Abstract
Objective
Evaluation of 18F-FDG uptake value via PET is central to current methods of diagnosis and staging of non-small cell lung cancer (NSCLC) due to its ability to evaluate expression levels of key regulators associated with glucose metabolism in tumor cells. Tp53-induced glycolysis and apoptosis regulator (TIGAR) is an important P53-induced protein that can inhibit glycolysis; however, there have been few clinical studies on its mechanism. Here we have investigated the relationship between TIGAR expression and 18F-FDG PET in tumors, along with its relationship with the clinical characteristics of NSCLC.
Methods
We analyzed SUVmax in 79 patients with NSCLC through immunohistochemical staining of TIGAR and five other biological markers associated with tumor cell glycolysis, in order to evaluate the correlation between their expression and SUVmax. We also plotted Kaplan-Meier survival curves to assess TIGAR expression with the prognosis and survival of patients with NSCLC.
Results
The key findings were as follows: SUVmax was negatively correlated with the expression of TIGAR (r = −0.31, p<0.01); TIGAR expression was correlated with tumor size (p = 0.01), histological type (p<0.01), differentiation degree (p<0.01) and lymph node metastasis(p<0.01) in patients with NSCLC; and the survival time of patients whose TIGAR was negatively expressed was significantly shorter than for those whose TIGAR was positively expressed (P = 0.023).
Conclusions
The expression of TIGAR in primary tumors is significantly correlated with SUVmax, and low expression of TIGAR may predict a worse clinical outcome in patients with NSCLC.
Introduction
Unlike normal cells, most cancer cells depend on a high rate of glycolysis for energy production during malignant progression. This is known as the Warburg effect and is considered the seventh hallmark of cancer [1]. This effect has been exploited in 18F-fluorodeoxyglucose positron emission tomography/computerized tomography (18F-FDG PET/CT) technologies, which has proved highly successful in clinical practice and is widely applied in tumor diagnosis [2], [3].
The maximal standardized uptake value (SUVmax) determined through PET imaging is a simple and reliable method of evaluating the glucose uptake capacity of tumors in vivo. It is defined as the ratio of activity in tissue per unit volume to the activity in the injected dose per patient body weight [4]. Recent reports have demonstrated that the SUVmax of primary tumors is correlated with the stage, nodal status, histologic type, differentiation and progression of tumors [5], [6]. Furthermore, a high SUVmax has been linked with poor prognosis in cancer patients [7].
Despite these observations, further investigations are required to establish the clinical value of 18F-FDG PET/CT imaging for tumor diagnosis, by identifying the metabolic enzymes and abnormal expressions of cancer genes that underlie SUVmax changes in cancer tissues. Therefore, many studies have focused on defining the relationship between FDG uptake and the expression of tumor biomarkers, including nuclear- associated antigen Ki-67, cyclooxygenase-2 (Cox-2), vascular endothelial growth factor (VEGF) and the glucose transporter 1 (GLUT1) in relation to lung cancer [4], [8], [9]. To date, these have reported a correlation between SUVmax and tumor biomarkers and have contributed to understanding the Warburg effect and identifying tumor-related genes that are associated with the SUV, thereby providing a robust basis for staging, prognosis and personalized treatment of cancers.
Tp53-induced glycolysis and apoptosis regulator (TIGAR) is a novel gene related to the glucose metabolism in tumor cells [10]. TIGAR inhibits glycolysis by limiting the level of fructose-2, 6-bisphosphate (Fru-2, 6-BP) in the cell by functioning as a Fru-2, 6-bisphosphatase (Fru-2, 6-BPase). It was commonly believed that induction of TIGAR led to glycolysis inhibition of the cancer suppressor, P53 [11]. More recent studies have suggested that TIGAR expression increases NADPH levels through activation of the pentose phosphate pathway (PPP), thereby promoting antioxidant function which reduces reactive oxygen species (ROS)-associated apoptosis and enhances cancer cell survival [12]. However, these studies were conducted on a limited number of cell lines through intervening transient expression experiments; therefore, it remains to be established whether TIGAR expression is correlated with SUV on FDG-PET and tumor prognosis in clinical practice, by acting as a suppressor gene of glucose metabolism.
