Patients treated with low gonadotoxic therapies show clear impairment of their ovarian reserves over longer times when evaluated within few years from the end of the therapy and compared with age-matched controls. This study stresses the importance of accurate counseling at the time of diagnosis of cancer and emphasizes the risks of infertility with low gonadotoxic therapies that may reduce the reproductive window of survivors.
Keywords: Fertility preservation, Hematological malignancies, Anti-Müllerian hormone, Antral follicle count
Abstract
The impact of cancer therapy on the reproductive potential of patients is increasingly recognized because survival rates of patients have clearly improved in recent years. Different fertility preservation methods, either generally accepted or still experimental, are currently available, and counseling of patients requires a delicate balance between the efficacy and side effects of the proposed method and the characteristics of both the tumor and the therapy. Deeper knowledge of the effects of cancer therapy on the reproductive potential of patients over time is required to identify the most appropriate fertility preservation method. In this paper, we report a case-control study in which female patients who were diagnosed with hematological malignancies and treated with chemotherapy and/or radiotherapy were compared with age-matched controls in terms of ovarian reserve, as measured by ultrasound examination and hormonal status. By stratifying patients for gonadotoxicity of the therapy received and time elapsed from the end of the therapy, we report that patients treated with low gonadotoxic therapies, while being similar to age-matched controls in their ovarian reserve when evaluated within a few years from the end of the therapy, show a clear impairment over longer times. We also report that anti-Müllerian hormone is the most sensitive hormonal parameter in detecting changes in ovarian reserve when compared with follicle-stimulating hormone or inhibin-B. This study stresses the importance of accurate counseling at the time of diagnosis of cancer and emphasizes the risks of infertility with low gonadotoxic therapies that may reduce the reproductive window of survivors.
Implications for Practice:
The need for accurate and personalized counseling of women diagnosed with hematological malignancies and exposed to anticancer therapy in their reproductive age is the major clinical implication of our study. More specifically, our study implies that adequate fertility preservation methods should be discussed when therapies with a low toxicity to the reproductive system are used. Indeed, patients may experience impairment of their reproductive potential, even several years post-therapy, hampering their fertility wishes. Patients should undergo frequent evaluation of their fertility potential to detect eventual impairments in a timely manner.
Introduction
The relationship between cancer and patient fertility has been the object of extensive research in recent years as it became clear that cancer treatment should not be limited to the eradication of the tumor itself but also should aim to preserve the quality of life of the increasing percentages of survivors. Especially for tumors occurring during prepubertal or reproductive ages, quality of life is strongly related to the possibility of having children once the tumor is cured [1].
The presence of regular menstrual cycles after cancer therapy is not equivalent to normal fertility; even if menstrual cycles reappear, a premature ovarian failure (POF) might occur, leading to premature menopause [2]. It has become clear that the effects of chemotherapy and radiotherapy (RT) on gonadal function are strongly related to the type of drugs and/or radiation received (alkylating drugs, such as cyclophosphamide, and total body irradiation [TBI] being more damaging), the amount of drugs and/or radiation, the type of pathology, and the patient's age [3, 4]. The evaluation of the ovarian reserve in women exposed to gonadotoxic therapies is difficult because only limited data on the value of hormonal status and ultrasound examination in cancer survivors are currently available [5, 6].
Hematological malignancies compose a heterogeneous group of diseases with incidence rates of 24.5 per 100,000 for lymphoid malignancies and 7.55 per 100,000 for myeloid malignancies [7]. Although the incidence generally increases with age, Hodgkin lymphoma (HL), lymphoblastic lymphoma/acute lymphoblastic leukemia, and Burkitt lymphoma/leukemia peak in childhood or in the age range of 15–44 years [7]. Importantly, the survival rates of patients treated for hematological malignancies have clearly improved over the past 30 years, with 86% of patients surviving ≥5 years in the case of HL [8].
In a recent large retrospective study, patients in ongoing remission for at least 1 year after therapy for either early-stage or advanced-stage HL were evaluated for gonadal function and fertility [9]. As expected, the results showed that the intensity of chemotherapy correlated with hormonal parameters (anti-Müllerian hormone [AMH] and follicle-stimulating hormone [FSH]) and menstrual cycle and that age was a significant factor. Indeed, >90% of patients treated for early-stage HL reported a regular cycle after therapy, independent of age, whereas the presence of regular cycles dropped from 82% in patients younger than age 30 to 45% in older patients after treatment for advanced-stage HL. As an outcome of gonadal function and fertility, pregnancies were fewer in patients treated for advanced-stage HL than for early-stage HL. Although the results are reassuring for patients treated with less intensive chemotherapy, the mean time from the end of chemotherapy is 4 years (range: 1–8 years), and it is not clear how gonadal function and fertility change over longer times.
We performed a case-control study in patients treated for hematological malignancies and compared the ovarian reserve not only in relation to the intensity of the chemotherapy and RT received but also in relation to the time elapsed from the end of the therapy. The objective of this study is to provide more detailed information about the changes that occur in the ovarian reserve following different therapeutic regimens to help physicians better inform patients about the risks of infertility and the opportunity to pursue fertility preservation methods in a timely manner.
Materials and Methods
Patients and Study Design
Patients were identified from the patients' registry of the hematology department of Verona University Hospital. Inclusion criteria were female sex, hematological malignancy, aged <36 years at diagnosis and between 18 and 41 years at survey, in ongoing remission at least 1 year after therapy, and presence of both the uterus and the ovaries. Exclusion criteria were ovarian surgery, polycystic ovarian syndrome, and presence of secondary amenorrhea before therapy. Eligible patients were asked to participate in the study by the principal investigator (R.D.P.) through a telephone call. Written informed consent was obtained from all participants at the time of outpatient consultation at the department of life and reproduction sciences, section of obstetrics and gynecology, at Verona University Hospital.
