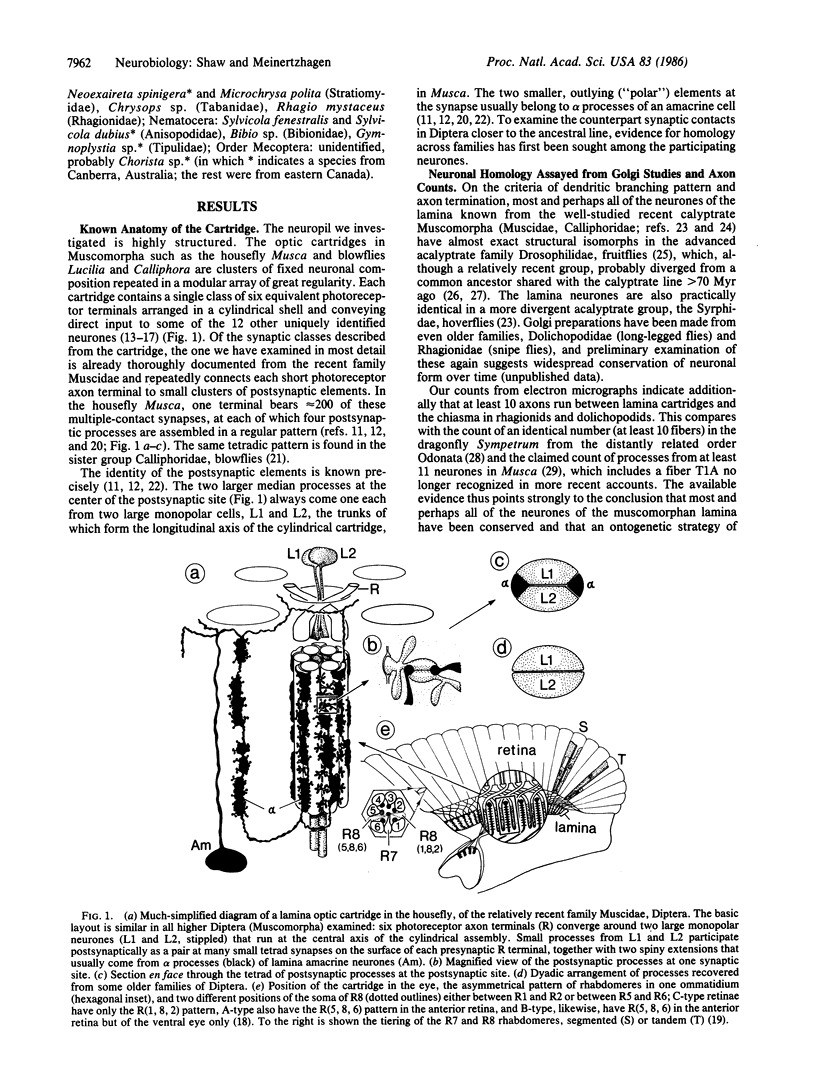
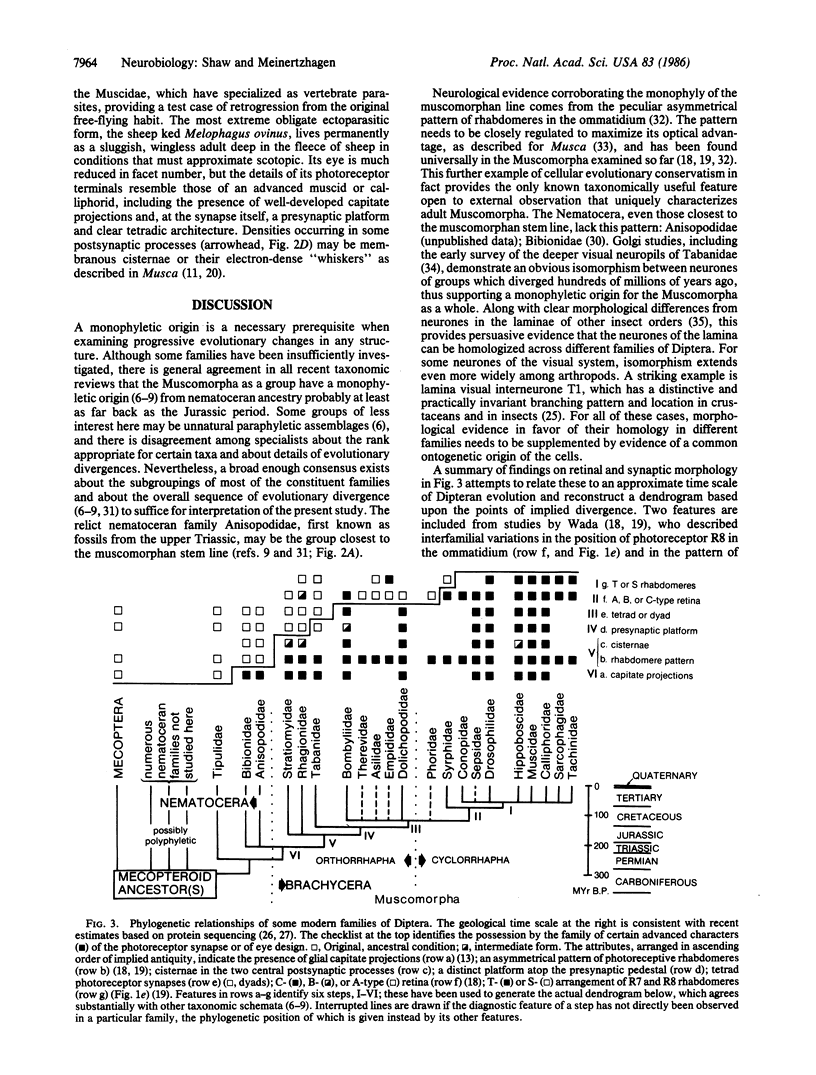
Abstract
A comparative ultrastructural study of photoreceptor synapses formed upon homologous postsynaptic neurones in insects has been made by using serial-section electron microscopy in representative Diptera from a monophyletic series of 14 families. At all of the synaptic contacts there is a presynaptic dense bar, surmounted in phylogenetically more recent families by a presynaptic platform. Opposite the bar lies a pair of postsynaptic elements that invariably originate one each from two unique monopolar neurones L1 and L2. Both elements contain increasingly elaborate cisternae in more recent flies. Within the phylogenetic series, the postsynaptic ensemble itself changes from the original dyad to a tetradic configuration in more recent Muscomorpha by the addition of two new postsynaptic elements from an amacrine cell. This transition occurs once only in the series, which, gauged by the fossil record, covers divergences from the stem line extending back greater than 200 million years.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley S. M., Wilson A. C. Ancient origin for Hawaiian Drosophilinae inferred from protein comparisons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4753–4757. doi: 10.1073/pnas.82.14.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Wilson A. C. Molecular evolution in Drosophila and the higher Diptera II. A time scale for fly evolution. J Mol Evol. 1984;21(1):1–13. doi: 10.1007/BF02100622. [DOI] [PubMed] [Google Scholar]
- Boschek C. B. On the fine structure of the peripheral retina and lamina ganglionaris of the fly, Musca domestica. Z Zellforsch Mikrosk Anat. 1971;118(3):369–409. doi: 10.1007/BF00331193. [DOI] [PubMed] [Google Scholar]
- Burkhardt W., Braitenberg V. Some peculiar synaptic complexes in the first visual ganglion of the fly, Musca domestica. Cell Tissue Res. 1976 Oct 13;173(3):287–308. doi: 10.1007/BF00220317. [DOI] [PubMed] [Google Scholar]