The purpose of this present study was to assess the correlation between SUVmax and the expression of selected markers associated with tumor metabolism, namely, glucose transporter 1(GLUT1), hexokinase 2 (HK2), pyruvate kinase M2 (PKM2), lactate dehydrogenase A (LDHA), protein kinase B (AKT) and TIGAR. In addition, the relationship between the TIGAR expression and prognosis in non-small cell lung carcinoma (NSCLC) was investigated. We selected 79 NSCLC cases for this study, with the aim of identifying novel biological indictors for clinical diagnosis and personalized treatment options, by analyzing the relationship between SUV and key regulatory factors in glucose metabolism.
Patients and Methods
Ethics Statement
The Human Investigation Ethical Committee of Shanghai Jiao Tong University affiliated Renji Hospital and Shanghai Chest Hospital approved this study. All procedures involving human specimens were performed with written informed consent according to the Declaration of Helsinki.
Study Population
This was a retrospective study of all patients who were confirmed to have NSCLC based on histopathological finding and underwent surgery after 18F-FDG PET/CT between December 2006 and December 2009 at Shanghai Jiaotong University affiliated Renji Hospital and Shanghai Chest Hospital. Eligibility criteria were (1) without receiving chemotherapy/ radiotherapy before PET/CT scanning; (2) tumor pathology of NSCLC (excluded benign lung lesions and small cell lung cancers); (3) complete case records; (4) performed PET/CT scanning no more than 2 weeks prior to surgery; (5) available tissue specimen for IHC staining. Finally, 79 patients (50 male and 29 female) with a median age of 61 (range, 30–79 years) were evaluated in this study. All clinical and pathological findings were reviewed from three hospitals in Shanghai, China (Renji Hospital, Shanghai Chest Hospital, Ruijin Hospital).
PET/CT imaging
A dedicated whole-body PET/CT tomography (SIMENS) was used for all PET/CT imaging. Image acquisition was performed with an integrated PET/CT device. Immediately after CT scanning, PET was performed to cover the identical axial field of view. PET-image data sets were reconstructed iteratively with segmented correction for attenuation using the CT data. For semi-quantitative analysis of the 18F-FDG uptake, irregular regions of interest (ROIs) were placed over the most intense area of 18F-FDG accumulation. The maximum SUV (SUVmax) was calculated using following formula: maximum pixel value with the ROI activity (MBq/kg)/(injected dose [MBq]/body weight [kg]). Two experienced nuclear medicine physicians, blinded to the clinical history, independently evaluated the PET images and reached a consensus on all image results.
Immunohistochemical Staining
Immunohistochemical analyses were performed on paraffin-embedded lung cancer tissues. After microtome sectioning (4 µm slices), the slides were processed for staining. All primary antibodies (TIGAR, GLUT-1, HK-2, PKM-2, LDHA, AKT) were purchased from Abcam. Sections were assessed using a light microscope (BX51TR, Olympus, Japan) by 2 independent observers. The expression of each marker protein was examined and statistical software (DFC320 CCD system, Leica, Germany) was used for semi-quantitative analysis of IHC staining. The slides were scored for intensity of staining (0 to 3) and the percentages of cells with scores of 0 (0%), 1 (1% to 9%), 2 (10% to 49%), and 3 (50% to 100%) were determined. The immunohistochemistry (IHC) score (0 to 9) was defined as the product of the intensity and percentage of cells. Protein expression was judged as positive when the IHC score was greater than or equal to 4. All IHC results were evaluated by 2 experienced observers who were blind to the condition of the patients. Where discrepancies occurred by 2 readers, the 2 readers reached a consensus.
Statistical Analysis
Continuous variables were analyzed by the Student's t test, and the results were expressed as mean ± standard deviation. Dichotomous variables were analyzed by the x2 test. We used Spearman rank correlation to examine the association between SUVmax and protein expression. To explore the association between recurrence-free survival and TIGAR expression, a Kaplan-Meier survival analysis was performed. Two- sided p values of less than 0.05 were considered to be statistically significant. All analyses were performed using SPSS software, version 16.0 (SPSS Inc, Chicago, IL).
Results
Clinical and pathological characteristics of patients in relation to SUVmax
The clinical and pathological characteristics of the 79 patients with NSCLC in this study are illustrated in Table 1. The patients were aged between 30 and 79 years (mean; 60.85±10.40 years) and included 50 males and 29 females. The mean SUVmax was 7.24±4.58, the maximum, minimum and median values were 24.1, 0.7 and 6.6 respectively, and 10 patients had a SUVmax <2.5. Statistical analysis showed that there were no significant differences in SUVmax between patients ≥60 years old and patients <60 years old or between male and females patients.