Detailed data regarding demographics, type of hematological malignancy, stage, chemotherapy and/or RT, and dates of diagnosis and disease relapse were obtained from patients' medical records. Consent to the use of medical records for research purposes was obtained from all patients at the time of diagnosis, as part of the informed consent to treatment and according to the forms revised and approved by our institutional review board. Demographic characteristics of enrolled patients and treatment data are reported in Table 1. Controls were selected as follows: age between 18 and 41 years, presence of regular menstrual cycles (21–35 days), not using oral contraceptives at the time of survey and during the previous 6 months, and without a history of gonadal surgery and exposure to antitumoral drugs.
Table 1.
Patients and controls characteristics
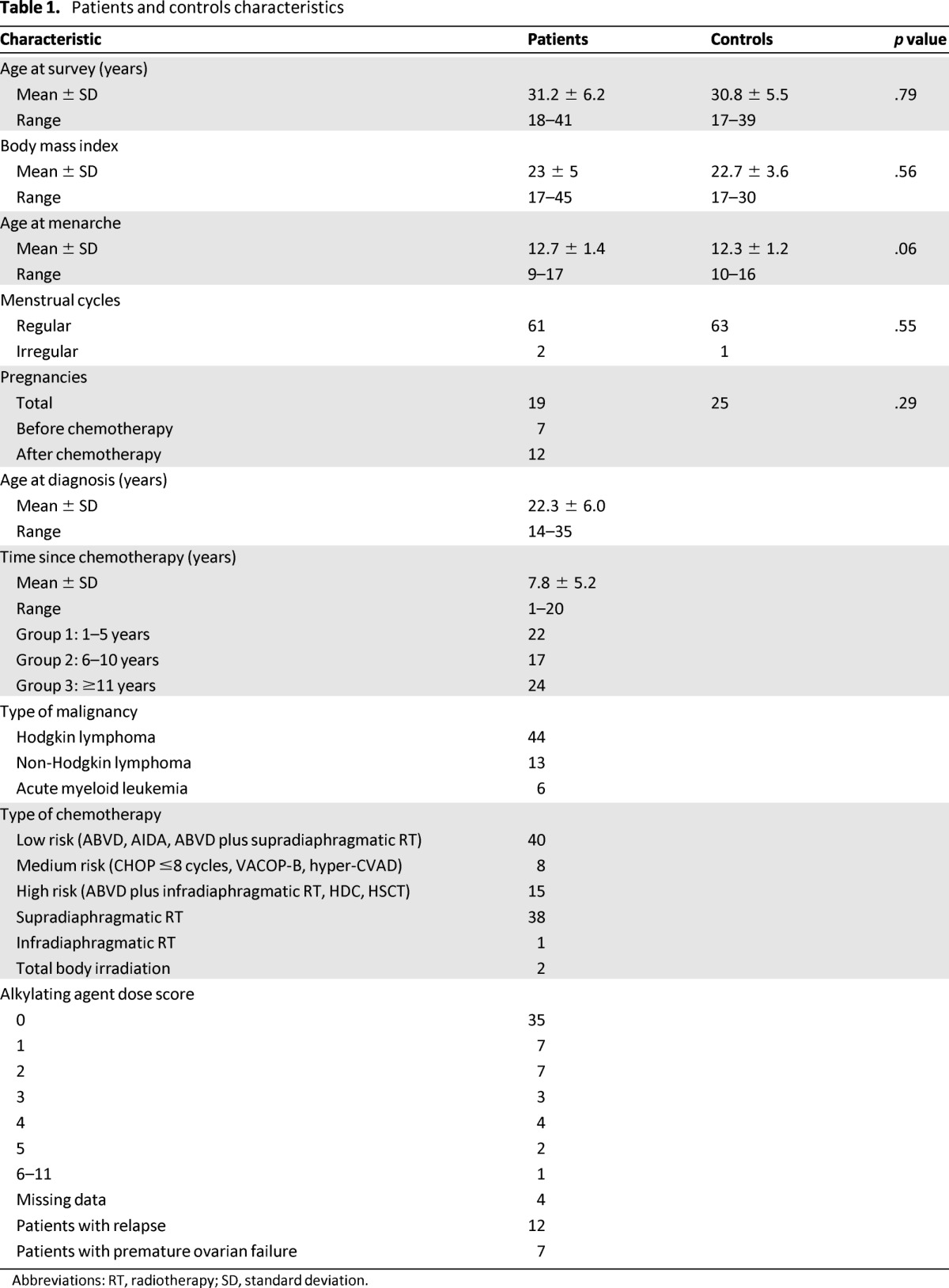
Abbreviations: RT, radiotherapy; SD, standard deviation.
Patients affected by acute myeloid leukemia (AML) underwent the AIDA regimen [10] if affected by AML-M3 (four cases) or cytarabin plus idarubicin (7 + 3 regimen) [11] plus high-dose Ara-C (two cycles) in case of other AML (two cases). Patients affected by HL underwent the ABVD regimen (Adriamycin, bleomycin, vinblastine, dacarbazine) [12] with or without involved-field RT (IF-RT) according to the stage of disease [13]. In particular, 9 patients received four ABVD cycles, 25 patients received six ABVD cycles, and 10 patients received eight ABVD cycles. Patients affected by high-grade non-HL (NHL) underwent the CHOP regimen (cyclophosphamide [Cytoxan], hydroxy doxorubicin [Adriamycin], vincristine [Oncovin], prednisone) [14] (three cases), rituximab-CHOP (one case), VACOP-B [15] (five cases), R-VACOP-B (two cases), and hyper-CVAD [16] (two cases).
Sequential high-dose chemotherapy [17] was administered to 12 relapsed HL or NHL patients. Two patients, one affected by AML/M5 and one affected by high-risk NHL, underwent allogeneic hematopoietic stem cell transplant.
The distribution of cumulative dose for each of the agents was divided into tertiles. Among patients exposed to an alkylating agent, the alkylating agent dose score was calculated by adding the tertile score for each of the alkylating agents given to a particular patient [18].
Supradiaphragmatic RT (median: 32 Gy; range: 18–45 Gy) was delivered to 38 patients, infradiaphragmatic RT (30 Gy) was delivered to 1 patient, and TBI (12 Gy) was administered to 2 patients as part of hematopoietic stem cell transplant conditioning.