- Fröhlich A. Freeze-fracture study of an invertebrate multiple-contact synapse: the fly photoreceptor tetrad. J Comp Neurol. 1985 Nov 15;241(3):311–326. doi: 10.1002/cne.902410306. [DOI] [PubMed] [Google Scholar]
- Fröhlich A., Meinertzhagen I. A. Synaptogenesis in the first optic neuropile of the fly's visual system. J Neurocytol. 1982 Feb;11(1):159–180. doi: 10.1007/BF01258010. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K. Die Projektion der optischen Umwelt auf das Raster der Rhabdomere im Komplexauge von MUSCA. Exp Brain Res. 1967;3(3):248–270. doi: 10.1007/BF00235588. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen I. A., Armett-Kibel C. J., Frizzell K. L. The number and arrangement of elements in the lamina cartridge of the dragonfly Sympetrum rubicundulum. Cell Tissue Res. 1980;206(3):395–401. doi: 10.1007/BF00237969. [DOI] [PubMed] [Google Scholar]
- Nicol D., Meinertzhagen I. A. An analysis of the number and composition of the synaptic populations formed by photoreceptors of the fly. J Comp Neurol. 1982 May 1;207(1):29–44. doi: 10.1002/cne.902070104. [DOI] [PubMed] [Google Scholar]
- Shaw S. R. Early visual processing in insects. J Exp Biol. 1984 Sep;112:225–251. doi: 10.1242/jeb.112.1.225. [DOI] [PubMed] [Google Scholar]
- Strausfeld N. J., Campos-Ortega J. A. Vision in insects: pathways possibly underlying neural adaptation and lateral inhibition. Science. 1977 Mar 4;195(4281):894–897. doi: 10.1126/science.841315. [DOI] [PubMed] [Google Scholar]
- Thomas J. B., Bastiani M. J., Bate M., Goodman C. S. From grasshopper to Drosophila: a common plan for neuronal development. Nature. 1984 Jul 19;310(5974):203–207. doi: 10.1038/310203a0. [DOI] [PubMed] [Google Scholar]
- Wada S. Morphological duality of the retinal pattern in flies. Experientia. 1975 Aug 15;31(8):921–923. doi: 10.1007/BF02358853. [DOI] [PubMed] [Google Scholar]
- Wilson J. A., Phillips C. E., Adams M. E., Huber F. Structural comparison of a homologous neuron in gryllid and acridid insects. J Neurobiol. 1982 Sep;13(5):459–467. doi: 10.1002/neu.480130507. [DOI] [PubMed] [Google Scholar]



