The occurrence of malignant disease increases the risk for venous thromboembolism (VTE). This study sought to evaluate the risk for VTE in a large unselected cohort of patients with cancer receiving chemotherapy. Those undergoing chemotherapy as outpatients are at high risk for VTE and for major bleeding complications, and thromboprophylaxis should be considered for these patients.
Keywords: Cancer, Chemotherapy, Health care costs, Thromboprophylaxis, Venous thromboembolism
Abstract
Introduction.
The occurrence of malignant disease increases the risk for venous thromboembolism (VTE). Here we evaluate the risk for VTE in a large unselected cohort of patients with cancer receiving chemotherapy.
Methods.
The United States IMPACT health care claims database was retrospectively analyzed to identify patients with a range of solid tumors who started chemotherapy from January 2005 through December 2008. International Classification of Diseases, 9th revision, Clinical Modification Codes were used to identify cancer location, presence of VTE 3.5 months and 12 months after starting chemotherapy, and incidence of major bleeding complications. Health care costs were assessed one year before initiation of chemotherapy and one year after initiation of chemotherapy.
Results.
The overall incidence of VTE 3.5 months after starting chemotherapy was 7.3% (range 4.6%–11.6% across cancer locations) rising to 13.5% at 12 months (range 9.8%–21.3%). The highest VTE risk was identified in patients with pancreatic, stomach, and lung cancer. Patients in whom VTE developed had a higher risk for major bleeding at 3.5 months and at 12 months (11.0% and 19.8% vs. 3.8% and 9.6%, respectively). Health care costs were significantly higher in patients in whom VTE developed.
Conclusion.
Those undergoing chemotherapy as outpatients are at increased risk for VTE and for major bleeding complications. Thromboprophylaxis may be considered for such patients after carefully assessing the risks and benefits of treatment.
Implications for Practice:
This large observational study of unselected patients receiving cancer chemotherapy demonstrates considerably greater rates of venous thromboembolism (VTE) than commonly reported in patients accrued to clinical trials. The risk of VTE appears to increase progressively over the year following initiation of treatment. Cancer patients developing VTE also experience a greater risk of major bleeding and greater health care costs than patients without VTE. Patients considered at high risk for VTE should be considered for thromboprophylaxis after assessing the balance of potential benefits and harms.
Introduction
Malignant disease is associated with a hypercoagulable state that increases the risk for development of venous thromboembolism (VTE) by at least 4-fold [1–3]. The risk for VTE varies according to other factors, including age, obesity, history of thrombosis, recent reduced mobility, or major surgery [4]. The type of cancer treatment also has an important impact on the development of VTE, with chemotherapy increasing the VTE risk by up to 6.5-fold [1, 5]. In addition, the VTE risk associated with cancer varies throughout the course of the disease. The risk is particularly high in the first few months following diagnosis and there is an estimated 4-fold to 13-fold increase in risk for VTE in late-stage metastatic disease [5–7]. VTE incidences ranging from 2% to 12% have been reported in different populations of patients with cancer [5, 7–10]. Types of tumor associated with the highest VTE risk are hematologic cancers, followed by lung, pancreatic, stomach, ovarian, uterine, bladder, and brain tumors [3, 5–7].
It is therefore anticipated that a subset of patients with cancer who have additional VTE risk factor(s) and are undergoing chemotherapy are at high risk for VTE. Thromboprophylaxis use is sporadic or not routine, and patients with cancer are usually treated with curative doses when symptomatic VTE events occur. Patients with cancer are more likely to have recurrent thromboembolic complications and major bleeding during anticoagulant treatment than those without malignancy [11]. However, United States and European guidelines do not recommend routine thromboprophylaxis for ambulatory patients receiving chemotherapy on an outpatient basis [12–15]. Recently revised guidelines on VTE prevention in oncology from the United States National Comprehensive Cancer Network [13, 14] and from the American Society of Clinical Oncology [16] suggest that thromboprophylaxis should be considered for high-risk ambulatory patients with cancer receiving chemotherapy.
This retrospective study aimed to evaluate the risk for VTE in an unselected cohort of patients from the United States IMPACT health care insurance claims database with high-risk solid tumors who were starting chemotherapy treatment. Secondary objectives were the assessment of the risk for bleeding and the economic burden of VTE in this patient cohort.
Data Source and Study Design
The IMPACT claims database is a fully de-identified, United States Health Insurance Portability and Accountability Act-compliant national database that includes the complete medical history for >100 million individuals with managed care health plans. The IMPACT database was used to identify patients retrospectively who had cancer of the lung, pancreas, stomach, colon/rectum, bladder, or ovary who initiated chemotherapy between January 1, 2005, and December 31, 2008. Because the claims data used were fully de-identified, approval from a local human investigations committee was not required.
