Abstract
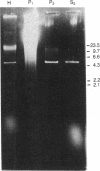
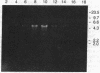
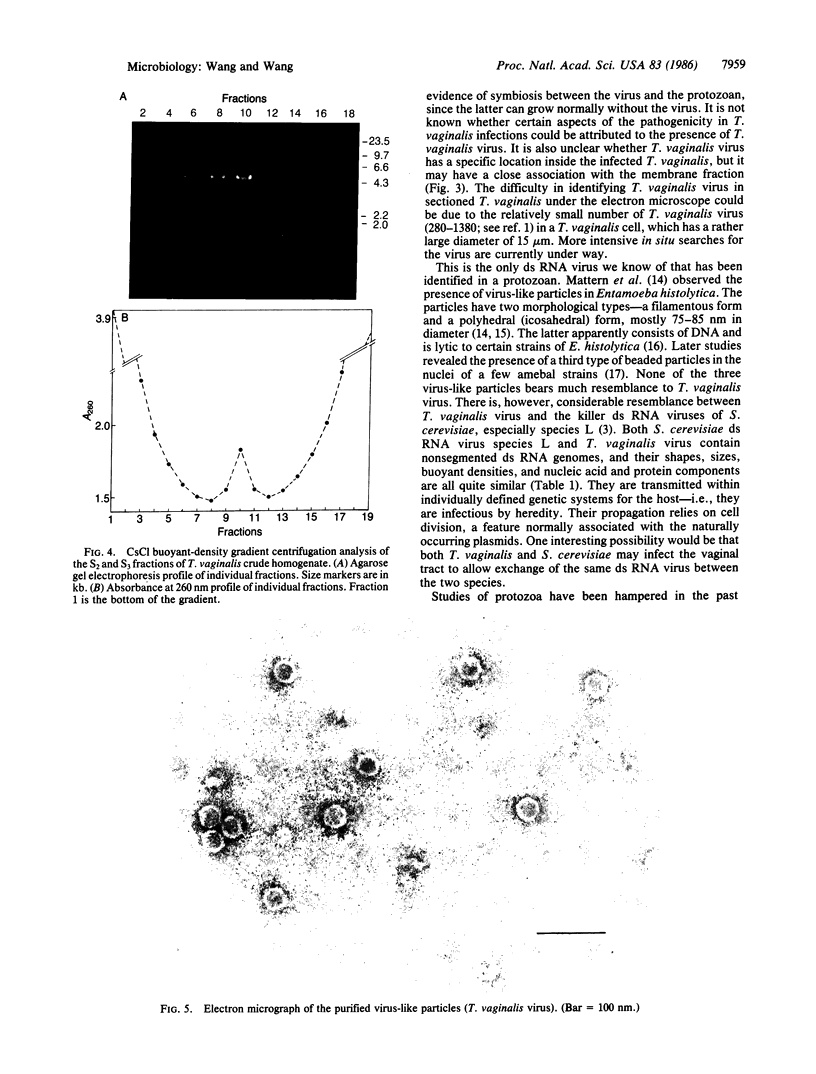
A linear 5.5-kilobase double-stranded RNA, identified in many strains and isolates of the parasitic protozoan Trichomonas vaginalis in a previous study, is found largely intact in ribonuclease-treated homogenates of the parasite. It can be pelleted with membranes from the homogenate at 12,500 X g and further purified in CsCl buoyant density-gradient centrifugations. The purified sample contains the double-stranded RNA as well as one major protein with an estimated molecular mass of 85 kDa in NaDodSO4/PAGE. Electron microscopic examinations indicated the presence of icosahedral virus-like particles of 33-nm diameter in the purified preparation. The exact location of the virus in T. vaginalis is not clear, except that it is not found in the nuclear fraction and is probably membrane-bound. No free virus can be recovered from the culture medium of T. vaginalis, and no successful infection of virus-free T. vaginalis strains by purified virus has yet been accomplished. There is no viral genomic sequence identifiable in host DNA. So far as we know, it is the first time a double-stranded RNA virus has been identified in a protozoan.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruenn J. A., Brennan V. E. Yeast viral double-stranded RNAs have heterogeneous 3' termini. Cell. 1980 Apr;19(4):923–933. doi: 10.1016/0092-8674(80)90084-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J. A. Virus-like particles of yeast. Annu Rev Microbiol. 1980;34:49–68. doi: 10.1146/annurev.mi.34.100180.000405. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Diamond L. S., Mattern C. F., Bartgis I. L. Viruses of Entamoeba histolytica. I. Identification of transmissible virus-like agents. J Virol. 1972 Feb;9(2):326–341. doi: 10.1128/jvi.9.2.326-341.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L. S., Mattern C. F. Protozoal viruses. Adv Virus Res. 1976;20:87–112. doi: 10.1016/s0065-3527(08)60502-3. [DOI] [PubMed] [Google Scholar]
- Hruska J. F., Mattern C. F., Diamond L. S. Viruses of Entamoeba histolytica. IV. Studies on the nucleic acids of the filamentous and polyhedral viruses. J Virol. 1974 Jan;13(1):205–210. doi: 10.1128/jvi.13.1.205-210.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGRIDGE R., GOMATOS P. J. The structure of RNA. Reovirus RNA and transfer RNA have similar three-dimensional structures, which differ from DNA. Science. 1963 Aug 23;141(3582):694–698. doi: 10.1126/science.141.3582.694. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Diamond L. S., Daniel W. A. Viruses of Entamoeba histolytica. II. Morphogenesis of the polyhedral particle (ABRM 2 leads to HK-9) leads to HB-301 and the filamentous agent (ABRM) 2 leads to HK-9. J Virol. 1972 Feb;9(2):342–358. doi: 10.1128/jvi.9.2.342-358.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Gorrell T. E. Metabolism and metronidazole uptake in Trichomonas vaginalis isolates with different metronidazole susceptibilities. Antimicrob Agents Chemother. 1983 Nov;24(5):667–673. doi: 10.1128/aac.24.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper N., Bussey H. Mode of action of yeast toxins: energy requirement for Saccharomyces cerevisiae killer toxin. J Bacteriol. 1977 Feb;129(2):668–677. doi: 10.1128/jb.129.2.668-677.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Isolation and characterization of DNA from Tritrichomonas foetus and Trichomonas vaginalis. Mol Biochem Parasitol. 1985 Mar;14(3):323–335. doi: 10.1016/0166-6851(85)90060-x. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Cheng H. W. Salvage of pyrimidine nucleosides by Trichomonas vaginalis. Mol Biochem Parasitol. 1984 Feb;10(2):171–184. doi: 10.1016/0166-6851(84)90005-7. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]