Abstract
Background
Women who use antiretroviral therapy (ART) solely for the prevention of mother-to-child transmission of HIV discontinue postpartum. We hypothesized that women discontinuing ART by 6 weeks postpartum (“discontinuers”) would have elevated postpartum inflammatory biomarker levels relative to women remaining on ART postpartum (“continuers”).
Methods
Data from HIV-infected pregnant women enrolled in the International Maternal Pediatric Adolescent AIDS Clinical Trials Group P1025 with CD4 counts >350 cells per cubic millimeter before initiating ART or first pregnancy CD4 counts >400 cells per cubic millimeter after starting ART and with available stored plasma samples at >20 weeks of gestation, delivery, and 6 weeks postpartum were analyzed. Plasma samples were tested for highly sensitive C-reactive protein, D-dimer, and interleukin-6. We used longitudinal linear spline regression to model biomarkers over time.
Results
Data from 128 women (65 continuers and 63 discontinuers) were analyzed. All biomarkers increased from late pregnancy to delivery, then decreased postpartum (slopes different from 0, P < 0.001). Continuers had a steeper decrease in log D-dimer between delivery and 6 weeks postpartum than discontinuers (P = 0.002).
Conclusions
In contrast to results from treatment interruption studies in adults, both ART continuers and ART discontinuers had significant decreases in the levels of D-dimer, highly sensitive C-reactive protein, or interleukin-6 postpartum. Continuation was associated with a more rapid decline in D-dimer levels compared with discontinuation.
Keywords: HIV/AIDS, pregnancy and postpartum, inflammation, antiretroviral therapy
INTRODUCTION
The treatment guidelines of the United States for HIV-infected adults and adolescents recommend antiretroviral therapy (ART), once initiated, be continued lifelong.1 The recommended CD4 threshold for initiation of ART in adolescents and adults with HIV infection has evolved over time (<200 to < 350 to <500 cells/mm3), and ART is now considered for all adolescents and adults, irrespective of CD4 count.1
In contrast to treatment guidelines for adolescents and adults,1 US guidelines for HIV-infected pregnant women incorporate ART as prophylaxis of mother-to-child transmission (MTCT) of HIV and ART as treatment.2 Historically, pregnant women who met criteria for treatment based on CD4 thresholds were started on ART as soon as possible and continued lifelong, whereas pregnant women who did not meet criteria for treatment were initiated on ART after the first trimester of pregnancy solely for the prevention of MTCT and then discontinued postpartum. As thresholds for initiating ART have increased over time, it has become unclear whether pregnant women with high CD4 counts who have traditionally been started on ART for prophylaxis of MTCT of HIV should continue or discontinue ART postpartum.
Several studies of structured treatment interruption (TI), in which ART was selectively started and stopped in men and nonpregnant women based on CD4 thresholds, have shown excess morbidity and mortality from AIDS and non-AIDS clinical events.3–5 One of these studies (Strategies for Management of Antiretroviral Therapy or SMART) has demonstrated associations between inflammatory and coagulation biomarkers and clinical outcomes.6 In this study, a comparison of levels of interleukin-6 (IL-6) and D-dimer at study entry in participants who died and those who survived showed that entry elevations in these markers were associated with a large increase in the likelihood of death, with unadjusted odds ratios for those in the highest quartile compared with the lowest of 8.3 for IL-6 and 12.4 for D-dimer. Adjustment for a variety of factors including age, race, viral load, CD4 count, smoking, body mass index, diabetes, and hepatitis B or C coinfection strengthened these associations. IL-6 and D-dimer increased in discontinuers, particularly in those with virological suppression at entry, and the increases were related to viral load 1 month after discontinuation. These biomarkers were more strongly associated with all-cause mortality than with cardiac death, suggesting that these markers are surrogates of a pathway that is globally detrimental to health. Based on these data, it has been recommended to avoid TI for HIV-infected individuals.1 It has been argued that extrapolation of findings from TI studies conducted among men and nonpregnant women to pregnant and postpartum women is suboptimal given significant differences in age, overall health status, CD4 nadir levels, and unique physiological drivers of inflammation among this population; however, discontinuation of ART postpartum among women who initiated ART for prevention of MTCT could be considered a unique form of TI because most HIV-infected postpartum women would be expected to restart ART eventually, either for treatment of the woman herself or for transmission prophylaxis during a subsequent pregnancy.
Because of uncertainty surrounding postpartum ART strategy for pregnant women with high nadir CD4 counts, we sought to characterize inflammatory and coagulation biomarkers in pregnant and postpartum women with HIV infection starting ART during pregnancy with pre-ART CD4 counts >350 cells per cubic millimeter or first on ART CD4 count of >400 cells per cubic millimeter and to compare biomarker trajectories in women who continued or discontinued ART after delivery. We also evaluated biomarker levels and associations with subsequent pregnancy and delivery complications and clinical events up to 6 months postpartum. We hypothesized that postpartum ART discontinuation would be associated with marked elevations in biomarkers among discontinuers due to uncontrolled viral replication and triggering of inflammatory and coagulation pathways and that these elevations in biomarkers would be associated with higher rates of postpartum clinical events resulting from the detrimental end-organ effects of these pathways. We also hypothesized that higher levels of third trimester biomarkers, reflective of higher levels of inflammation and coagulation despite ART, would be associated with subsequent late pregnancy and delivery events.
METHODS
P1025 Protocol
The International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) P1025 is a prospective cohort study that aims to assess maternal and infant safety and effectiveness of new and existing interventions prescribed for prevention of MTCT of HIV. Starting in 2002, participants aged ≥13 years from 56 clinical sites in the United States and Puerto Rico were enrolled during pregnancy (≥8 weeks of gestation) or within 2 weeks of delivery, with follow-up continued until at least 6 months after delivery. The number of antepartum study visits was a maximum of 4 completed at least 4 weeks apart during windows corresponding to 14–20, >20–30, and >30 weeks of gestation to delivery. Postpartum study visits were performed at 6 ± 4 weeks and then at 12 and 24 weeks ± 8 weeks, with a minimum of 4 weeks between visits through 6 months postpartum. Study visits included biologic specimen collection, medical record abstraction, and questionnaire administration for adherence assessment. All women provided written informed consent. Detailed study schedules and evaluations have been described in previously published studies.7,8
Study Population and Definitions for This Analysis
The study population for this analysis comprised HIV-infected women enrolled in P1025 during pregnancy who initiated ART during pregnancy (including triple nucleoside combinations), were followed through at least the 6-week postpartum study visit, and had a pre-ART CD4 counts >350 cells per cubic millimeter or first on ART CD4 count of >400 cells per cubic millimeter. Women who had at least 1.5 mL of stored plasma collected at each of 3 time points—late pregnancy (>20 weeks of gestation), delivery (+2 weeks), and 6 weeks postpartum (±4 weeks)—were included in the analysis.