Table 1. Clinical and pathological characteristics in relation to SUVmax in patients with NSCLC.
| Characteristic | No. patients | SUVmax | P-value |
| Age | |||
| 60 years | 39 | 6.85±3.81 | 0.58 |
| 60 years | 40 | 7.42±4.23 | |
| Gender | 0.37 | ||
| Male | 50 | 7.65±4.27 | |
| Female | 29 | 6.54±4.84 | |
| Tumor size (cm) | <0.01 | ||
| ≤3.0 | 54 | 5.62±3.82 | |
| >3.0 | 25 | 10.7±4.18 | |
| Pathology | <0.01 | ||
| Squamous | 24 | 10.60±4.73 | |
| Adenocarcinoma | 55 | 5.94±4.04 | |
| Tumor differentiation | <0.01 | ||
| Well | 13 | 4.4±3.1 | |
| Moderate | 34 | 7.2±4.2 | |
| Poor | 32 | 12.7±4.1 | |
| Pathological N stage | <0.01 | ||
| N0 | 55 | 6.33±4.60 | |
| N1 | 24 | 9.31±3.89 | |
| Outcome | <0.01 | ||
| Alive | 42 | 6.13±3.89 | |
| Dead | 20 | 9.66±4.15 |
SUVmax = maximal standardized uptake value.
However, there were significant differences in SUVmax between patients with tumors >3 cm and those with tumors ≤3 cm in size. In addition, SUVmax was statistically different between patients with well, moderate and poorly differentiated tumors; between patients with squamous carcinoma and adenocarcinoma and between patients with pathological stage N0 and N1 tumors (p<0.01).
Relationship between FDG-PET and immunohistochemical scores of proteins involved in glucose metabolism in tumor cells
We carried out immunohistochemical (IHC) analysis in order to assess the correlation between PET SUVmax and the expression of selected proteins associated with glucose metabolism in tumor cells according to their IHC staining scores.
The results are shown in Figure 1 and presented in Table 2. SUVmax of FDG showed a negative correlation with TIGAR expression (r = −0.31, p<0.01). In addition, SUVmax was significantly correlated with expression levels of GLUT1(r = 0.37, p<0.01). However, SUVmax was weakly correlated with PKM2 and not correlated with glucose metabolism genes, LDHA, HK2 or oncogenes, AKT.
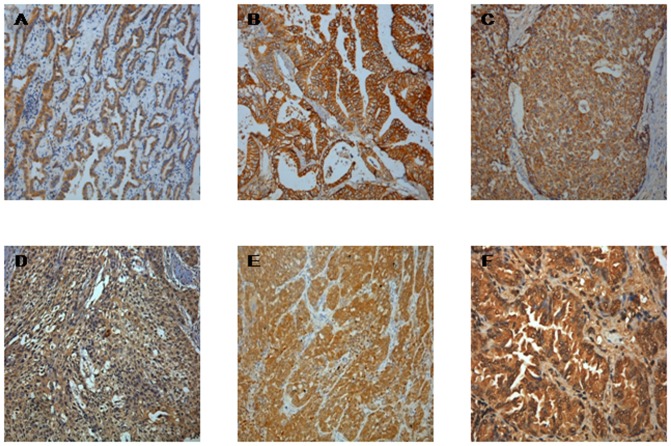
Figure 1. Immunohistochemical analysis showed positive staining.
A: TIGAR, B: GLUT, C: HK2, D: PKM, E: LDHA, F: AKT (magnification, ×400).
Table 2. Pearson correlation coefficients and p-value between the immunohistochemistry (IHC) staining scores for genes expression associated with glucose metabolism in tumors with SUVmax.
| Factor | SUVmax | ||
| correlation coefficients | P-value | ||
| TIGAR expression | 0.31 | <0.01 | |
| GLUT-1 expression | 0.37 | <0.01 | |
| HK-2 expression | 0.08 | 0.45 | |
| PKM-2 expression | 0.21 | 0.06 | |
| LDHA expression | 0.09 | 0.39 | |
| AKT expression | 0.04 | 0.71 |
There was a significant difference in SUVmax between patients with positive and negative expression of TIGAR (P<0.01). The mean value of SUVmax was 8.29±3.64 in the TIGAR negative cases and 6.31±3.14 in the TIGAR positive cases. Similarly, SUVmax was significantly different between patients with positive and negative expression of GLUT1 (P<0.01). The mean value of SUVmax was 6.09±3.60 in the GLUT1 negative cases and 8.83±5.33 in the GLUT1 positive cases (Table 3).
