to manuscript. This study was funded by the Bill and Melinda Gates Foundation as part of the “HPV Vaccines: Evidence for Impact” project and coordinated by PATH. We acknowledge the contribution of our research collaborators: Dr. Harriet Mutonyi and Barbara Nyanzi-Wakholi (Kampala, Uganda) and Pham Vu Thien (Centre for Creative Initiatives in Health and Population, Hanoi, Vietnam). We would also thank Colleen Kuehl for assisting in the preparation of the manuscript, Dr. Marjorie Murray for editing, and Dr. Vivien D. Tsu for critical review. Finally, we express our sincere gratitude to all the individuals who volunteered to participate in this research.
Author Contributions
Conception/Design: Proma Paul, Jennifer L. Winkler, Jose Jeronimo
Provision of study material or patients: Trinh Thu Huong, Le Thi Nga, Edward Kumakech, Edward Mugisha
Collection and/or assembly of data: Rosario M. Bartolini, Mary E. Penny, Trinh Thu Huong, Le Thi Nga, Edward Kumakech, Edward Mugisha
Data analysis and interpretation: Proma Paul, Jennifer L. Winkler, Jose Jeronimo
Manuscript writing: Proma Paul, Jennifer L. Winkler, Jose Jeronimo
Final approval of manuscript: Proma Paul, Jennifer L. Winkler, Jose Jeronimo, Rosario M. Bartolini, Mary E. Penny, Trinh Thu Huong, Le Thi Nga, Edward Kumakech, Edward Mugisha
Disclosures
Mary E. Penny: Merck, Sharp, Dohme (RF); Astra Zeneca (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(suppl S):3/11–3/25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Sauvaget C, Fayette JM, Muwonge R, et al. Accuracy of visual inspection with acetic acid for cervical cancer screening. Int J Gynaecol Obstet. 2011;113:14–24. doi: 10.1016/j.ijgo.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Sankaranaryan R, Nessa A, Esmy PO, et al. Visual inspection methods for cervical cancer prevention. Best Pract Res Clin Obstet Gynaecol. 2012;26:221–232. doi: 10.1016/j.bpobgyn.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Sankaranarayanan R, Gaffikin L, Jacobs M, et al. A critical assessment of screening methods for cervical neoplasia. Int J Gynecol Obstet. 2005;89(suppl 2):4–12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Denny L, Kuhn L, De Souza M, et al. Screen-and-treat approaches for cervical cancer prevention in low-resource settings. JAMA. 2005;294:2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 7.Sankaranarayanan R, Shyamalakumary B, Wesley R, et al. Visual inspection with acetic acid in the early detection of cervical cancer and precursors. Int J Cancer. 1999;80:161–163. doi: 10.1002/(sici)1097-0215(19990105)80:1<161::aid-ijc28>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Belinson JL, Pretorius RG, Zhang WH, et al. Cervical cancer screening by simple visual inspection after acetic acid. Obstet Gynecol. 2001;98:441–444. doi: 10.1016/s0029-7844(01)01454-5. [DOI] [PubMed] [Google Scholar]
- 9.Megevand E, Denny L, Dehaeck K, et al. Acetic acid visualization of the cervix: An alternative to cytologic screening. Obstet Gynecol. 1996;88:383–386. doi: 10.1016/0029-7844(96)00189-5. [DOI] [PubMed] [Google Scholar]
- 10.Kitchener HC, Symonds P. Detection of cervical intraepithelial neoplasia in developing countries. Lancet. 1999;353:856–857. doi: 10.1016/s0140-6736(98)00417-6. [DOI] [PubMed] [Google Scholar]
- 11.Visual inspection with acetic acid for cervical-cancer screening: Test qualities in a primary-care setting. University of Zimbabwe/JHPIEGO Cervical Cancer Project. Lancet. 1999;353:869–873. [PubMed] [Google Scholar]
- 12.Phosngsavan K, Phenqsavanh A, Wahlstrom R, et al. Safety, feasibility and acceptability of visual inspection with acetic acid and immediate treatment with cryotherapy in rural Laos. Int J Gynecol Obstet. 2011;114:268–272. doi: 10.1016/j.ijgo.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Amhed T, Ashrafunnessa, Rahman J. Development of visual inspection programme for cervical cancer prevention in Bangladesh. Reprod Health Matters. 2008;16:78–85. doi: 10.1016/S0968-8080(08)32419-7. [DOI] [PubMed] [Google Scholar]