Patient Evaluation
All patients went through a single evaluation performed, when possible, during the follicular phase. Gonadal function was assessed based on a complete clinical history (age at menarche, regularity of menstrual cycles, previous pregnancies and live births, fertility workup, and presence of menopausal symptoms), transvaginal ultrasound examination for antral follicle count (AFC), and measurement of hormone values (inhibin-B, FSH, and AMH).
Transvaginal Ultrasound Examination
Participants had a transvaginal ultrasound examination during the early follicular phase of the menstrual cycle, when possible, for AFC by using a Voluson 730 PRO ultrasound machine (GE Healthcare, Zipf, Austria, http://www.gehealthcare.com). All follicles between 2 mm and 10 mm in both ovaries were included in AFC.
Hormonal Measurement
Participants had a baseline blood drawing during the early follicular phase of the menstrual cycle, when possible, for AMH, FSH, and inhibin-B measurements. Serum levels of AMH and inhibin-B were determined using the AMH Gen II and the inhibin-B Gen II enzyme-linked immunosorbent assays (Beckman Coulter, Inc., Webster, TX, http://www.beckmancoulter.com), respectively, both assessed on a Triturus enzyme immunoassay analyzer (Grifols, Barcelona, Spain, http://www.grifols.com). FSH measurement was performed by using an automated chemiluminescence assay system (Immulite 2000; Siemens, Los Angeles, CA, http://www.medical.siemens.com).
Statistical Analysis
Statistical analysis was performed with PASW-SPSS Statistics v.18 (2009; IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/). Differences between groups were tested with the chi-square test and the Fisher's exact test (in 2 × 2 tables). Kolmogorov-Smirnov and Shapiro-Wilk tests were used to test for normality of variables, and significance was calculated by using either parametric (t test, ANOVA) or nonparametric (Wilcoxon-Mann-Whitney, Kruskal-Wallis) statistical analyses. Receiver operating characteristic curves were obtained to evaluate sensitivity and specificity of AMH and FSH in relation to AFC and to determine the related cutoff values. Statistical significance was achieved at p < .05.
Results
Overall, 112 survivors met the inclusion criteria and were contacted: 30 (27%) refused to participate to the study, 13 (12%) could not be reached, 4 (3%) were pregnant, and 2 (2%) were recently diagnosed with a relapse. A total of 63 survivors (56%; age range: 18–41 years) were included in the study. As controls, 64 women of reproductive age and without any previous exposure to antitumoral therapies were also recruited. Mean age at survey was 31 years, and the difference with the mean age of controls was not significant (p = .79). Among the survivors, menstrual cycles were regular before cancer therapy in 61 cases (96%); mean age at menarche was 12.7 years; mean body mass index was 23; and the mean number of total pregnancies was 19, of which 7 (37%) occurred before chemotherapy at a mean age of 23 years and 12 (63%) occurred after chemotherapy, at a mean age of 29 years. All of these characteristics were not significantly different between survivors and controls (Table 1).
Forty-four survivors (69.9%) were diagnosed with HL, 13 (20.6%) were diagnosed with NHL, and 6 (9.5%) were diagnosed with AML. At survey, all survivors were in complete remission. Seven survivors (11%) were in amenorrhea secondary to POF and were using hormone replacement therapy; their mean age was 31 years, with a mean time since chemotherapy of 9 years. Survivors were stratified based on time since chemotherapy and gonadotoxicity of the therapy (Table 1). There were 22 survivors (35%) between 1 year and 5 years since chemotherapy, 17 (27%) between 6 and 10 years since chemotherapy, and 24 (38%) more than 10 years since chemotherapy at the time of the evaluation. In terms of gonadotoxicity of the therapy, 40 survivors (63%) received a low-toxicity therapy, 8 (13%) received a medium-toxicity therapy, and 15 (24%) received a high-toxicity therapy. Twelve survivors (19%) had a relapse, and two of them received TBI. Patient characteristics are reported in Table 1.
AMH, FSH, inhibin-B, and AFC values were compared between cases and controls. As expected, controls showed a significant correlation between AMH and AFC values and age. Cases and controls were significantly different for AFC and hormone values (mean AFC: 9.8 vs. 16.0 [p = .0001; Fig. 1A]; mean AMH: 2.02 vs. 2.97 ng/mL [p = .02; Fig. 1B]; mean FSH: 16.9 vs. 8.1 μ/mL [p = .03; Fig. 1C]; inhibin-B: 33.7 vs. 69.4 ng/L [p < .0001; data not shown]).
Figure 1.
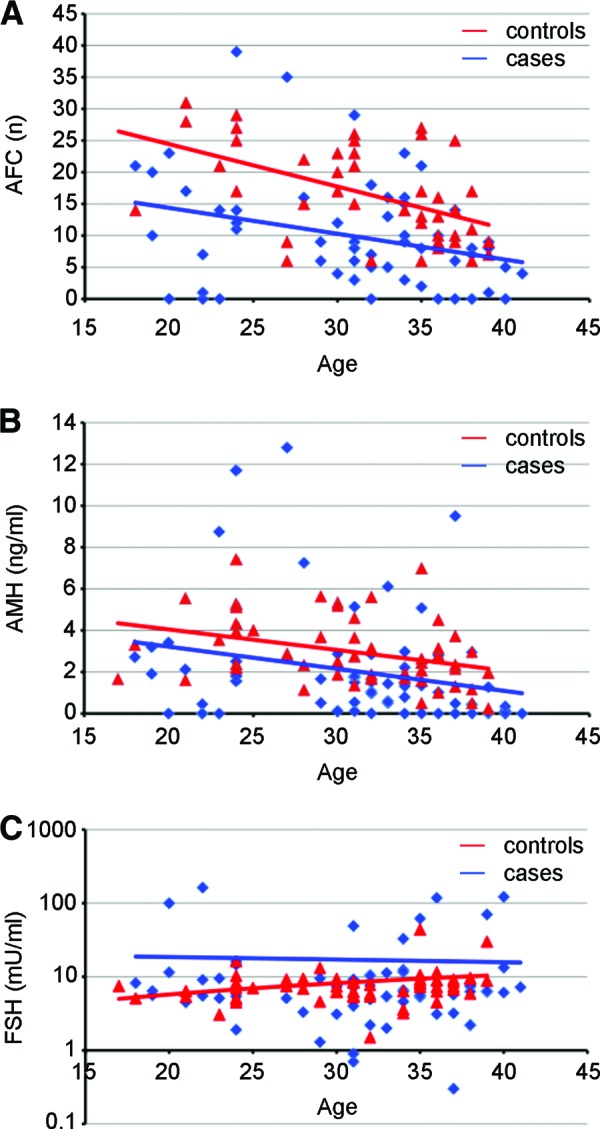
Gonadotoxic therapies affect AFC and hormonal status. Patients treated for hematological malignancies (blue) and normal controls (red) were evaluated for levels of AFC (A), AMH (B), and FSH (C). Triangles stand for controls, rhombuses represents cases. Solid lines represent linear regression of data.