For this manuscript, ART refers to potent 3-drug antiretroviral combinations used in pregnant women for either prophylaxis of MTCT or for treatment. The primary exposure of interest was “ART status,” which categorized women into 3 groups according to continuation or discontinuation of ART after delivery: (1) continuers, defined as continuation of ART beyond 6 weeks postpartum and on ART for all 3 biomarker time points; (2) early discontinuers, defined as discontinuation after delivery and before the delivery biomarker measurement; and (3) late discontinuers, defined as discontinuation after delivery and the delivery biomarker measurement, but before the 6-week postpartum measurement. The primary outcomes of interest were biomarkers of inflammation and coagulation: highly sensitive C-reactive protein (hsCRP), IL-6, and D-dimer.
Other important characteristics examined in the analysis included maternal age, race/ethnicity, earliest CD4 count during pregnancy, plasma HIV RNA concentration (viral load) before initiation of ART, CDC clinical classification at entry,9 ART regimen, complications, and mode of delivery (defined as vaginal delivery, Cesarean section after labor and/or rupture of membranes, and Cesarean section before labor and before rupture of membranes). For the analysis of pregnancy and delivery complications, events included gestational hypertension, gestational diabetes, pre-eclampsia, premature rupture of membranes (<37 weeks), and preterm labor (<37 weeks). For the analysis of postpartum complications, a clinical event was defined as any World Health Organization stage 1–4 diagnosis10 or any other confirmed clinical diagnosis, including non–AIDS-defining events (defined as liver, kidney, cardiovascular disease, and non–AIDS-associated malignancies).
Biomarker Determination and Validation of D-dimer Measurement From Ethylenediaminetetraacetic Acid Plasma
Samples stored at − 70°C were transported from the IMPAACT repository (Rockville, MD) to Quest Diagnostics (Chantilly, VA) for determination of hsCRP, IL-6, and D-dimer. Testing for hsCRP was performed with nephelometry and testing for IL-6 with a highly sensitive enzyme-linked immunosorbent assay.11 Because the only validated sample type for D-dimer testing performed at Quest Diagnostics was citrated plasma and stored samples were collected from ethyl-enediaminetetraacetic acid (EDTA) plasma, before study samples were analyzed, a correlation study was performed to compare D-dimer values obtained from citrate and EDTA (performed with STA-R analyzer/immunoturbidometric method). An excellent correlation between the 2 different sample types was obtained with an R value of 0.99. EDTA plasma samples yielded a slightly higher result in comparison with the citrate plasma samples, with an average bias of 0.1 µg/mL.
Statistical Analysis
Sociodemographic, HIV, and pregnancy characteristics by ART status were compared using χ2 tests, Kruskal-Wallis tests, or Fisher’s exact tests as appropriate. Biomarker distributions and median levels of each biomarker were determined at each time point. Because biomarker measurements at each time point had skewed distributions, all analyses were performed on the natural log transformed values. When the biomarker measurement was below the limit of detection, an imputed value was set to the midpoint between the lower limit of detection and 0 (before transformation).
We expected the biomarker slopes to behave differently before and after delivery and therefore used longitudinal linear regression with a spline knot at time of delivery to model each biomarker over time, where time was calculated from exact dates relative to pregnancy. In all regression analyses, we first fitted models, which allowed the biomarker slopes to differ for each period and according to a given characteristic, and assessed whether slopes differed significantly using an F test. If no significant differences were observed, we assessed whether there was an overall difference in biomarker level over time between groups. We compared biomarker slopes by ART status in 2 ways, first across the 3 ART status groups, then between continuers and all discontinuers (early and late discontinuers combined). To explore the possible misclassification of women’s ART status postpartum due to nonadherence, we repeated the regression analyses and compared biomarker trajectories between those with viral loads ≤400 copies per milliliter versus >400 copies per millimeter at 6 weeks postpartum. With n = 50 subjects per group, we had 80% power to detect a medium effect size linear change (0.5 SD). This effect size is in line with that observed by Kuller et al6 for D-dimer and IL-6 in men and nonpregnant women in the SMART study. We also explored the relationship between biomarker levels over time with age, race/ethnicity, and maternal pregnancy and delivery complication status. In the analysis of pregnancy and delivery complications women were excluded if their first reported complication was before their first biomarker measurement. For postpartum clinical event analysis, we compared the frequency of events by ART group using a χ2 test. We compared mean biomarker levels in the third trimester and at delivery between those women with and without events up to 6 months postpartum using t tests with unequal variance. All statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC). A 2-sided P value<0.05 was considered statistically significant.
RESULTS
Size and Characteristics of the Study Population
Of 2057 women enrolled in P1025 as of August 1, 2010, 1549 were enrolled during pregnancy, had delivered, and had been followed through at least 6 weeks postpartum. Of these, 1221 initiated ART during pregnancy. In this group, 307 had antepartum CD4 counts >350 cells per cubic millimeter before initiation of ART. An additional 124 women without pre-ART CD4 measurements had earliest pregnancy CD4 counts >400 cells per cubic millimeter after ART initiation [median weeks on ART before CD4 count: 8.2, interquartile range (IQR): 4.0– 13.9]. Of the 431 women, 128 had ≥1.5 mL of stored plasma collected at each of the specified time points and were included in the analyses. Characteristics of the 128 women included versus the 303 women excluded due to lack of at least 1 repository sample (166 ART continuers and 137 discontinuers) revealed no significant differences except that the women included were more likely to be on boosted protease inhibitors (PI), whereas those excluded were more likely to be on non-nucleoside reverse transcriptase inhibitors (data not shown).
Of the 128 women studied, 65 were continuers and 63 were discontinuers. Of the 63 discontinuers, 30 stopped after delivery and before the delivery biomarker measurement (“early discontinuers,” median duration of ART after delivery of 0 days, IQR: 0–1) and 33 stopped after delivery and after the delivery biomarker measurement and before the 6-week postpartum biomarker measurement (“late discontinuers,” median duration of ART after delivery of 13 days, IQR: 3– 21). The first biomarker measurement during pregnancy was taken at a median gestational age of 29 weeks (IQR: 25–34). The delivery measurement was taken within 3 days of delivery in 75% of the group. The final measurement was taken at a median of 6.9 weeks postpartum (IQR: 6.0–7.7).