International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) codes were used to identify cancer location and the presence of VTE (supplemental online Table 1) and bleeding (supplemental online Table 2). To reduce the effects of diagnostic imprecision associated with VTE diagnosis based on ICD-9-CM codes [17], the analyses of VTE incidence were performed using three different definitions. Definition A was an all-inclusive analysis comprising all individuals with an ICD-9-CM code relating to VTE who met the inclusion and exclusion criteria for the study (i.e., study period, tumor location). This definition considered a VTE case as a patient who had ≥1 VTE claim. Definition B considered a VTE case as only patients who had ≥2 outpatient claims ≥30 days apart, or one inpatient claim, or a single outpatient claim in which an anticoagulant was administered within 90 days, and excluded the ICD-9-CM code 997.2 (peripheral vascular complications not elsewhere classified). The most stringent definition, definition C, further excluded from definition B any VTE events within 90 days of any major or invasive surgery. Codes relating to esophageal, renal, and uterine cancer were excluded.
The first day of chemotherapy treatment following cancer diagnosis was defined as the index date, and data from patients with ≥12 months of continuous medical coverage prior to the index date (baseline period) and ≥3.5 months of medical insurance coverage during follow-up were included. Patients with a history of VTE within 12 months, major bleeding within 3 months, or anticoagulant treatment within 2 weeks prior to the index date were excluded.
Statistical Analysis
Patient baseline characteristics were determined during the one year prior to the index date to determine any prior relevant comorbidities or medication use. The Charlson Comorbidity Index score [18] was used to assess the overall burden of comorbidity. The index assigns a weighted score of 1 to 6 to various comorbid conditions based on their impact on one-year mortality, and it has been widely used and validated across a range of major cancers [19]. The higher the overall score, the more severe the burden of comorbidity and the higher the risk for mortality [18]. Summary statistics are presented as percentages in the case of categoric variables and as means with standard deviations in the case of continuous variables.
VTE incidence and the proportion of patients with deep vein thrombosis (DVT), pulmonary embolism (PE), or both were assessed at 3.5 and 12 months post-index. The 3.5-month follow-up period was chosen to reflect the follow-up period used in SAVE-ONCO, which is currently the largest trial of VTE prophylaxis in patients with cancer receiving chemotherapy [20]. The 12-month follow-up was used to assess annual costs both before and after the index date.
Bleeding complications were assessed using both the International Society on Thrombosis and Haemostasis (ISTH) definition of major bleeding and an expanded version of the ISTH definition (supplemental online Table 2). The ISTH definition classified major bleeding in patients who did not undergo surgery as fatal bleeding and/or symptomatic bleeding in a critical area or organ (i.e., intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular bleeding with compartment syndrome) and/or any bleeding resulting in a decrease in hemoglobin levels of ≥20 g/L or leading to a transfusion of ≥2 units of whole blood or red blood cells [21]. Confidence intervals (CIs) at 95% confidence level were derived using Clopper-Pearson (exact) method under binomial distribution for dichotomous response. The 95% CI for categoric variables with more than two levels was derived under multinomial distribution.
For the overall population, health care costs (2008 US $), including pharmacy, inpatient, emergency department, and outpatient costs were assessed at one year pre-index and post-index by VTE status. To address the skewed distribution of cost that does not follow a symmetric bell-shaped normal distribution, and to account for the potential imbalance at baseline between patients with and without VTE, a multivariate generalized linear model with a logarithmic link function and gamma variance was used for cost comparison during the follow-up period. Covariates in the model included demographics (age and gender), cancer location, comorbidities, baseline health care utilization, and baseline health care expenditures. Adjusted annual cost and 95% CIs were reported by performing exponentiation of the least-square means estimated from the generalized linear model. The cumulative VTE risk during follow-up was derived from one minus survival function estimated by the Kaplan-Meier method for overall population and by cancer location, which accounts for the timing of VTE and censoring as a result of loss to follow-up.
Given the descriptive nature of this study and the large number of potential comparisons, the analysis mainly reported point estimates along with 95% CI to reflect the variability, and p values reflecting specific hypothesis tests were not included routinely. The analyses were based on data observed and no missing data were imputed. The software used in this study was SAS Enterprise Guide Version 4.2 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
Baseline Characteristics
A total of 27,479 eligible patients were identified for inclusion in this analysis (supplemental online Fig. 1). Baseline characteristics of and medications used by patients undergoing chemotherapy who did or did not have VTE at 3.5 and 12 months according to definition A are summarized in Table 1. Baseline characteristics of patients according to VTE definitions B and C were similar to those for definition A (data not shown). Mean age was 63 years, approximately 52% of patients were male, and half of the total population had metastatic disease. Few patients had a central venous catheter and were receiving thromboprophylaxis at inclusion. The most prevalent comorbidity was chronic pulmonary disease (>30%). Overall, the mean Charlson Comorbidity Index score was approximately 6 to 7. Demographics and comorbidities of patients in whom VTE developed post-index were similar to those of patients in whom VTE did not develop. However, metastatic disease was present in a notably higher proportion of the patients on chemotherapy in whom VTE developed at 3.5 months post-index compared with patients without VTE (62% vs. 50%, respectively). There were no clinically important differences in the non-chemotherapy medications or in the number of patients who had central venous catheters among patients in whom VTE developed post-index and those in whom VTE did not develop.