Abbreviations: AFC, antral follicle count; AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone.
A linear regression was calculated for either AMH or FSH and AFC in patients. The predictive value of each hormone with respect to AFC was significant (AMH: p < .001 [Fig. 2]; FSH: p = .003 [data not shown]). After correction for age, AMH showed a stronger association than FSH in a correlation test (r = 0.78 [p < .0001] vs. r = −.4045 [p = .001]).
Figure 2.
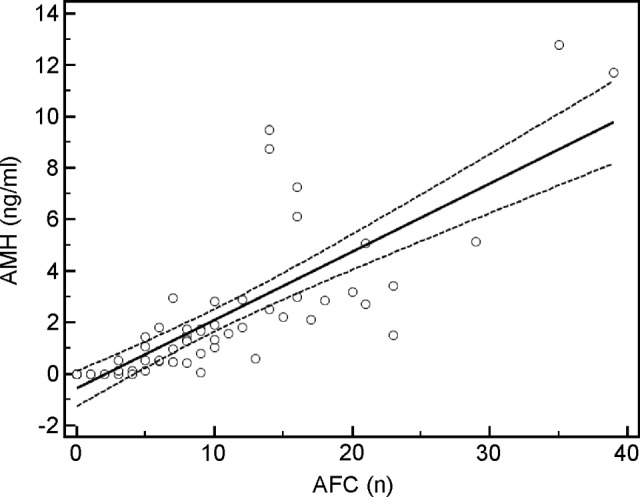
AFC and AMH levels in patients are correlated. Patients who had undergone gonadotoxic therapies for hematological malignancies were evaluated for AFC and AMH levels. Solid line represents linear regression of data. Dashed lines represent 95% confidence interval.
Abbreviations: AFC, antral follicle count; AMH, anti-Müllerian hormone.
When restricted to patients with normal FSH values (<10 UI/L), the median for AMH and AFC was significantly different between cases and controls with the Mann-Whitney test (AMH: 1.5 vs. 2.7 ng/mL [p = .0005]; AFC: 9 vs. 15 [p = .0005]) (Fig. 3).
Figure 3.
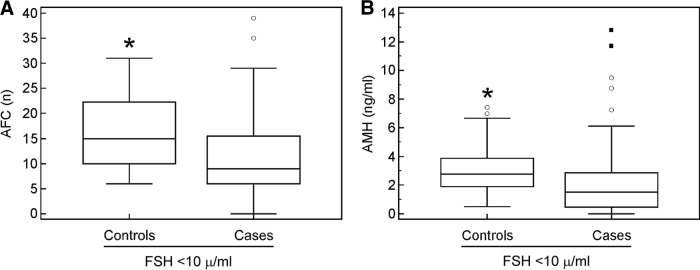
Gonadotoxic therapies affect AFC and AMH levels regardless of normal FSH values. Patients and controls with FSH values <10 μ/mL were evaluated for levels of AFC (A) and AMH (B), with box plots showing interquartile range, whiskers, and maximum and minimum outliers.
Abbreviations: *, p = .0005; AFC, antral follicle count; AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone.
AFC and AMH values were compared with an analysis of covariance test after adjustment for age of survivors according to the gonadotoxicity of the therapy received. Coefficient of determination for AFC was 0.3233 (p < .0001 between high and low toxicity; p = .0045 between high and medium toxicity; not significant between medium and low toxicity). Coefficient of determination for AMH was 0.1919 with p = .008 between high and low toxicity. The difference did not reach statistical significance between high and medium toxicity, likely because of the small number of cases (p = .29) (Fig. 4).
Figure 4.
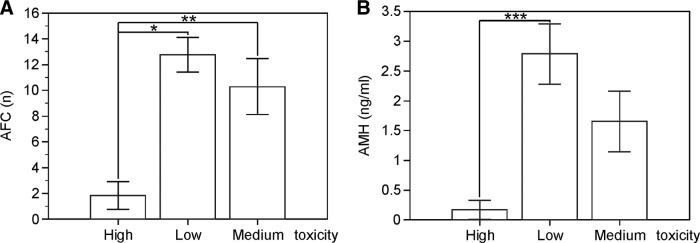
The degree of gonadotoxicity of the therapy influences AFC and AMH levels. Patients exposed to low, medium, or high gonadotoxic therapies were evaluated for levels of AFC (A) and AMH (B). Values are mean plus or minus standard error of the mean.
Abbreviations: *, p < .0001; **, p < .005; ***, p < .01; AFC, antral follicle count; AMH, anti-Müllerian hormone.
Receiver operating characteristic curves were calculated for AMH and FSH when AFC <8, the latter being the mean value in the AFC nomogram [19]. Area under the curve was 0.904 for AMH and 0.678 for FSH, and the difference was statistically significant (p = .0013) (Fig. 5).
Figure 5.