The baseline characteristics of study participants are shown in Table 1. No statistically significant differences were observed among the 3 ART status groups according to age, race/ethnicity, earliest CD4 count during pregnancy, CDC clinical classification at study entry,9 or mode of delivery. The median CD4 count was 574 cells per cubic millimeter and 28% of women had earliest pregnancy CD4 counts >700 cells per cubic millimeter. Continuers were more likely to be on a boosted PI regimen during pregnancy (P = 0.03) compared with discontinuers. We observed no differences by ART regimen in pretreatment viral load, viral load at time of delivery, or CD4 count at time of delivery.
TABLE 1.
Characteristics of Women by ART Status Group
| Characteristic | ART Group | Total, N =128 | P | ||
|---|---|---|---|---|---|
| Continuers N = 65 |
Discontinuers | ||||
| Early, N = 30 | Late, N = 33 | ||||
| Age (yrs), n (%) | |||||
| 13–24 | 21 (32) | 15 (50) | 13 (39) | 49 (38) | 0.14* |
| 25–30 | 17 (26) | 8 (27) | 13 (39) | 38 (30) | |
| 31+ | 27 (42) | 7 (23) | 7 (21) | 41 (32) | |
| Race/ethnicity, n (%) | |||||
| Black non-Hispanic | 31 (48) | 22 (73) | 20 (61) | 73 (57) | 0.20* |
| Hispanic | 26 (40) | 6 (20) | 9 (27) | 41 (32) | |
| White non-Hispanic/other | 8 (12) | 2 (7) | 4 (12) | 14 (11) | |
| Earliest CD4 count during pregnancy (cells/mm3), n (%) | |||||
| Median (Q1, Q3) | 540 (478, 670) | 568 (467, 719) | 663 (524, 773) | 574 (478, 720) | 0.17† |
| 350–399 | 1 (2) | 2 (7) | 2 (6) | 5 (4) | 0.21‡ |
| 400–499 | 21 (32) | 8 (27) | 6 (18) | 35 (27) | |
| 500–599 | 19 (29) | 7 (23) | 4 (12) | 30 (23) | |
| 600–699 | 10 (15) | 5 (17) | 7 (21) | 22 (17) | |
| 700+ | 14 (22) | 8 (27) | 14 (42) | 36 (28) | |
| Pre-ART viral load during pregnancy (copies/mL), n (%)§ | |||||
| ≤400 | 8 (20) | 6 (24) | 7 (26) | 21 (23) | 0.05* |
| 401–1000 | 4 (10) | 3 (12) | 6 (22) | 13 (14) | |
| 1001–10,000 | 9 (22) | 10 (40) | 11 (41) | 30 (32) | |
| >10,000 | 20 (49) | 6 (24) | 3 (11) | 29 (31) | |
| CDC clinical classification at entry, n (%) | |||||
| A | 58 (89) | 28 (93) | 33 (100) | 119 (93) | 0.25‡ |
| B | 3 (5) | 2 (7) | 0 (0) | 5 (4) | |
| C | 4 (6) | 0 (0) | 0 (0) | 4 (3) | |
| Initial ART regimen during pregnancy, n (%)‖ | |||||
| 2 NRTIs + 1 NNRTI | 2 (3) | 0 (0) | 2 (6) | 4 (3) | 0.08‡ |
| 2 NRTIs + 1 PI | 16 (25) | 9 (30) | 5 (15) | 30 (23) | |
| 2 NRTIs + 1 boosted PI | 39 (60) | 16 (53) | 14 (42) | 69 (54) | |
| 3 NRTIs | 8 (12) | 5 (17) | 12 (36) | 25 (20) | |
| Mode of delivery, n (%) | |||||
| Vaginal | 32 (49) | 16 (53) | 17 (52) | 65 (51) | 0.78* |
| Cesarean section after labor and/or rupture of membranes | 10 (15) | 7 (23) | 6 (18) | 23 (18) | |
| Cesarean section before labor and before rupture of membranes | 23 (35) | 7 (23) | 10 (30) | 40 (31) | |
Chi square test; comparison for all 3 groups (continuers, early discontinuers, and late discontinuers).
Kruskal–Wallis test; comparison for all 3 groups (continuers, early discontinuers, and late discontinuers).
The Fisher exact test; comparison for all 3 groups (continuers, early discontinuers, and late discontinuers).
Pre-ART viral load was unavailable in n = 35 women.
Classified by first regimen during pregnancy; 102 women (80%) were only on 1 regimen during pregnancy, 23 (18%) were on 2 regimens, and 3 (2%) were on 3 or 4 regimens during pregnancy.
NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Biomarker Levels and Trajectories
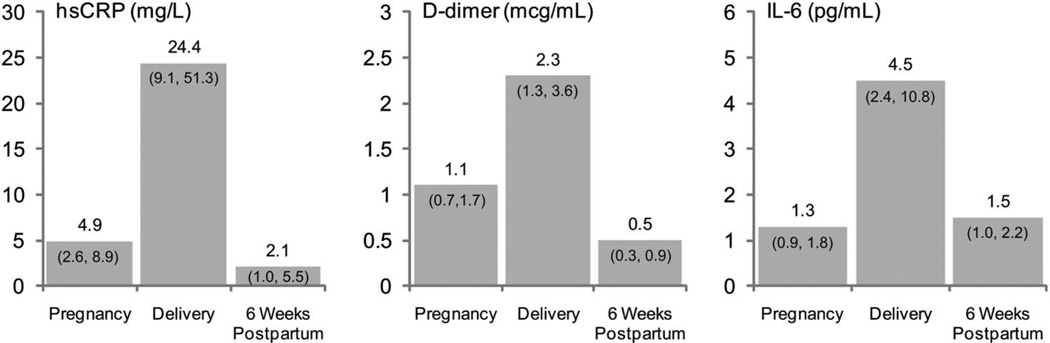
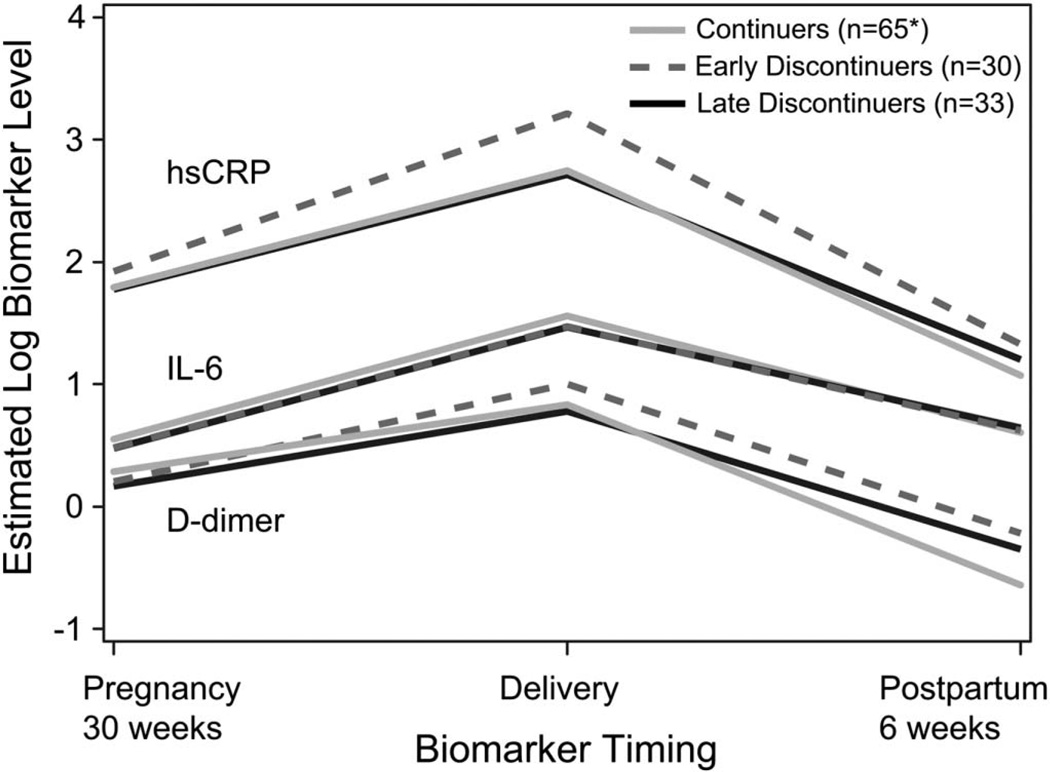
Median biomarker levels were elevated above the upper limit of the reference range at all time points tested, with highest levels observed at delivery with median hsCRP, D-dimer, and IL-6 of 24.4 mg/L, 2.3 µg/mL, and 4.5 pg/mL, respectively (Fig. 1). All biomarkers increased from late pregnancy to delivery, then decreased postpartum (slopes all significantly different from 0, P < 0.001). No significant differences in biomarker trajectories across the 3 ART status groups were observed (Fig. 2).
FIGURE 1.
Median (Q1, Q3) biomarker levels. All biomarker levels were highest at time of delivery, then decreased postpartum to a level comparable with or lower than the level during pregnancy. Quest reference range: (1) hsCRP: >3–10 mg/L is considered high risk and <1 mg/L low risk; (2) D-dimer upper limit of normal is 0.50 µg/mL; (3) IL-6 (highly sensitive) lower limit of detection is −0.31pg/mL. Data gathered from http://www.questdiagnostics.com/testcenter and http://www.specialtylabs.com/clients/gbmc/details.asp?id=S51587&spt=true.
FIGURE 2.
Estimated log biomarker trajectories by ART status group. Displays predicted values at selected time points from longitudinal regression models. All biomarkers increased from the third trimester of pregnancy to delivery and decreased by 6 weeks postpartum to levels below those seen in pregnancy. *One woman was missing the D-dimer measurements.
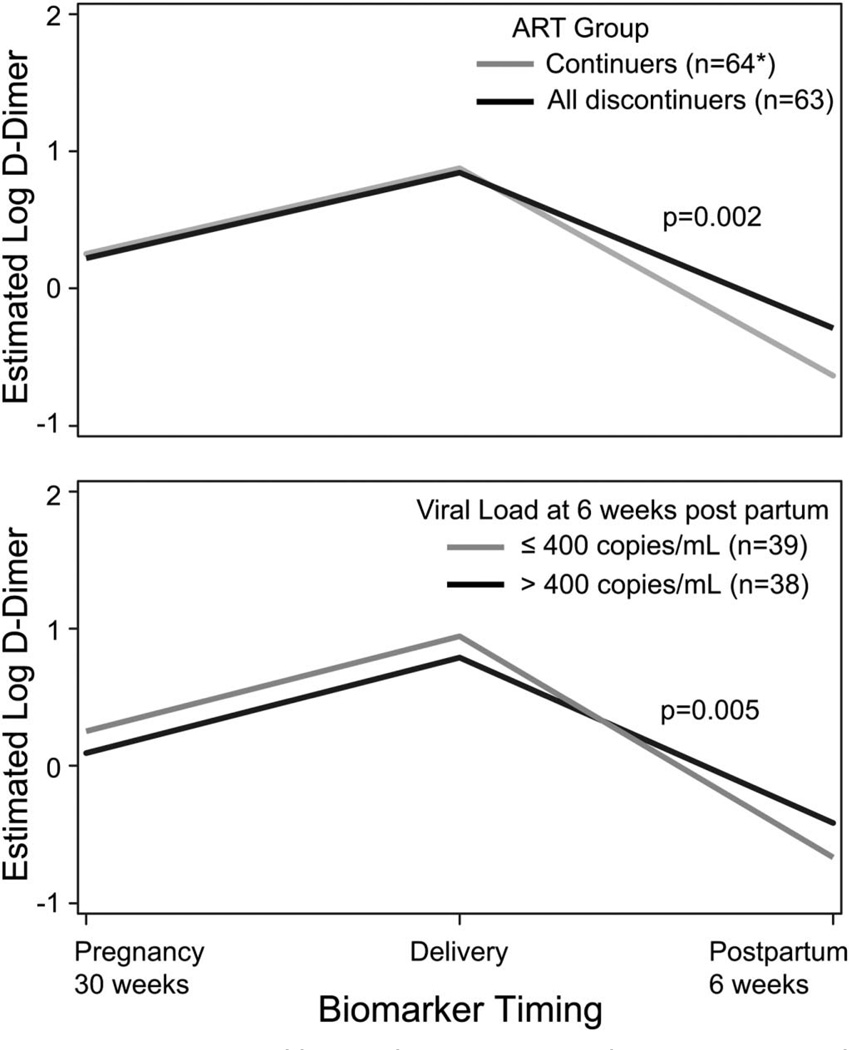
In the regression analysis combining early and late discontinuers into a single group, continuers had a greater decrease in log D-dimer postpartum than discontinuers (estimated change: − 1.51 versus − 1.13, P = 0.002) (Fig. 3).