Table 1.
Patient characteristics at baseline (1-year pre-index date) in patients who developed VTE and in those who did not at 3.5 months and 12 months post-index (definition A)
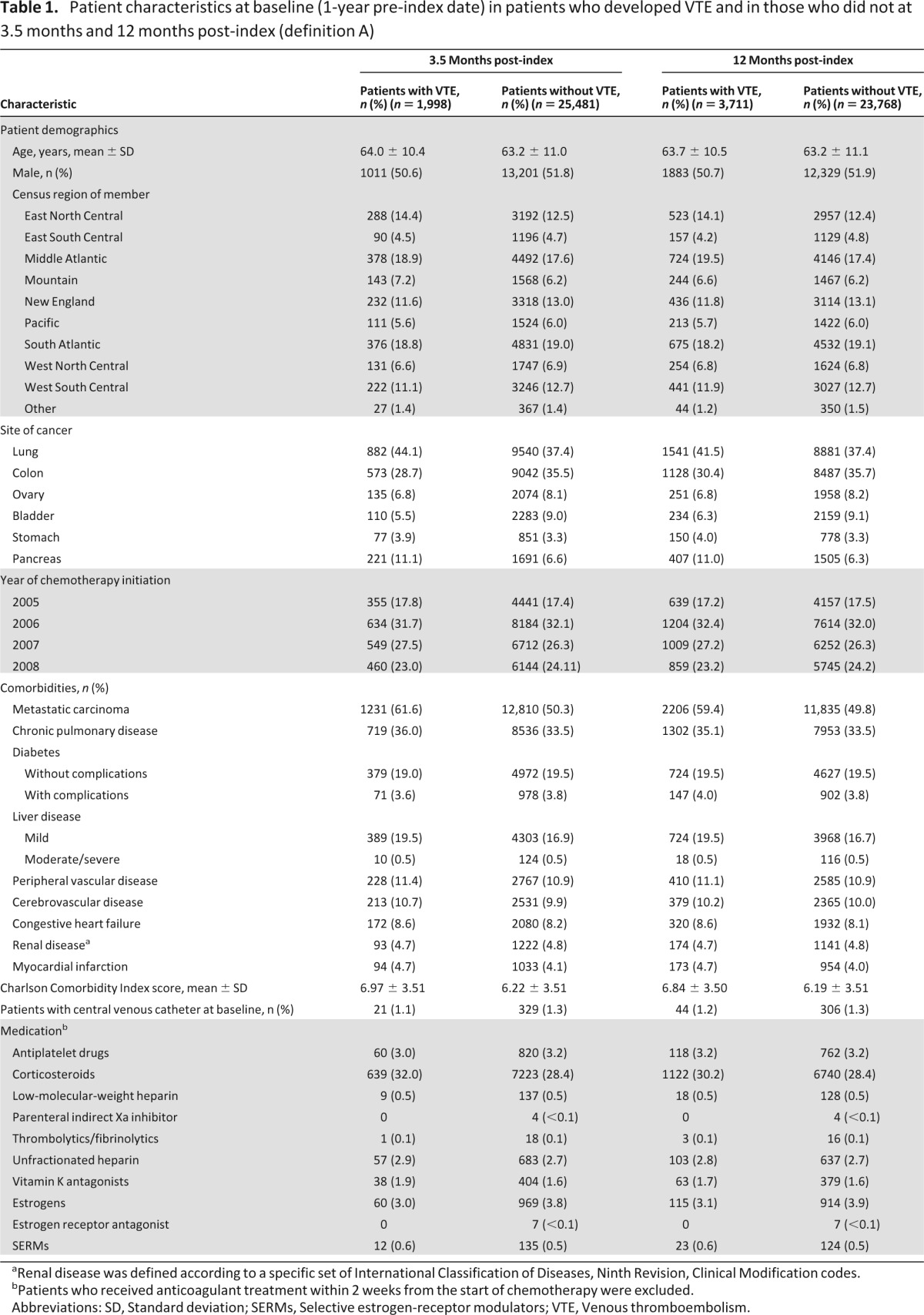
aRenal disease was defined according to a specific set of International Classification of Diseases, Ninth Revision, Clinical Modification codes.
bPatients who received anticoagulant treatment within 2 weeks from the start of chemotherapy were excluded.
Abbreviations: SD, Standard deviation; SERMs, Selective estrogen-receptor modulators; VTE, Venous thromboembolism.