- 14.Sanghvi H, Limpaphayom KK, Plotkin M, et al. Cervical cancer screening using visual inspection with acetic acid: Operational experiences from Ghana and Thailand. Reprod Health Matters. 2008;16:67–77. doi: 10.1016/S0968-8080(08)32401-X. [DOI] [PubMed] [Google Scholar]
- 15.Agurto I, Bishop A, Sanchez G, et al. Perceived barriers and benefits to cervical cancer screening in Latin America. Prev Med. 2004;39:91–98. doi: 10.1016/j.ypmed.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Bradley J, Correy P, Arrossi S, et al. Women's perspectives on cervical screening and treatment in developing countries: Experiences with new technologies and service delivery strategies. Women Health. 2006;43:103–121. doi: 10.1300/J013v43n03_06. [DOI] [PubMed] [Google Scholar]
- 17.Ansink AC, Tolhurst R, Haque R, et al. Cervical cancer in Bangladesh: Community perceptions of cervical cancer and cervical cancer screening. Trans R Soc Trop Med Hyg. 2008;102:499–505. doi: 10.1016/j.trstmh.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Mtuyaba T, Faxelid E, Mirembe F, et al. Influences on uptake of reproductive health services in Nsangi community of Uganda and their implications for cervical cancer screening. Reprod Health. 2007;4:4. doi: 10.1186/1742-4755-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bingham A, Bishop A, Coffey P, et al. Factors affecting utilization of cervical cancer prevention service in low-resource settings. Salud Publica Mex. 2003;45(suppl 3):408–416. doi: 10.1590/s0036-36342003000900015. [DOI] [PubMed] [Google Scholar]
- 20.Lewis KD, Sellors JW, Dawa A, et al. Report on a cryotherapy service for women with cervical intraepithelial neoplasia in a district hospital in western Kenya. Afr Health Sci. 2011;11:370–376. [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell H, MacDonald S, McKiernan M. Promotion of cervical screening uptake by health visitor follow-up of women who repeatedly failed to attend. J Public Health Med. 1996;18:94–97. doi: 10.1093/oxfordjournals.pubmed.a024468. [DOI] [PubMed] [Google Scholar]
- 22.Aldana JM, Piechulek H, al-Sabir A. Client satisfaction and quality of health care in rural Bangladesh. Bull World Health Organ. 2001;79:512–517. [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob M, Broekhuizen FF, Castro W, et al. Experience using cryotherapy for treatment of cervical precancerous lesions in low-resource settings. Int J Gynecol Obstet. 2005;89(suppl 2):13–20. doi: 10.1016/j.ijgo.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 24.Gage JC, Rerreccio C, Gonzales M, et al. Follow-up care of women with an abnormal cytology in a low-resource setting. Cancer Prev Detect. 2003;27:466–471. doi: 10.1016/j.cdp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Gaffikin L, Blumenthal PD, Emerson M, et al. Safety, acceptability, and feasibility of a single visit approach to cervical cancer prevention in rural Thailand: A demonstration project. Lancet. 2003;361:814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- 26.Nene BM, Hiremath PS, Kane S, et al. Effectiveness, safety, and acceptability of cryotherapy by midwives for cervical intraepithelial neoplasia in Maharashtra, India. Int J Gynecol Obstet. 2008;103:232–236. doi: 10.1016/j.ijgo.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Sankaranarayanan R, Rajikumar R, Esmy PO, et al. Effectiveness, safety and acceptability of ‘see and treat’ with cryotherapy by nurses in a cervical screening study in India. Br J Cancer. 2007;96:738–743. doi: 10.1038/sj.bjc.6603633. [DOI] [PMC free article] [PubMed] [Google Scholar]