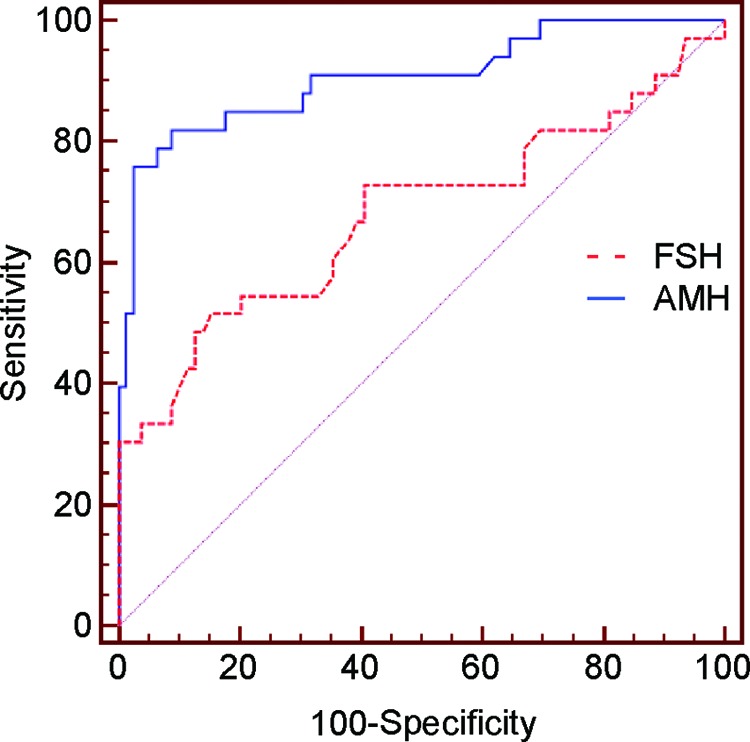
AMH has a higher sensitivity and specificity than FSH. Patients who underwent gonadotoxic therapies for hematological malignancies with antral follicle count <8 were evaluated for AMH and FSH values, and receiver operating characteristic curves were calculated. The areas under the curves are 0.904 for AMH and 0.678 for FSH.
Abbreviations: AMH, anti-Müllerian hormone; FSH, follicle-stimulating hormone.
AFC, AMH, and FSH values were compared among survivors according to the years elapsed between the end of the chemotherapy and the time of the survey (small gap: 1–5 years; medium gap: 6–10 years; large gap: ≥11 years). The difference between cases and controls was significantly different for AMH in the large-gap group (p = .0003) but not in the small-gap group (p = .94). In the medium-gap group, the difference did not reach statistical significance, despite being close, likely because of the small number of cases (p = .06). The difference between cases and controls was significantly different for AFC in the large- and medium-gap groups (p = .0001 and p = .0005, respectively) but not in the small-gap group (p = .18). FSH values were not significantly different between cases and controls in all of the groups (p = .22, p = .30, and p = .16 in the large-, medium-, and small-gap groups, respectively) (Table 2).
Table 2.
Hormonal parameters and antral follicle count in relation to the gonadotoxicity of the therapeutic regimen and the time since therapy
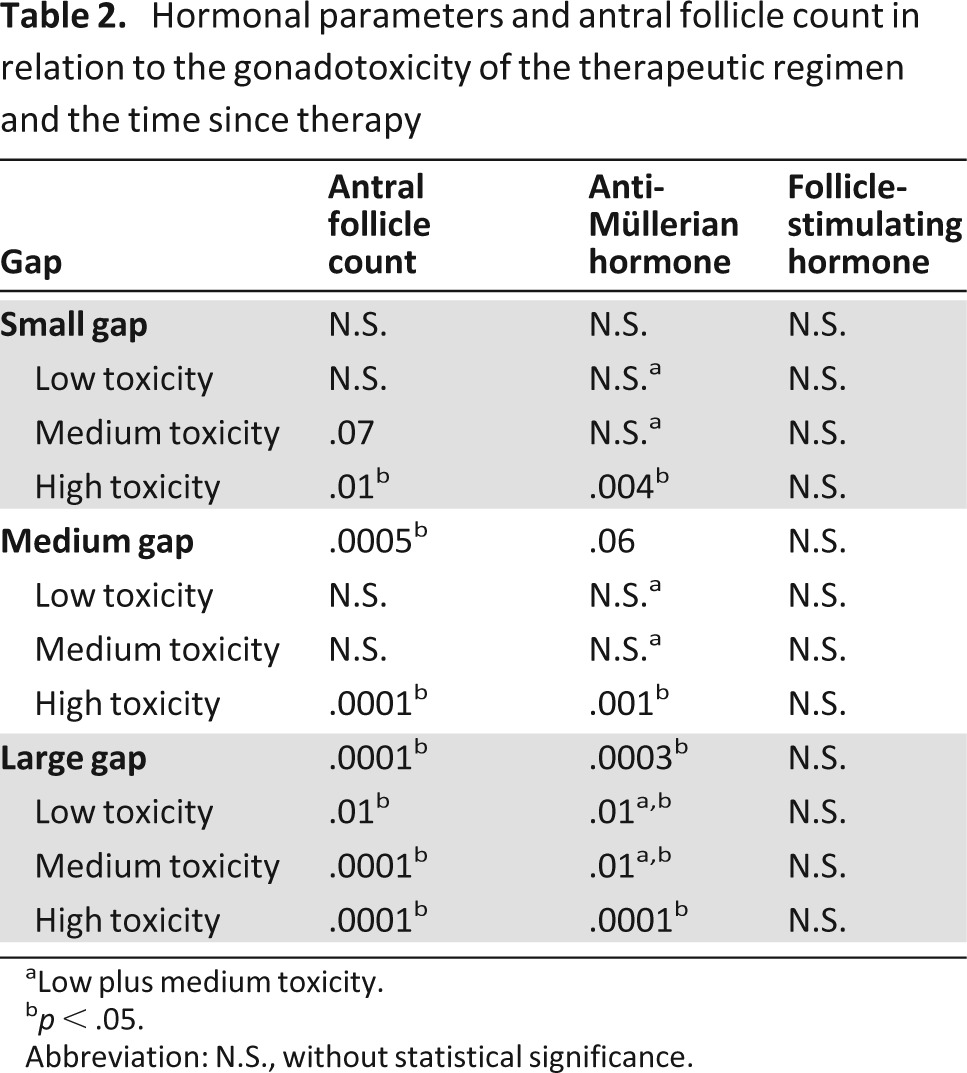
aLow plus medium toxicity.
bp < .05.