FIGURE 3.
Estimated log D-dimer trajectory by ART status and viral load at 6 weeks postpartum. Displays predicted values at selected time points from longitudinal regression models. D-dimer levels were significantly higher among women when early and late discontinuers were combined into 1 group (above) and in women with viral loads greater than 400 copies per milliliter (below). *One woman was missing the D-dimer measurements. Viral load at 6 weeks postpartum was only available in 77 women (43 continuers and 34 discontinuers).
Of the 77 women with viral load results available at 6 weeks postpartum, 49% had viral loads >400 copies per milliliter [continuers: 30% (n = 13), discontinuers: 74% (n = 25), P < 0.001]. No significant differences were observed in biomarker trajectories for hsCRP and IL-6 by viral load. Women with viral loads ≤400 copies per milliliter had a greater decrease in log D-dimer postpartum compared with those with viral loads >400 copies per milliliter (estimated change: −1.61 versus −1.20, P = 0.005) (Fig. 3). There were differences in D-dimer by age and ethnicity. Women in the highest age quartile (32 years and older) had a higher log D-dimer at all 3 time points compared with younger women (difference = 0.25, P = 0.02). Hispanic women had a greater decrease in log D-dimer postpartum than non-Hispanic women (estimated change: − 1.60 versus − 1.19, P = 0.002).
Biomarkers and Pregnancy or Delivery Complications
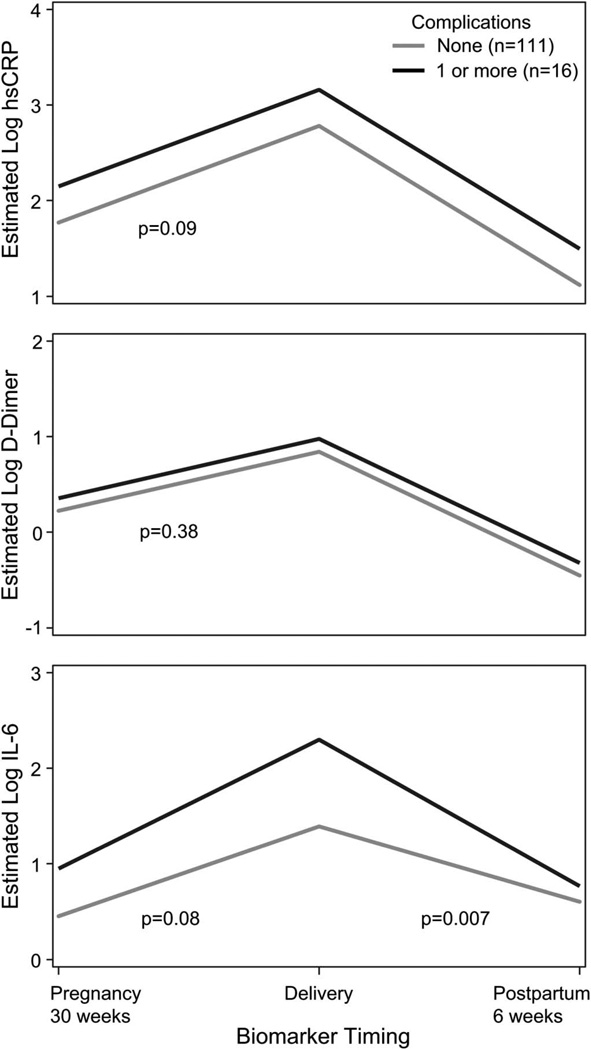
Seventeen women (13% of the study population) had 20 pregnancy or delivery complications. One woman was excluded from this analysis because her first event occurred before her first biomarker measurement. The median time from biomarker collection to complication was 46 days (IQR: 30–75). Women who experienced at least 1 complication had higher levels of IL-6 at all 3 time points and a greater decrease in estimated log IL-6 postpartum (− 1.53 versus − 0.80, P = 0.007) compared with women who had no complications. Women who experienced at least 1 complication also had a higher log hsCRP at each time point compared with those with no complications and a higher log D-dimer at each time point, although these differences were not statistically significant (difference = 0.37, P = 0.09, and difference = 0.14, P = 0.36, respectively) (Fig. 4).
FIGURE 4.
Biomarker trajectories in women with and without pregnancy/delivery complications. Displays predicted values at selected time points from longitudinal regression models. IL-6 trajectory was significantly different in women who experienced at least 1 pregnancy/delivery complication compared with those who did not. At each time point, D-dimer and hsCRP levels were higher in women who experienced at least 1 pregnancy/delivery complication compared with those who did not, but these differences were not statistically significant.
Biomarkers and Complications up to 6 Months Postpartum
Sixteen women had 24 incident postpartum clinical events, but no World Health Organization stage 4 events, significant non–AIDS-defining events, or deaths. No significant increase in the risk of events was observed for ART discontinuers compared with continuers. The most common clinical diagnoses were cervical dysplasia (10 events), bronchitis (4), and pneumonia (3). No significant association was observed between biomarker levels in the third trimester or at delivery and the incidence of postpartum clinical events.
DISCUSSION
Does ART Mediate Markers of Inflammation and Coagulation in Postpartum Women?
In this cohort of HIV-infected women from the United States and Puerto Rico with high CD4 counts, postpartum biomarkers markedly declined among all women, regardless of ART status. Unlike biomarker results from TI studies of nonpregnant women and men with HIV infection, the primary drivers of biomarker levels among our population were pregnancy and delivery. The contribution of inflammation from HIV infection versus pregnancy in our study is unclear because no HIV-uninfected subjects were included. Lack of virological suppression was associated with higher D-dimer levels, supporting the important role of virological suppression in the inflammatory milieu of HIV-infected individuals.
Studies of HIV-uninfected pregnant women show that uncomplicated pregnancy and delivery are characterized by a maternal inflammatory response.12,13 Elevations in D-dimer also have been described during normal pregnancy and uncomplicated delivery.14,15 Mikyas et al16 evaluated serum neopterin, beta-2-microglobulin, tumor necrosis factor alpha, and cell surface activation markers HLA-DR and CD38 on CD8 T cells in 99 HIV-infected women and 46 HIV-uninfected women during pregnancy and up to 6 months postpartum. Evidence of inflammation and cellular immune activation was found in all women at later stages of pregnancy, and the increases in HIV-infected women paralleled those seen in HIV-uninfected women, but at more marked levels. Truong et al17 also found increased levels of serum activation markers in HIV-infected and -uninfected pregnant women, with a rise postpartum despite various antiretrovirals, although postpartum ART blunted the effect. Taken together, these studies suggest that HIV-infected women experience an inflammatory response beyond that seen in healthy women with uncomplicated pregnancies. Our study lends support to the hypothesis that ART attenuates postpartum inflammation, with continuation of treatment associated with more rapid declines in postpartum D-dimer levels.