VTE Rate at 3.5 Months and 12 Months Post-Index
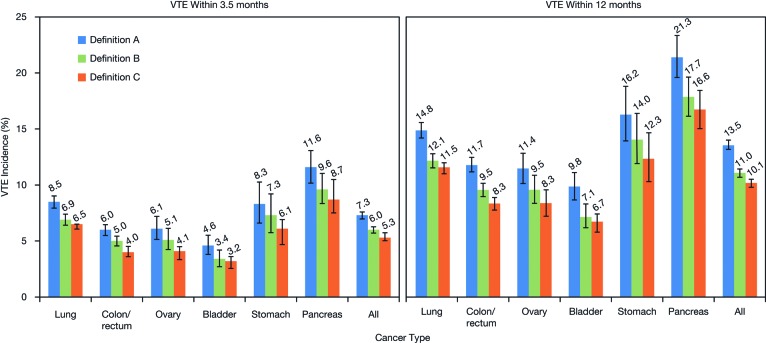
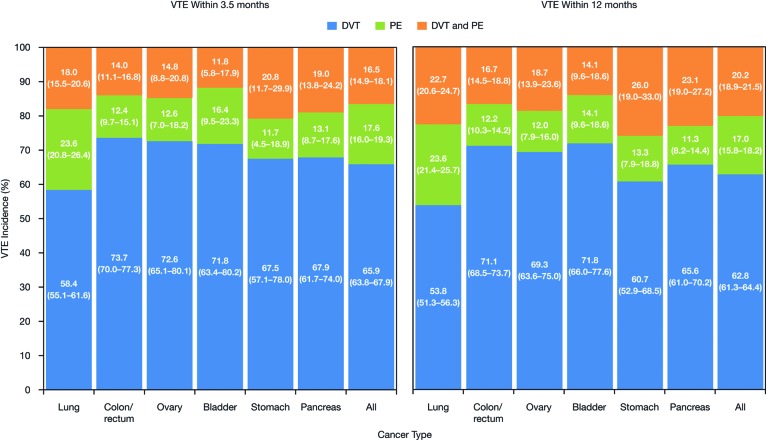
The incidence of VTE by cancer site and by VTE definition at 3.5 months and 12 months post-index for definitions A to C are shown in Figure 1. The overall incidence of VTE in patients with cancer 3.5 months after chemotherapy initiation according to definition A was 7.3% (range 4.6%–11.6% across cancer locations). According to definition A, the highest incidence of VTE was observed in patients with pancreatic (11.6%), lung (8.5%), and stomach cancers (8.3%). The incidence of VTE in patients on chemotherapy continued to increase over time, with an overall incidence of 13.5% at 12 months post-index (range 9.8%–21.3% across cancer locations) in patients identified using definition A. Similar VTE rates were observed for definitions B and C (which were both lower than definition A) at 3.5 months (ranges 3.4%–9.6% and 3.2%–8.7%, respectively) and at 12 months (ranges 7.1%–17.7% and 6.7%–16.6%, respectively) post-index (Fig. 1). The majority of cases of VTE observed were deep vein thrombosis (DVT), with a smaller proportion of pulmonary embolism (PE). For definition A, the proportion of VTE cases that were DVT or PE only at 3.5 months post-index was 65.9% and 17.6%, respectively (16.5% of patients had both PE and DVT). Across the cancer locations at 3.5 months, within the definition A cohort, the proportion of patients with VTE patients who had DVT only varied from 58% to 74% (highest in colon cancer); for PE only, the figures were 12% to 24% of patients (highest in lung cancer), and for both DVT and PE, the proportions varied from 12% to 21% (highest in stomach cancer) (Fig. 2). The pattern of distribution of DVT and PE remained similar 12 months post-index. A similar pattern of distribution of DVT and PE was also observed for definitions B and C (data not shown).
Figure 1.
VTE incidence (95% confidence interval) by cancer site and VTE definition (see text) (A, B, and C) at 3.5 months and 12 months post-index. Data labels indicate point estimates. Abbreviation: VTE, venous thromboembolism.
Figure 2.
Proportions of VTE cases (95% confidence interval) that were DVT only, PE only, or both DVT and PE according to definition A at 3.5 months and 12 months post-index. Abbreviations: DVT, deep-vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
In terms of the cumulative risk for VTE in patients with cancer undergoing chemotherapy, for definition A, no plateau or reduction in VTE risk was seen within 12 months of starting chemotherapy (Fig. 3). The cumulative risk for VTE in the sensitivity analyses (definitions B and C) and across all definitions is shown in supplemental online Fig. 2.
Figure 3.