Abbreviation: N.S., without statistical significance.
Within each gap group, survivors were compared with controls according to the gonadotoxicity of the therapy received. In the case of low and medium toxicity, AMH values were similar to controls in the small- and medium-gap groups (p = .45 and p = .60, respectively) but were significantly reduced in the large-gap group (p = .01). In the case of high toxicity, AMH values were significantly different from controls in all of the groups (p < .0001, p < .001, and p = .004 in the large-, medium-, and small-gap groups, respectively). Because the high-toxicity group also included three patients who received RT to the abdomen and pelvis (one received 30 Gy infradiaphragmatically, two received 12 Gy to ovaries as a result of TBI) with a lethal dose to ovaries, we repeated the statistical analysis after exclusion of these patients and obtained similar results (data not shown).
Similar to AMH, AFC values were significantly reduced in the large-gap group (low toxicity: p = .01; medium toxicity: p < .0001) but not in the small- and medium-gap groups in the case of low and medium toxicity (low toxicity: p = .83 and p = .07, respectively; medium toxicity: p = .45 and p = .16, respectively) and were significantly different in all of the gap groups in the case of high toxicity (p < .0001, p = .0001, and p = .0015 in large-, medium-, and small-gap groups, respectively) (Table 2). FSH values were not significantly different from controls within each gap group independent of the toxicity of the therapy received (Table 2).
Discussion
The risk for infertility secondary to chemotherapy and RT should be clearly discussed with oncological patients at the time of diagnosis [4, 20–22]. Notably, patients treated with the same chemotherapy regimen show an increased incidence of POF starting from 30 years of age [4, 23]. Another major role is played by the presence of alkylating drugs. Meirow et al. observed a 42% incidence of early menopause after therapies in which alkylating agents were used [24]. The therapeutic regimen ABVD, widely used as the initial chemotherapy treatment for HL, has <10% incidence of POF, especially in patients younger than 25 years of age [25]. In a cohort study including 518 women aged 14–40 years at the time of diagnosis with HL and evaluated after at least 5 years since the end of the treatment, the presence of alkylating drugs, especially cyclophosphamide and procarbazine, showed the highest correlation with POF. After 10 years, the incidence of POF was 64% and 15% with high and low doses, respectively. The therapeutic regimens MOPP (mechlorethamine [Mustargen], vincristine [Oncovin], procarbazine, prednisone) or ChlVPP (chlorambucil, vincristine [Oncovin], procarbazine, prednisone) for HL cause ovarian dysfunction in 19%–63% of patients. Amenorrhea is common in older women, but young women can experience early menopause [26]. The therapeutic regimen BEACOPP (bleomycin, etoposide, doxorubicin [Adriamycin], cyclophosphamide, vincristine [Oncovin], procarbazine, prednisone) causes amenorrhea in 20% of cases, increasing to 67% with eight cycles, and the correlation is dependent on age, with an incidence of amenorrhea of 95% in patients older than 30 years of age and 51% in the younger patients [27]. Regimens with high doses of chemotherapeutics and RT, especially TBI before stem cell transplantation, cause POF in 70%–100% of cases [21, 28]. Recovery of ovarian function could be observed in only 10%–14% of patients, with pregnancy rates <3% [29–32].
The impact of chemotherapy on ovarian reserve is twofold: first, a rapid depletion of growing follicles and amenorrhea occur during treatment; second, a late loss of primordial follicles may cause POF. When the loss of primordial follicles is partial, this long-term effect will become evident only after several years following the end of the treatment. The function of the ovary is impaired according to the “two-hits” model described in a prospective study by Petrek et al. [2]. Several studies have evaluated ovarian damage after antitumor therapies, with the recovery of menstrual cycles after transient amenorrhea as main outcome for gonadal function; however, the recovery of regular menstrual cycles is not equivalent to normal fertility, even if long-lasting amenorrhea is a good predictor of infertility. Accurate evaluation of ovarian reserve is essential to estimate the risk for therapy-induced gonadal damage and to identify the best criteria for fertility preservation.
AMH is secreted by small and large preantral follicles and therefore is representative of the gonadotropin-independent pool of follicles. For this reason, AMH decreases with age and, unlike FSH and inhibin-B, has little intra- and intercycle variation. Several studies have proved the accuracy of AMH, currently considered the best marker for the pool of primordial follicles [33–36]. Recent prospective studies have clearly established that AMH can usefully detect chemotherapy-induced ovarian damage in patients treated for cancer; however, whether AMH can predict the ovarian reserve over the long term is still unclear [21].
In our study, patients treated for hematological malignancies had lower AMH, AFC, and inhibin-B and higher FSH values than age-matched controls. When the comparison was performed based on the time elapsed from the end of the therapy, AMH and AFC were significantly lower when the gap was >10 years, and AFC was also lower when the gap was between 5 and 10 years. In contrast, FSH did not show any significant correlation with the time elapsed from the end of the therapy. Our results have shown that AMH and AFC in survivors were significantly lower after 10 years of treatment (in the case of AFC, the decrease was evident even after 5 years) but similar to controls in the short-term period. These results are in line with the literature; indeed, the recovery of ovarian function after the initial damage induced by chemotherapy is stable up to 5–10 years following chemotherapy, and a progressive decline initiates thereafter, leading to a premature depletion of ovarian function. In other words, patients treated with chemotherapy have a shorter reproductive window, showing late iatrogenic gonadal damage. In particular, in our study, this result was evident in the most numerous group of patients that received therapies considered to have low gonadotoxicity and with an alkylating agent dose score ranging from 0 to 1. Consequently, it is important to discuss methods for fertility preservation, even with patients without any particular risk factor. During the first 5 years after the therapy, AMH and AFC were not significantly different from controls; after 5 years and 10 years for AFC and AMH, respectively, significant differences between cases and controls could be observed. When the toxicity of the therapy was also considered, AFC and AMH were significantly lower in patients who received low- or medium-gonadotoxic therapy with respect to controls but only 10 years after the therapy. In patients who received therapy with high toxicity, both AMH and AFC were significantly lower than controls independent of the time elapsed from the end of the therapy. In the latter patients, AMH could not be detected in serum and ovaries did not contain any follicles. These patients were in hormone replacement therapy and had amenorrhea. In the case of FSH, no significant differences could be detected between patients and controls, regardless of both the time elapsed since the end of the chemotherapy and the degree of toxicity of the therapy received.