Table 3. Correlation between TIGAR or GULT-1 expression and SUVmax.
| Factor | No. patients | SUVmax | P-value |
| TIGAR expression | <0.01 | ||
| Negative | 37 | 8.29±3.64 | |
| Positive | 42 | 6.31±5.14 | |
| GLUT-1 expression | <0.01 | ||
| Negative | 46 | 6.09±3.60 | |
| Positive | 33 | 8.83±5.33 |
We further analyzed the impacts of the different expression level of TIGAR and GLUT1 on glucose uptake (Table 4). The maximum SUVmax (mean, 10.20±3.40) was observed in patients with negative expression of TIGAR and positive expression on GLUT1. Conversely, the minimum SUVmax (mean, 5.47±3.89) was observed in patients with positive expression of TIGAR and negative expression of GLUT1.
Table 4. Correlation between expression of TIGAR and GLUT-1 with SUVmax, based on immunohistochemical (IHC) score.
| TIGAR (IHC score) | |||
| No. patients | No. patients | ||
| Negative | positive | ||
| Negative | 6.82±3.17 | 5.47±3.89 | |
| GLUT-1 | 46 | 21 | 25 |
| Positive | 10.20±3.40 | 7.54±6.51 | |
| 33 | 16 | 17 | |
Relationship between TIGAR expression and the biological characteristics and staging grade of NSCLC tumors
Although TIGAR is closely linked to glucose uptake in tumor tissues, there have been few reports on its relationship with the biological characteristics of tumor tissues. Therefore we analyzed TIGAR expression levels in tissue samples from 79 patients with NSCLC in relation to age, gender, tumor size, histological type, histological degree and tumor staging. The results are presented in Table 5. These showed that the difference between TIGAR expression in respect of patient gender or age (<60 or ≥60 years) was not significant. Conversely, the difference was significant between TIGAR expression in patients with respect to tumor size (≤3 or >3 cm), histological type (squamous or adenocarcinoma), differentiation degree (well or poor) and staging (N0 or N1).
Table 5. The relationship between TIGAR expression and tumor characteristics, based on immunohistochemical (IHC) score.
| TIGAR (IHC score) | |||
| Characteristic | No. patients (n = 79) | ||
| Negative | Positive | P-value | |
| Age | 0.22 | ||
| <60 years | 21 | 18 | |
| ≥60 years | 16 | 24 | |
| Gender | 0.09 | ||
| Male | 27 | 23 | |
| Female | 10 | 19 | |
| Tumor size (cm) | 0.01 | ||
| ≤3 | 20 | 34 | |
| >3 | 17 | 8 | |
| Pathology | <0.01 | ||
| Squamous | 18 | 7 | |
| Adenocarcinoma | 19 | 35 | |
| Tumor differentiation | <0.01 | ||
| poor | 21 | 11 | |
| well | 16 | 31 | |
| Pathological N stage | <0.01 | ||
| N0 | 19 | 36 | |
| N1 | 18 | 6 | |
The relationship between TIGAR expression with survival and prognosis of patients with NSCLC
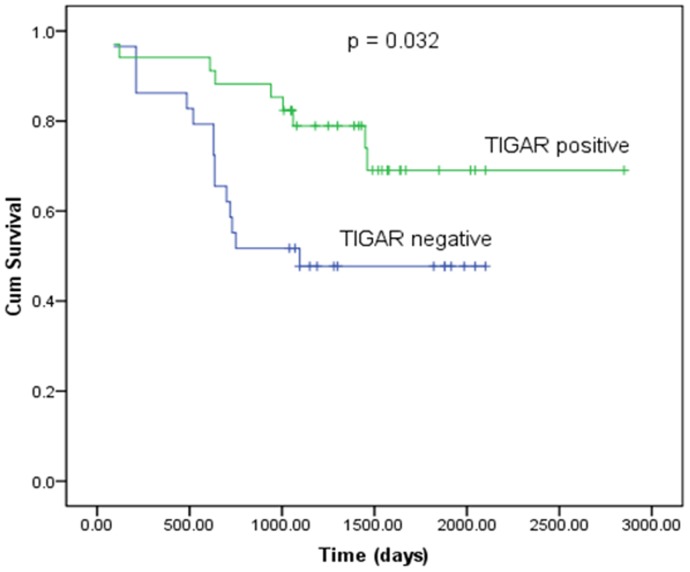
In order to assess the impact of TIGAR expression on patients' survival, we plotted Kaplan-Meier survival curves for patients with positive expression and negative expression of TIGAR (Figure 2). The results showed that the survival time of patients whose TIGAR was negatively expressed was significantly shorter than for those whose TIGAR was positively expressed (P = 0.023 according to the log-rank test).