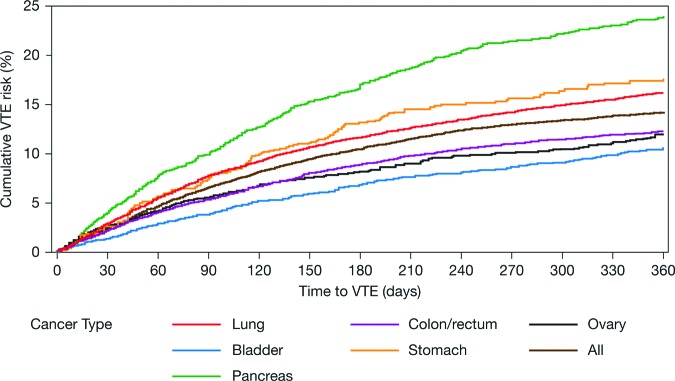
Cumulative risk for VTE using definition A in patients with cancer undergoing chemotherapy. Abbreviation: VTE, venous thromboembolism.
Bleeding Analysis
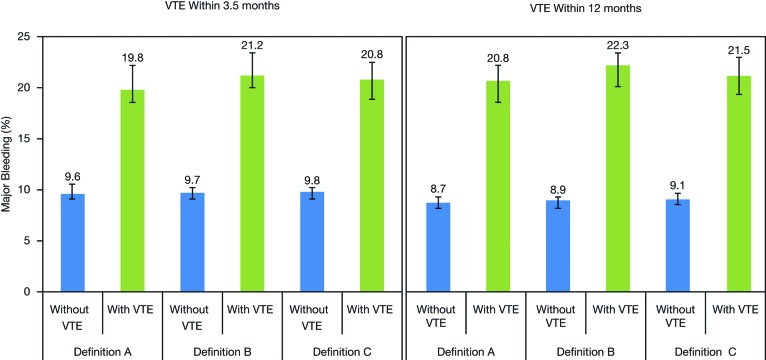
Across all three definitions, patients with VTE had a higher risk for major bleeding within 12 months after starting chemotherapy compared with patients without VTE. Those receiving chemotherapy as outpatients in whom VTE developed (definition A) at 3.5 months had a higher risk for major bleeding complications within 3.5 months than those who were not receiving chemotherapy on an outpatient basis (11.0% vs. 3.8%, respectively). According to definition A, at 3.5 months, the incidence of major bleeding (using the expanded ISTH definition) within 12 months post-index was 19.8% in patients with VTE and 9.6% in patients without VTE complications (Fig. 4). The risk for major bleeding varied across the tumor locations studied; in patients with VTE at 3.5 months, the incidence of major bleeding within 12 months of starting chemotherapy ranged from 17.3% (colon cancer) to 27.3% (stomach cancer).
Figure 4.
Incidence (95% confidence interval) of major bleeding (using the expanded International Society on Thrombosis and Haemostasis definition) within 12 months after starting chemotherapy (for definitions of VTE A, B, and C) in patients with and without VTE within 3.5 months and 12 months post-index. Data labels indicate point estimates. Abbreviation: VTE, venous thromboembolism.
Health Care Costs
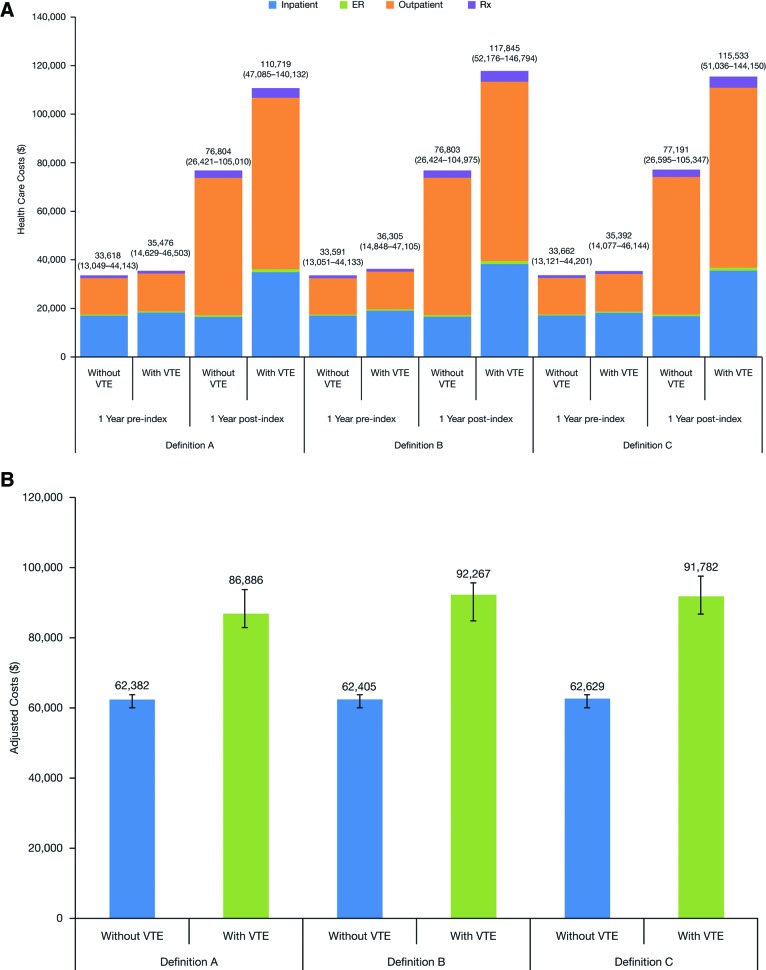
Patients in whom VTE developed within 3.5 months post-index had baseline health care costs (i.e., overall costs one year pre-index) comparable to those without VTE (definition A: US $35,476 vs. $33,618, respectively) (Fig. 5). During the first 12 months post-index, costs in patients with VTE were significantly higher than in those without VTE (definition A: US $110,719 vs. $76,804, respectively) (p < .0001), primarily driven by higher inpatient and outpatient costs (Fig. 5A). The overall cost of US $110,719 was made up of VTE-related inpatient, outpatient, and emergency department costs (US $7,964; $3,415; and $149, respectively). Similar results were observed for the VTE definitions B and C for patients in whom VTE developed within 12 months post-index (Fig. 5A). Adjusted annual costs during one-year follow-up were also higher in patients in whom VTE developed compared with those without VTE, and were similar for all definitions (Fig. 5B).