Several studies have shown the higher benefit of AMH and AFC in predicting the ovarian reserve with respect to age, even in the general population. A recent study by Gracia et al. evaluating women of reproductive age could show that the sensitivity of AMH and AFC was higher in the quantification of chemotherapy-induced ovarian damage [37].
In our study, the numbers of pregnancies for cases and controls were not significantly different. In addition, three spontaneous pregnancies occurred in patients with low AMH values (<0.5 ng/mL) measured 2 years after delivery. Thus the reduction of the number of primordial follicles probably does not imply infertility in terms of oocyte quality but acts on the biological age of women, making it greater than actual chronological age. A limitation of our study is the absence of pretreatment values for markers of ovarian reserve; a prospective study is currently ongoing to fill this gap. Another limitation is the limited number of patients treated with a therapeutic regimen of medium toxicity, although the homogeneity between type of pathology and therapeutic regimen has allowed the evaluation of the gonadotoxicity in the context of similar chemotherapeutic regimens. Finally, based on a recent study by Mazonakis et al. [38] evaluating the risk of hereditary effects in women undergoing therapeutic irradiation, supradiaphragmatic RT (received by 60% of our patients in addition to chemotherapy) implies a radiation dose up to 25 centigrays to the ovaries. Although 25 centigrays is beneath the lethal ovarian dose [39], and data from the literature seem to exclude gonadal toxicity in patients treated with traditionally viewed low-gonadotoxic regimens such as ABVD plus supradiaphragmatic RT [40], no studies have prospectively established whether such a combination can actually be considered more damaging to the ovaries.
Conclusion
The depletion of primordial follicles can explain the different behaviors of the parameters used to evaluate the ovarian reserve. This depletion is not followed by a long-term recovery, as shown by the differences in the AMH and AFC values, and is predictive of the shorter reproductive window of the patients; interestingly, this is also evident in patients who underwent low-gonadotoxic therapies at 10 years after the end of the therapy. In women with normal FSH values, AMH and AFC were significantly lower in patients with respect to controls, as expected by the depletion of the chemosensitive follicles, which correspond to the primordial follicles. Most women with cancer present with concerns about future fertility; the optimal approach to fertility preservation must be individualized, and management requires multidisciplinary collaboration between oncologists and assisted reproductive techniques teams.
Acknowledgments
C.C. is a recipient of a fellowship in gynecology and reproductive medicine from the Fondazione Cesare Serono.
Author Contributions
Conception/Design: Rossana Di Paola, Claudio Costantini, Cristina Tecchio, Massimo Franchi
Provision of study material or patients: Cristina Tecchio, Gian Luca Salvagno, Rachele Montemezzi, Giovanni Pizzolo
Collection and/or assembly of data: Rossana Di Paola, Claudio Costantini, Cristina Tecchio, Rachele Montemezzi, Alessio Perandini
Data analysis and interpretation: Rossana Di Paola, Claudio Costantini, Alessio Perandini, Stefano Zaffagnini, Massimo Franchi
Manuscript writing: Rossana Di Paola, Claudio Costantini, Massimo Franchi
Final approval of manuscript: Rossana Di Paola, Claudio Costantini, Cristina Tecchio, Gian Luca Salvagno, Rachele Montemezzi, Alessio Perandini, Giovanni Pizzolo, Stefano Zaffagnini, Massimo Franchi
Disclosures
The authors indicated no financial relationships.
References
- 1.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 2.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D, Schiff E. Appraisal of chemotherapy effects on reproductive outcome according to animal studies and clinical data. J Natl Cancer Inst Monogr. 2005:21–25. doi: 10.1093/jncimonographs/lgi025. [DOI] [PubMed] [Google Scholar]
- 4.Meirow D, Biederman H, Anderson RA, et al. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 5.Buyuk E, Seifer DB, Younger J, et al. Random anti-Mullerian hormone (AMH) is a predictor of ovarian response in women with elevated baseline early follicular follicle-stimulating hormone levels. Fertil Steril. 2011;95:2369–2372. doi: 10.1016/j.fertnstert.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 6.Bath LE, Wallace WH, Shaw MP, et al. Depletion of ovarian reserve in young women after treatment for cancer in childhood: Detection by anti-Mullerian hormone, inhibin B and ovarian ultrasound. Hum Reprod. 2003;18:2368–2374. doi: 10.1093/humrep/deg473. [DOI] [PubMed] [Google Scholar]
- 7.Sant M, Allemani C, Tereanu C, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: Results of the HAEMACARE project. Blood. 2010;116:3724–3734. doi: 10.1182/blood-2010-05-282632. [DOI] [PubMed] [Google Scholar]
- 8.Lichtman MA. Battling the hematological malignancies: The 200 years' war. The Oncologist. 2008;13:126–138. doi: 10.1634/theoncologist.2007-0228. [DOI] [PubMed] [Google Scholar]
- 9.Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31:231–239. doi: 10.1200/JCO.2012.44.3721. [DOI] [PubMed] [Google Scholar]
- 10.Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: Results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010;116:3171–3179. doi: 10.1182/blood-2010-03-276196. [DOI] [PubMed] [Google Scholar]
- 11.Dillman RO, Davis RB, Green MR, et al. A comparative study of two different doses of cytarabine for acute myeloid leukemia: A phase III trial of Cancer and Leukemia Group B. Blood. 1991;78:2520–2526. [PubMed] [Google Scholar]