Association of Biomarkers With Subsequent Late Pregnancy and Delivery Complications Among HIV-Infected Women
CRP levels are elevated in healthy HIV-uninfected women with pre-eclampsia,18 rupture of membranes with chorioamnionitis,19 and preterm delivery,20 and elevated CRP at 9–13 weeks of gestation has been shown to be associated with the subsequent development of gestational diabetes.21 We found an association with IL-6 and pregnancy and delivery complications and a trend toward significance for hsCRP and D-dimer. If confirmed in larger studies, biomarker measurements could be useful for counseling when added to other known risks for adverse pregnancy outcomes (prior history of complications, smoking, hypertension, PI use) to encourage behavior change for modifiable risk factors and inform the need for closer follow-up for those at highest risk of complications.
Biomarkers in Pregnant and Postpartum Women With HIV: Lack of Association With Clinical Events up to 6 Months Postpartum
Controversy exists over whether pregnancy modifies HIV disease in regard to immunologic, virological, and/or clinical outcomes. CD4% seems to remain stable during pregnancy in HIV-infected women.22–25 Viral loads in women without primary acute infection have also been shown to remain stable during pregnancy in the absence of ART.23 Additionally, HIV-infected pregnant women do not seem to have an increased risk of virological failure on ART compared with nonpregnant women, regardless of whether they conceive on ART or initiate after pregnancy.26,27 Therefore, although existing data do not suggest that pregnancy itself accelerates HIV disease progression or that pregnancy increases the risk for virological failure, the potential contribution of a start–stop event or TI to HIV disease progression remains unclear. A recent study evaluated 206 ART-naïve pregnant women with CD4 counts >350 cells per cubic millimeter. No difference in CD4 counts and viral loads was observed over 1 year postpartum regardless of whether women continued or discontinued postpartum antiretrovirals, but more marked CD8+ T-cell immune activation (defined by CD38+ and DR+) was seen among the 59 women who discontinued antiretrovirals.28 In this study, the majority of women discontinued zidovudine monotherapy, and only 18 discontinued 3-drug ART postpartum, limiting the power to detect differences among ART continuers and discontinuers, and reducing the generalizability of the results.
Data on poor outcomes after TI have been obtained from men and nonpregnant women in studies across the globe, including 2 studies from Africa, Trivacan and Development of Antiretroviral Therapy in Africa, both stopped early due to an increased rate of morbidity and/or disease progression.3,5 SMART involved randomization of more than 5000 patients from 33 countries with baseline CD4 counts >350 cells per cubic millimeter (both ART-naïve and on ART), to take therapy continuously or interrupt therapy, with reinitiation for a CD4 decline to <250 cells per cubic millimeter. This trial was also stopped early for a higher risk of serious AIDS-related and non-AIDS events in the TI arm (relative risk: 5.80 and 1.63, respectively).4 We observed high peak median levels of all 3 biomarkers at delivery, over 4 times the latest median hsCRP level (24.0 versus 5.26 mg/L) observed before death among those who died in the SMART study, and over 3 times the latest D-dimer level (2.3 versus 0.70 µg/mL) observed before death in SMART. Caution must be used in generalizing data from these TI studies to pregnant and postpartum women who are younger and healthier with higher CD4 nadir and a shorter duration of ART exposure. IMPAACT’s Promoting Maternal and Infant Survival Everywhere (PROMISE 1077HS) study is an ongoing trial in which women who do not meet criteria for long-term ART (by country-specific guidelines) are randomized to continue or discontinue ART postpartum. Pending data from this trial, information is needed that can help to identify women with high CD4 counts who would most benefit from ART continuation postpartum.
Study Limitations and Weaknesses
Our study has several important limitations. Because the study population did not include HIV-uninfected women, we cannot comment on the contribution of HIV to absolute elevations in biomarkers. Approximately 70% of P1025 participants were excluded from our analysis due to missing samples, and although we did not detect differences other than ART regimen, we cannot exclude the possibility of other unmeasured differences between participants who did versus did not have plasma stored during the study. Additionally, of those women included, we do not have complete data on reasons for continuation versus discontinuation. Therefore, it is possible that women who continued ART in our sample may have been different, either clinically (ie, sicker) or in other ways (providers, insurance, social issues) than those who discontinued. Early in our analysis, we recognized that a subset of ART continuers were not virologically suppressed at 6 weeks postpartum and therefore evaluated groups using viral load data. This analysis was limited to the 77 women who had viral load data and may have been underpowered to detect differences in IL-6 and hsCRP and biased by the large amount of data missing from the analysis. Finally, preconception biomarker data and long-term data on biomarkers and clinical events are important to our understanding of inflammation in women during pregnancy and postpartum but were not collected in our study.
CONCLUSIONS
In contrast to results from TI studies in men and nonpregnant women, both ART continuers and ART discontinuers in this population of women with high nadir CD4 counts had significant decreases in the levels of D-dimer, hsCRP, and IL-6 postpartum. We did find a significantly more rapid decline in D-dimer levels in postpartum ART continuers compared with discontinuers and among those in whom viral loads were ≤400 copies per milliliter at 6 weeks postpartum. We found a significant association between IL-6 and subsequent pregnancy and delivery complications, but no significant associations between biomarker levels and AIDS and non–AIDS-defining clinical events up to 6 months postpartum. Future studies should evaluate preconception biomarker levels and incorporate longer postpartum follow-up to better characterize associations between biomarkers, ART status, and clinical events. Questions about use of ART in postpartum women must be answered through direct, carefully planned, randomized trials that inform clinical decision making for this unique subset of the HIV-infected population.
ACKNOWLEDGMENTS
Supported by the University of California, Los Angeles, Iris Cantor Center for Women’s Health Seed Grant Program and National Institutes of Health (NIH, grant AI56933; J.S.C., Principle Investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (AI068632). This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under NIAID cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group and #1 U01AI068616 with the IMPAACT. Support of the sites was provided by the NIAID and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Appendix
Members of the NWCS 101-P1025 Protocol Team include the following: R. M. Hoffman, E. Leister, D. Kacanek, D. E. Shapiro, J. S. Read, Y. Bryson, and J. S. Currier.