Figure 5.
One-year pre- and post-index health care costs according to definitions A, B, and C of VTE. (A): One-year pre- and post-index health care costs (mean values [interquartile range]) within 3.5 months post-index in patients with and without VTE, using definitions A, B, and C of VTE. Adjusted annual cost during one-year follow-up post-index according to definitions A, B, and C of VTE. (B): Adjusted annual cost (95% confidence interval) during one-year follow-up post-index in patients with and without VTE, using definitions A, B, and C of VTE (multivariate generalized linear model). Abbreviations: ER, emergency room; Rx, prescription medication; VTE, venous thromboembolism.
Discussion
In this large unselected cohort of patients with cancer, the overall risk for VTE 3.5 months after chemotherapy initiation was 7.3% (range 4.6%–11.6% across cancer locations). The cumulative risk for VTE continued to increase, with an estimated risk of 13.5% (range 9.8%–21.3%) at 12 months after starting chemotherapy. Those receiving chemotherapy on an outpatient basis in whom VTE developed also had a higher risk for major bleeding complications than those who did not, and this risk increased during the 12 months after starting chemotherapy. Patients with cancer in whom VTE developed experienced a significant economic burden for health care expenditure.
The highest VTE risk was observed in those patients receiving chemotherapy for tumors of the pancreas, stomach, and lung. Although this analysis did not include all cancer types, the solid tumors in this analysis are those previously identified as carrying an increased risk for VTE [3, 5, 7]. These tumors are responsible for millions of cancer diagnoses each year worldwide [22], and lung cancer remains a leading cause of cancer-related death in both men and women in the United States [22]. Therefore, the increase in VTE rates associated with chemotherapy in these solid tumors provides an important indicator of the increased thrombotic risk faced by many patients with cancer who are ambulatory and receive outpatient therapy.
In this cohort of patients with solid tumors, the observed risk for VTE was 7.3%, which is higher than that reported for placebo arms in randomized clinical trials [20, 23]. In the Prophylaxis of Thromboembolism during Chemotherapy (PROTECHT) trial, the incidence of VTE in the placebo arm was 3.9% (15/381 patients) [23]. Similarly, in the SAVE-ONCO study, VTE occurred in 3.4% of patients who received placebo [20]. This could be the result of the selection of lower-risk patients for inclusion in clinical trials. Moreover, incidences of VTE observed in cancer studies [20, 23] are higher than those observed after orthopedic surgery or in medical patients registries—clinical settings where thromboprophylaxis is routinely used (GLORY, 1.7% following major hip surgery and 2.3% following knee surgery; IMPROVE, 1.0%) [24, 25].
One factor that could partially explain the higher rate of VTE in our cohort compared with that of clinical trials is the identification of asymptomatic incidental VTE events. The duration of follow-up in our analysis was up to 12 months, allowing time for incidental VTE events to become clinically apparent. Incidental VTE events are often detected in patients with cancer through routine cancer staging scans [26]. These asymptomatic VTE events have been shown to develop in unusual and clinically important sites (e.g., mesenteric veins, cava inferior vein) [26], and they are associated with similar mortality and morbidity rates comparable to symptomatic VTE [27]. In the PROTECHT study, 1% of the 381 patients in the placebo group were diagnosed with incidental thrombi [23]. The burden of VTE is, therefore, likely to be underestimated in patients receiving chemotherapy because of the presence of unsuspected incidental thrombi, which may embolize, causing fatal PE.