Figure 2. Kaplan-Meier recurrence-free survival curve according to TIGAR expression in patients with NSCLC.
The survival time of patients with negative expression of TIGAR is significantly shorter than those with positive expression of TIGAR.
Discussion
Lung cancer has the highest incidence rate of human malignancies and is the leading cause of cancer mortality worldwide. Patients with NSCLC account for 80 to 85% of all patients with lung cancer; therefore, early diagnosis and targeted treatments are critical to improving survival rates of lung cancer patients [13]. In this respect, FDG-PET molecular imaging techniques have provided major advances in clinical diagnosis of lung cancer. In accordance with previous reports, our study showed that SUVmax was closely correlated with tumor size, differentiation degree and pathological type in NSCLC patients. In addition, patients with higher SUVmax had an increased probability of lymph node metastasis and a worse prognosis. High SUVmax is correlated with an increase in glucose metabolism and the Warburg effect in tumor cells. It is generally acknowledged that the Warburg effect is associated with abnormal expression of glucose metabolism enzymes (GLUT1, HK2,PKM2, LDHA), oncogenes (Ras, MET), signal transduction kinases (AMP, AKT) and mitochondrial proteins (IDH3, COX2), as well as a hypoxic microenvironment in which the expression level of hypoxia-inducible factor 1 (HIF-1) increases [14]–[16]. However, the mechanisms underlying the Warburg effect are not yet fully understood.
In our present study, the expression levels of seven metabolic enzymes associated with glucose metabolism in tumor cells were compared with SUVmax in 79 patients with NSCLC. Our results showed that SUVmax of FDG uptake was significantly correlated with expression levels of GLUT1 and TIGAR, weakly correlated with PKM2 and not correlated with glucose metabolism genes, LDHA, HK2 or oncogenes, AKT.
Previous reports have suggested that GLUT1 has an important role in 18F-FDG uptake in patients with lung cancer, soft tissue sarcoma and pancreatic cancer [9], [17], [18]. Conversely, Tohma et al. reported low correlation between 18F-FDG uptake and GLUT1 expression in esophageal cancer [19]. Our data demonstrated that GLUT1 expression was significantly associated with high SUVmax, supporting earlier reports that GLUT1 expression contributes to 18F-FDG uptake in NSCLC patients.
Notably, this study has provided the first evidence of a correlation between TIGAR expression and SUVmax on FDG-PET in patients with NSCLC. Our data showed that TIGAR expression is negatively correlated with tumor SUVmax. Furthermore, SUVmax was highest in patients who had positive expression of GLUT1 with negative expression of TIGAR. This further demonstrated that TIGAR involved in glycolysis in lung cancer and TIGAR inhibition could remove the negative regulation of glucose metabolism, thereby increasing glycolysis in tumor cells.
Many experiments that have been performed at the cellular level have confirmed that TIGAR can inhibit glycolysis. This is consistent with our clinical observations. It is generally considered that the glycolysis mechanism induced by TIGAR bears close similarity to that of the bisphosphatase (FBPase) domain of 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase (PFK-2/FBPase-2), in that TIGAR expression leads to a reduction in fructose-2, 6-bisphosphate (Fru-2, 6-BP) levels, thereby suppressing glycolytic flux and tumor growth. Fru-2, 6-BP is a potent allosteric regulator of PFK1, an early enzyme in the glycolytic pathway. Furthermore, the decrease in Fru-2, 6-BP levels may lead to decreased GK (the hepatic isoform of hexokinase) and increased G6Pase levels [20], providing an additional means by which TIGAR can negatively regulate glycolysis.