P1025 Team Acknowledgement: G. B. Scott, MD, University of Miami School of Medicine, Miami, FL; R. Tuomala, MD, Brigham and Women’s Hospital, Boston, MA; E. Smith, MD, National Institute of Allergy and Infectious Diseases Division of AIDS, Pediatric Medicine Branch, Bethesda, MD; H. Watts, MD, National Institute of Child Health and Human Development, Maternal and Pediatric Infectious Disease Branch, Bethesda, MD; K. M. Oden, MHS, International Maternal Pediatric Adolescent AIDS Clinical Trials Group, Silver Spring, MD; Y. Huo, MS, Harvard School of Public Health, Boston, MA; K. Patel, DSc, MPH, Harvard School of Public Health, Boston, MA; E. A. Barr, CPNP, CNM, MSN, University of Colorado Denver, The Children’s Hospital, Denver, CL; A. Bardeguez, MD, MPH, FACOG, University of Medicine & Dentistry of New Jersey, Newark, NJ; S. K. Burchett, MD, MSc, Harvard Medical School, Boston, MA; E. Livingston, MD, Duke University Medical Center, Durham, NC; A. M. Stek, MD, Keck School of Medicine, University of Southern California, Los Angeles, CA; M. T. Basar, BS, Frontier Science & Technology Research Foundation, Inc, Amherst, NY; A. Hernandez, MA, Frontier Science & Technology Research Foundation, Inc, Amherst, NY; A. Jennings, BS, Frontier Science & Technology Research Foundation, Inc, Amherst, NY; T. R. Cressey, PhD, BSc, Program for HIV Prevention & Treatment, Chang Mai, Thailand; and J. Bryant, MPA, Westat, Rockville, MD.
Participating sites and site personnel include the following: 5041 Children’s Hospital of Michigan NICHD CRS (T. B. Jones, MD; E. Brown, RN; and N. Woods, RD); 5052 University of Colorado Denver NICHD CRS (A. Katai, MHA; T. Kennedy, FNP-BC; K. Kinzie, MSN, FNP-BC; J. Wallace, MSW; and CTSI Grant Number UL1 TR000154); 5031 San Juan City Hospital PR NICHD CRS (R. Diaz- Velasco, MD, FACOG, AAHIVS; M. Acevedo-Flores, MD, MT; E. Pérez-Hernández, BS, MEd, MA, MPH; and A. Rodriguez-Mimoso, MD, FACOG); 5048 USC LA NICHD CRS (A. Stek, MD; F. Kramer, MD; L. Spencer, MD; and J. Homans, MD); 4601 UCSD Maternal, Child, and Adolescent HIV CRS (A. Hull, MD; M. Caffery, RN, MSN; J. M.Manning RN, BSN; and S. A. Spector, MD); 4101 Columbia IMPAACT CRS; 4201 University of Miami Pediatric Perinatal HIV/AIDS CRS (G. B. Scott, MD; C. D. Mitchell, MD; S. Yasin, MD; and Safia Khan, MD); 5083 Rush University Cook County Hospital Chicago NICHD CRS (M. Aziz, MD; L. Logan, MD; J. Schmidt, MD; and H. Cejtin, MD); 5096 University of Alabama Birmingham NICHD CRS (M. Crain, MPH, MD; S. Robbins, BA; M. Parks, CRNP; and Y. Gamble Duke, MA); 6901 Bronx-Lebanon Hosp. IMPAACT CRS (M. Purswani, MD; S. Hagmann, MD, MSc, FAAP; J. Gutierrez, MD; and M. Vachon, LMSW, MPH); 5012 NYU School of Medicine NICHD CRS (W. Borkowsky, MD; M. Minter, RN; A. Kaul, MD; and N. Deygoo, MS); 3801 Texas Children’s Hospital CRS (S. Buschur, RN, NMV; K. Pitts, CPNP; C. McMullen-Jackson, BSN, RN; and T. Aldape, LMSW; grant number AI069441); 4001 Chicago Children’s CRS (Donna McGregor, RN); 5009 Children’s Hospital of Boston NICHD CRS (S. K. Burchett, MD, MS; R. Tuomala, MD; A. Buck, RN; and C. Kneut, RN, CPNP); 5018 USF - Tampa NICHD CRS (P. Emmanuel, MD; K. Bruder, MD; and G. Lewis, RN); 6501 St Jude/UTHSC CRS (K. Knapp, MD; E. Thorpe, MD; N Sublette, FNP, PhD; and P. Finnie, MSN); 2802 NJ Medical School CRS (A. D. Bardeguez, MD, MPH; C. Calilap-Bernardo, RN; and L. Bettica, RN); 3601 UCLA-Los Angeles/Brazil AIDS Consortium CRS (J. G. Deville, MD; K. Nielsen-Saines, MD; N. Falgout, RN; and M. Carter, RN); 4005 Mt Sinai Hospital Med Center, Women’s & Children’s HIV Program (B. Wolfe, APN; and M. Hartrich, MPH); 5017 Seattle Children’s Hospital CRS; 5023 Washington Hospital Center NICHD CRS (S. Zeichner, MD, PhD; S. R. Parker, MD; P. Tanjutco, MD; and V. Emmanuel, BA); 5028 University of Illinois College of Medicine at Chicago, Department of Pediatrics (K. Rich, MD; K. Hayani, MD; and J. Camacho, RN); 5051 University of Florida College of Medicine, Jacksonville (M. Rathore, MD; A. Mirza, MD; N. Maraqa, MD; and K Thoma, MA, CCRP); 5094 University of Maryland Baltimore NICHD CRS (D. Watson, MD; and C. Hilyard); 6601 University of Puerto Rico Pediatric HIV/AIDS Research Program CRS (I. L. Febo, MD; V. Tamayo, MD; R. Santos, RN, MPH; and M. Cruz-Rodriguez); 5003 Metropolitan Hospital NICHD CRS; 5013 Jacobi Medical Center Bronx NICHD CRS (S. Gross, MD; M. Moore, MD; and C. Caines, RN); 5038 Yale University School of Medicine; 5045 Harbor UCLA Medical Center NICHD CRS (M. Keller, MD; S. Wettgen, RN, PNP; J. Hayes, RN; and Y. Gonzalez, RN); 5095 Tulane University New Orleans NICHD CRS (Y. Luster, RN; R. Maupin, MD; C. Dola, MD; and M. Silio, MD); 6701 The Children’s Hosp. of Philadelphia IMPAACT CRS (S. D. Douglas, MD; R. M. Rutstein, MD; and C. A. Vincent, CRNP, MSN).