In this unselected cohort of patients undergoing chemotherapy, the rate of VTE (7.3%) was higher than that reported for similar populations in clinical trials [20, 23, 28], and the major bleeding complications were also high (11.0% and 19.8% in patients in whom VTE developed 3.5 months and 12 months post-index, respectively). Higher bleeding rates were observed in patients with VTE compared with those without VTE. In patients with cancer, the cumulative 12-month risk for bleeding during anticoagulant treatment has previously been reported to be more than twice as high as in patients without cancer (12.4% vs. 4.9%, respectively) [11]. The use of preventive anticoagulant treatment was very low in our patient cohort, ranging from approximately 5% to 6% in patients with or without VTE 3.5 months post-index. Although there is concern regarding the risk for bleeding with anticoagulants, studies of VTE prophylaxis with low-molecular-weight heparins (LMWHs) and most recently with the ultra-LMWH (ULMWH), semuloparin, have not shown a significant increase in major bleeding rates compared with placebo [20, 23–30]. Indeed, in the FRAGEM study, the incidence of major bleeding was lower in the dalteparin group than in the control (gemcitabine) group (4.0% vs. 7.5%, respectively) [28]. Importantly, randomized clinical trials have shown that anticoagulant prophylaxis with LMWHs or a ULMWH significantly reduces the risk for symptomatic DVT and PE in patients with advanced solid tumors receiving chemotherapy [20, 23, 28, 31]. In line with these findings, a more recent meta-analysis of randomized clinical trials assessing parenteral anticoagulation in patients with cancer found that parenteral therapy was associated with a significant reduction in the risk for VTE in these patients without having an impact on bleeding or quality of life [32].
The health-economic findings of this real-world analysis highlight that patients with cancer in whom VTE develops experience a significant burden for health care expenditure. Despite similar pre-index health care costs, overall health care costs were significantly higher in patients in whom VTE developed, a finding that has been reported previously for patients with cancer [33]. In addition, in this analysis, metastatic disease was present in a higher proportion of patients in whom VTE developed. The presence of metastatic disease is associated with higher treatment and management costs, and may in part explain the increase in health care spending compared with patients without VTE. Interestingly, although the VTE incidence was approximately 20% lower using the more stringent VTE definitions, health care costs in patients with VTE were similar regardless of VTE definition. The 20% lower VTE rates translate into an approximate difference of 300 patients among the various VTE definitions used, which may not drastically change the mean total cost for a patient population of 17,500 patients. This finding may also imply that definition A captures most of the true VTE cases. Another factor contributing to the increased costs in definitions B and C relative to expectations is the capture of costs resulting from bleeding and other comorbidities as a consequence of the specificity of the definitions.
In terms of limitations, this was a retrospective analysis of a managed care database and therefore the data available were limited by the preset fields included in the IMPACT claims database, and overestimation of VTE events in the IMPACT claims database cannot be entirely excluded. Similarly, the absence of any mortality data with adjudication of cause of death may also have contributed to an underestimation of VTE risk, as VTE can manifest as fatal PE. A further limitation of the study is that patients who died or who changed health insurance plans during the study were not included in the analysis, which may have resulted in the exclusion of some patients with severe disease. In addition, the ICD-9-CM codes for esophageal, kidney, and uterine cancer surgery were missing. These tumors require surgery and represent approximately 20% of all cancer surgeries, which may have an impact on the interpretation of these results. Our cohort of patients was unselected within the limits of the study, not all cancers were included, and those patients not starting chemotherapy were excluded. This may limit the generalizability of our findings to some patient populations. Although patients receiving therapeutic anticoagulant therapy two weeks prior to entering the study were excluded, it cannot be ruled out that some of the anticoagulant treatment may have been therapeutic rather than prophylactic. Finally, full details of therapeutic regimens, including thromboprophylaxis and chemotherapy, were not tracked, and no comments can therefore be made on the association between specific regimens and VTE risk.
The VTE rates reported in our study are much higher than those observed in randomized clinical trials. Our analysis also showed that rates of major bleeding were higher in patients with cancer in whom VTE developed. The development of VTE in patients with cancer may interfere with planned active treatment, may increase patient morbidity and early death rates, and may worsen quality of life [34]. These results highlight that VTE prevention is not only important for reducing the thrombosis risk, but probably also for preventing the bleeding risk associated with thrombosis and its therapy. However, the decision to use thromboprophylaxis in patients undergoing chemotherapy must involve careful evaluation of the risk-benefit profile of anticoagulant use. Further studies highlighting the impact of chemotherapy on VTE risk are required to guide VTE risk assessment in patients undergoing cancer treatment.
Conclusion
The findings of this study of a real-world population highlight the increased VTE risk faced by patients receiving chemotherapy and the need for appropriate preventive measures.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The authors received editorial/writing support in the preparation of this manuscript provided by Marinella Calle, Ph.D., of Excerpta Medica, funded by Sanofi.