- 12.Santoro A, Bonfante V, Bonadonna G. Salvage chemotherapy with ABVD in MOPP-resistant Hodgkin's disease. Ann Intern Med. 1982;96:139–143. doi: 10.7326/0003-4819-96-2-139. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:589–597. doi: 10.6004/jnccn.2012.0061. [DOI] [PubMed] [Google Scholar]
- 14.Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993;328:1002–1006. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly SE, Hoskins P, Klimo P, et al. MACOP-B and VACOP-B in diffuse large cell lymphomas and MOPP/ABV in Hodgkin's disease. Ann Oncol. 1991;2(suppl 1):17–23. doi: 10.1093/annonc/2.suppl_1.17. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DA, Cortes J, O'Brien S, et al. Hyper-CVAD program in Burkitt's-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17:2461–2470. doi: 10.1200/JCO.1999.17.8.2461. [DOI] [PubMed] [Google Scholar]
- 17.Gianni AM, Siena S, Bregni M, et al. High-dose sequential chemo-radiotherapy with peripheral blood progenitor cell support for relapsed or refractory Hodgkin's disease—a 6-year update. Ann Oncol. 1993;4:889–891. doi: 10.1093/oxfordjournals.annonc.a058399. [DOI] [PubMed] [Google Scholar]
- 18.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Marca A, Spada E, Sighinolfi G, et al. Age-specific nomogram for the decline in antral follicle count throughout the reproductive period. Fertil Steril. 2011;95:684–688. doi: 10.1016/j.fertnstert.2010.07.1069. [DOI] [PubMed] [Google Scholar]
- 20.Meirow D, Dor J. Epidemiology and infertility in cancer patients. In: Tulandi T, Gosden R, editors. Preservation of fertility. London, UK: Taylor & Francis; 2004. pp. 21–38. [Google Scholar]
- 21.Donnez J, Kim SS. Principles and practice of fertility preservation. Cambridge, UK: Cambridge University Press; 2011. xviii,517. [Google Scholar]
- 22.Harel S, Ferme C, Poirot C. Management of fertility in patients treated for Hodgkin's lymphoma. Haematologica. 2011;96:1692–1699. doi: 10.3324/haematol.2011.045856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faddy MJ, Gosden RG. A mathematical model of follicle dynamics in the human ovary. Hum Reprod. 1995;10:770–775. doi: 10.1093/oxfordjournals.humrep.a136036. [DOI] [PubMed] [Google Scholar]
- 24.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–131. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 25.Decanter C, Morschhauser F, Pigny P, et al. Anti-Mullerian hormone follow-up in young women treated by chemotherapy for lymphoma: Preliminary results. Reprod Biomed Online. 2010;20:280–285. doi: 10.1016/j.rbmo.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Behringer K, Breuer K, Reineke T, et al. Secondary amenorrhea after Hodgkin's lymphoma is influenced by age at treatment, stage of disease, chemotherapy regimen, and the use of oral contraceptives during therapy: A report from the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2005;23:7555–7564. doi: 10.1200/JCO.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 27.Elis A, Tevet A, Yerushalmi R, et al. Fertility status among women treated for aggressive non-Hodgkin's lymphoma. Leuk Lymphoma. 2006;47:623–627. doi: 10.1080/10428190500353877. [DOI] [PubMed] [Google Scholar]
- 28.Jadoul P, Donnez J. How does bone marrow transplantation affect ovarian function and fertility? Curr Opin Obstet Gynecol. 2012;24:164–171. doi: 10.1097/GCO.0b013e328353bb57. [DOI] [PubMed] [Google Scholar]
- 29.Sanders JE, Hawley J, Levy W, et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood. 1996;87:3045–3052. [PubMed] [Google Scholar]
- 30.Salooja N, Szydlo RM, Socie G, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: A retrospective survey. Lancet. 2001;358:271–276. doi: 10.1016/s0140-6736(01)05482-4. [DOI] [PubMed] [Google Scholar]
- 31.Spinelli S, Chiodi S, Bacigalupo A, et al. Ovarian recovery after total body irradiation and allogeneic bone marrow transplantation: Long-term follow up of 79 females. Bone Marrow Transplant. 1994;14:373–380. [PubMed] [Google Scholar]
- 32.Brice P, Haioun C, Andre M, et al. Pregnancies after high-dose chemotherapy and autologous stem cell transplantation in aggressive lymphomas. Blood. 2002;100:736. doi: 10.1182/blood.v100.2.736. [DOI] [PubMed] [Google Scholar]
- 33.Weenen C, Laven JS, Von Bergh AR, et al. Anti-Mullerian hormone expression pattern in the human ovary: Potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 34.van Rooij IA, Tonkelaar I, Broekmans FJ, et al. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–606. doi: 10.1097/01.gme.0000123642.76105.6e. [DOI] [PubMed] [Google Scholar]
- 35.Nelson SM, Yates RW, Fleming R. Serum anti-Mullerian hormone and FSH: Prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22:2414–2421. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 36.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 37.Gracia CR, Sammel MD, Freeman E, et al. Impact of cancer therapies on ovarian reserve. Fertil Steril. 2012;97:134–140.e1. doi: 10.1016/j.fertnstert.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazonakis M, Damilakis J, Varveris H, et al. Therapeutic external irradiation in women of reproductive age: Risk estimation of hereditary effects. Br J Radiol. 2004;77:847–850. doi: 10.1259/bjr/88840344. [DOI] [PubMed] [Google Scholar]
- 39.Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod. 2003;18:117–121. doi: 10.1093/humrep/deg016. [DOI] [PubMed] [Google Scholar]
- 40.Brusamolino E, Baio A, Orlandi E, et al. Long-term events in adult patients with clinical stage IA-IIA nonbulky Hodgkin's lymphoma treated with four cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine and adjuvant radiotherapy: A single-institution 15-year follow-up. Clin Cancer Res. 2006;12:6487–6493. doi: 10.1158/1078-0432.CCR-06-1420. [DOI] [PubMed] [Google Scholar]