Footnotes
Presented in part at the 19th Conference on Retroviruses and Opportunistic Infections, March 5–8, 2012, Seattle, WA. Abstract 1056.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Washington, DC: Department of Health and Human Services; 2012. [Accessed April 15, 2013]. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. [Accessed April 15, 2013];Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2012 Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf.
- 3.Danel C, Moh R, Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 4.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 5.DART Trial Team. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts < 200 cells/microl. AIDS. 2008;22:237–247. doi: 10.1097/QAD.0b013e3282f2d760. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Read JS, Huo Y, Patel K, et al. Laboratory abnormalities among HIV-exposed, uninfected infants: IMPAACT Protocol P1025. J Pediatr Infect Dis Soc. 2012;1:92–102. doi: 10.1093/jpids/pis036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel K, Shapiro DE, Brogly SB, et al. Prenatal protease inhibitor use and risk of preterm birth among HIV-infected women initiating antiretroviral drugs during pregnancy. J Infect Dis. 2010;201:1035–1044. doi: 10.1086/651232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Guidelines for national human immunodeficiency virus case surveillance, including monitoring for human immunodeficiency virus infection and acquired immunodeficiency syndrome. MMWR Recomm Rep. 1999;48:1–27. 29–31. [PubMed] [Google Scholar]
- 10.World Health Organization. [Accessed April 15, 2013];WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-related Disease in Adults and Children. 2007 Available at: www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf.
- 11.Quest Diagnostics. [Accessed April 15, 2013];Interleukin-6 and Highly Sensitive CRP Test Information. 2012 Available at: http://www.questdiagnostics.com/testcenter/TestDetail.action?tabName=’ingInfo&ntc=34473 and http://www.questdiagnostics.com/testcenter/TestDetail.action?ntc=10124.
- 12.Veenstra van Nieuwenhoven AL, Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update. 2003;9:347–357. doi: 10.1093/humupd/dmg026. [DOI] [PubMed] [Google Scholar]
- 13.Sacks G, Sargent I, Redman C. An innate view of human pregnancy. Immunol Today. 1999;20:114–118. doi: 10.1016/s0167-5699(98)01393-0. [DOI] [PubMed] [Google Scholar]
- 14.Epiney M, Boehlen F, Boulvain M, et al. D-dimer levels during delivery and the postpartum. J Thromb Haemost. 2005;3:268–271. doi: 10.1111/j.1538-7836.2004.01108.x. [DOI] [PubMed] [Google Scholar]
- 15.Kline JA, Williams GW, Hernandez-Nino J. D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed. Clin Chem. 2005;51:825–829. doi: 10.1373/clinchem.2004.044883. [DOI] [PubMed] [Google Scholar]
- 16.Mikyas Y, Aziz N, Harawa N, et al. Immunologic activation during pregnancy: serial measurement of lymphocyte phenotype and serum activation molecules in HIV-infected and uninfected women. J Reprod Immunol. 1997;33:157–170. doi: 10.1016/s0165-0378(97)00018-1. [DOI] [PubMed] [Google Scholar]
- 17.Truong HM, Sim MS, Dillon M, et al. Correlation of immune activation during late pregnancy and early postpartum with increases in plasma HIV RNA CD4/CD8 T cells, and serum activation markers. Clin Vaccine Immunol. 2010;17:2024–2028. doi: 10.1128/CVI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teran E, Escudero C, Moya W, et al. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet. 2001;75:243–249. doi: 10.1016/s0020-7292(01)00499-4. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Jun JK, Park KH, et al. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996;88:1034–1040. doi: 10.1016/s0029-7844(96)00339-0. [DOI] [PubMed] [Google Scholar]
- 20.Hvilsom GB, Thorsen P, Jeune B, et al. C-reactive protein: a serological marker for preterm delivery? Acta Obstet Gynecol Scand. 2002;81:424–429. doi: 10.1034/j.1600-0412.2002.810509.x. [DOI] [PubMed] [Google Scholar]
- 21.Wolf M, Sandler L, Hsu K, et al. First-trimester C-reactive protein and subsequent gestational diabetes. Diabetes Care. 2003;26:819–824. doi: 10.2337/diacare.26.3.819. [DOI] [PubMed] [Google Scholar]
- 22.Tuomala RE, Kalish LA, Zorilla C, et al. Changes in total, CD4+, and CD8+ lymphocytes during pregnancy and 1 year postpartum in human immunodeficiency virus-infected women. The Women and Infants Transmission Study. Obstet Gynecol. 1997;89:967–974. doi: 10.1016/s0029-7844(97)00129-4. [DOI] [PubMed] [Google Scholar]
- 23.Burns DN, Landesman S, Minkoff H, et al. The influence of pregnancy on human immunodeficiency virus type 1 infection: antepartum and postpartum changes in human immunodeficiency virus type 1 viral load. Am J Obstet Gynecol. 1998;178:355–359. doi: 10.1016/s0002-9378(98)80025-2. [DOI] [PubMed] [Google Scholar]
- 24.Ekouevi DK, Inwoley A, Tonwe-Gold B, et al. Variation of CD4 count and percentage during pregnancy and after delivery: implications for HAART initiation in resource-limited settings. AIDS Res Hum Retroviruses. 2007;23:1469–1474. doi: 10.1089/aid.2007.0059. [DOI] [PubMed] [Google Scholar]
- 25.Miotti PG, Liomba G, Dallabetta GA, et al. T lymphocyte subsets during and after pregnancy: analysis in human immunodeficiency virus type 1-infected and -uninfected Malawian mothers. J Infect Dis. 1992;165:1116–1119. doi: 10.1093/infdis/165.6.1116. [DOI] [PubMed] [Google Scholar]
- 26.Keiser O, Gayet-Ageron A, Rudin C, et al. Antiretroviral treatment during pregnancy. AIDS. 2008;22:2323–2330. doi: 10.1097/QAD.0b013e3283189bf1. [DOI] [PubMed] [Google Scholar]
- 27.Westreich D, Evans D, Firnhaber C, et al. Prevalent pregnancy, biological sex, and virologic response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;60:489–494. doi: 10.1097/QAI.0b013e318256b310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watts DH, Lu M, Thompson B, et al. Treatment interruption after pregnancy: effects on disease progression and laboratory findings. Infect Dis Obstet Gynecol. 2009;2009:456717. doi: 10.1155/2009/456717. [DOI] [PMC free article] [PubMed] [Google Scholar]