Author Contributions
Conception/Design: Gary H. Lyman, Laurent Eckert, Yanxin Wang, Hongwei Wang, Alexander Cohen
Provision of study material or patients: Laurent Eckert, Yanxin Wang, Hongwei Wang
Collection and/or assembly of data: Laurent Eckert, Yanxin Wang, Hongwei Wang
Data analysis and interpretation: Gary H. Lyman, Yanxin Wang, Hongwei Wang, Alexander Cohen
Manuscript writing: Gary H. Lyman, Laurent Eckert, Yanxin Wang, Hongwei Wang, Alexander Cohen
Final approval of manuscript: Gary H. Lyman, Laurent Eckert, Yanxin Wang, Hongwei Wang, Alexander Cohen
Disclosures
Laurent Eckert: Sanofi R&D (E); Hongwei Wang: Sanofi (E, OI); Alexander Cohen: Bayer, Daiichi Sankyo, Jannsen (C/A, RF, H); Pfizer, BMS, Portola, XOD, Sanofi (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch Intern Med. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 3.Khorana AA, Francis CW, Culakova E, et al. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 4.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 5.Blom JW, Vanderschoot JP, Oostindiër MJ, et al. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: Results of a record linkage study. J Thromb Haemost. 2006;4:529–535. doi: 10.1111/j.1538-7836.2006.01804.x. [DOI] [PubMed] [Google Scholar]
- 6.Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 7.Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- 8.Sallah S, Wan JY, Nguyen NP. Venous thrombosis in patients with solid tumors: Determination of frequency and characteristics. Thromb Haemost. 2002;87:575–579. [PubMed] [Google Scholar]
- 9.Stein PD, Beemath A, Meyers FA, et al. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119:60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 11.Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 12.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: Recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–5505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 13.Mandalà M, Falanga A, Roila F, et al. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85–vi92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 14.Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw. 2011;9:714–777. doi: 10.6004/jnccn.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyman GH, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2013;31:2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- 17.Boulet SL, Grosse SD, Hooper WC, et al. Prevalence of venous thromboembolism among privately insured US adults. Arch Intern Med. 2010;170:1774–1775. doi: 10.1001/archinternmed.2010.336. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 19.Hall WH, Ramachandran R, Narayan S, et al. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agnelli G, George DJ, Kakkar AK, et al. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601–609. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Kearon C Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 22.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 23.Agnelli G, Gussoni G, Bianchini C, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: A randomised, placebo-controlled double-blind study. Lancet Oncol. 2009;10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 24.Warwick D, Friedman RJ, Agnelli G, et al. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: Findings from the Global Orthopaedic Registry. J Bone Joint Surg Br. 2007;89:799–807. doi: 10.1302/0301-620X.89B6.18844. [DOI] [PubMed] [Google Scholar]
- 25.Spyropoulos AC, Anderson FA, Jr., Fitzgerald G, et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706–714. doi: 10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 26.Di Nisio M, Ferrante N, De Tursi M, et al. Incidental venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Thromb Haemost. 2010;104:1049–1054. doi: 10.1160/TH10-05-0277. [DOI] [PubMed] [Google Scholar]
- 27.den Exter PL, Hooijer J, Dekkers OM, et al. Risk of recurrent venous thromboembolism and mortality in patients with cancer incidentally diagnosed with pulmonary embolism: A comparison with symptomatic patients. J Clin Oncol. 2011;29:2405–2409. doi: 10.1200/JCO.2010.34.0984. [DOI] [PubMed] [Google Scholar]
- 28.Maraveyas A, Waters J, Roy R, et al. Gemcitabine with or without prophylactic weight-adjusted dalteparin in patients with advanced or metastatic pancreatic cancer (APC): A multicentre, randomized phase IIB trial (the UK FRAGEM study) Eur J Cancer Suppl. 2009;7 abstract 6503. [Google Scholar]
- 29.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 30.Riess H, Pelzer U, Deutschinoff G, et al. A prospective, randomized trial of chemotherapy with or without the low molecular weight heparin (LMWH) enoxaparin in patients (pts) with advanced pancreatic cancer (APC): Results of the CONKO 004 trial. J Clin Oncol. 2009;27(Suppl) abstract LBA4506. [Google Scholar]
- 31.Akl EA, Schünemann HJ. Routine heparin for patients with cancer? One answer, more questions. N Engl J Med. 2012;366:661–662. doi: 10.1056/NEJMe1113672. [DOI] [PubMed] [Google Scholar]
- 32.Akl EA, Gunukula S, Barba M, et al. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2011;(4):CD006652. doi: 10.1002/14651858.CD006652.pub3. [DOI] [PubMed] [Google Scholar]
- 33.Elting LS, Escalante CP, Cooksley C, et al. Outcomes and cost of deep venous thrombosis among patients with cancer. Arch Intern Med. 2004;164:1653–1661. doi: 10.1001/archinte.164.15.1653. [DOI] [PubMed] [Google Scholar]
- 34.Lyman GH. Venous thromboembolism in the patient with cancer: Focus on burden of disease and benefits of thromboprophylaxis. Cancer. 2011;117:1334–1339. doi: 10.1002/cncr.25714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.