Activation of glycolysis is not only beneficial to tumor metabolism for energy production and proliferation, but can also activate multiple transcription factors associated with oncogenes and tumor development, such as sterol regulatory element binding protein (SREBP) which is involved in fatty acid synthesis [21]. Therefore, these glycolytic genes have also been the focus of studies on tumor development. It is increasingly apparent that TIGAR may play other significant roles in controlling processes involved in human malignancies; however, the mechanisms remain to be fully understood. It has been suggested that TIGAR inhibits tumor growth by acting in a similar manner to the tumor suppressor P53. López-Guerra et al. found that high expression of TIGAR was positively correlated with chronic lymphocytic leukemia (CLL) sensitivity to Fludarabine, which can induce apoptosis [22]. Madan et al. showed that TIGAR could stabilize the retinoblastoma protein and E2F transcription factor 1 (Rb-E2F1) complex leading to cell cycle arrest and inhibition of tumor growth [23]. Similarly, Hesegawa found that high expression of TIGAR promoted cell cycle arrest and cellular senescence, whereas a TIGAR knockout acted to reduce apoptosis [24]. However, a conflicting mechanism proposed by Bensaad et al. suggested that TIGAR inhibited glycolysis at the cellular level while activating the PPP, which inhibited ROS production and tumor cell apoptosis and autophagy; thereby promoting tumor growth in U2OS and RKO cell lines [12].
The majority of studies on TIGAR have been performed at the cellular level with only a few reports on clinical studies. Therefore, we conducted this study to compare TIGAR expression with the clinical characteristics of tumors, and its relationship to prognosis and survival in patients with NSCLC. We demonstrated that TIGAR expression was significantly higher in lung adenocarcinoma tissue than in squamous carcinoma (P<0.01), which is consistent with observations of lower SUV in lung adenocarcinoma. We found that well-differentiated NSCLC had higher expression levels of TIGAR compared to poorly differentiated NSCLC, indicating increased malignancy. In addition, we showed that patients with negative expression of TIGAR were more likely to develop lymph node metastasis than those with positive expression of TIGAR. These indicated that TIGAR expression was closely correlated with prognosis. Survival curve analysis showed that the survival rate declined significantly in NSCLC patients who had low levels of TIGAR expression (P = 0.023) compared those with high levels. These findings, based on clinical studies of NSCLC tissues, suggest that quantification of TIGAR protein levels may help predict prognosis of patients with NSCLC and cancer treatment targeting TIGAR might become feasible in the future. We have proposed to conduct a trial of a TIGAR activator for the treatment of lung cancer in PET-positive mice with the aim of developing potential gene targeted therapies for lung cancer.
In conclusion, the expression of GLUT1 and TIGAR was correlated to SUVmax in NSCLC. This is the first report that describes a significant correlation between the expression of TIGAR and SUVmax, and showed that low expression of TIGAR in primary NSCLC tumors was strongly correlated with a worse clinical outcome. These findings indicate that TIGAR may help predict prognosis of cancer patients and facilitate selection of patients for targeted therapies involving TIGAR in NSCLC. Further studies are required to identify lung cancer patients who are suitable for TIGAR-targeted therapy based on pre-evaluation of FDG uptake.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (Nos. 30830038, 30970842, and 81071180), the Major State Basic Research Development Program of China (973 Program) (No. 2012CB932604), and the New Drug Discovery Project (No. 2012ZX09506-001-005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The Next Generation. Cell 5: 646–674. [DOI] [PubMed] [Google Scholar]
- 2. Carins RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11: 85–95. [DOI] [PubMed] [Google Scholar]
- 3. Li L, Ren S, Zhang Y, Guan Y, Zhao J, et al. (2013) Risk factors for predicting the occult nodal metastasis in T1-2N0M0 NSCLC patients staged by PET/CT: Potential value in the clinic. Lung Cancer 81: 213–217. [DOI] [PubMed] [Google Scholar]
- 4. Katsuhiko S, Yuji H, Shinsuke S, Takuro Y, Maeda A (2012) Maximal Standardized Uptake Value on FDG-PET Is Correlated With Cyclooxygenase-2 Expression in Patients With Lung Adenocarcinoma. Ann Thorac Surg 93: 398–403. [DOI] [PubMed] [Google Scholar]
- 5. Minghuan L, Ningbo L, Man H, Fang S, Shuanghu Y, et al. (2009) Relationship between primary tumor fluorodeoxyglucose uptake and nodal or distant metastases at presentation in T1 stage non-small cell lung cancer. Lung Cancer 63: 383–386. [DOI] [PubMed] [Google Scholar]
- 6. Bille A, Pelosi E, Skanjeti A, Arena V, Errico L, et al. (2009) Preoperative intrathoracic lymph node staging in patients with non-small-cell lung cancer: accuracy of integrated positron emission tomography and computed tomography. Eur J Cardiothorac Surg 36: 440–445. [DOI] [PubMed] [Google Scholar]
- 7. Vesselle H, Freeman JD, Wiens L, Stern J, Nguyen HQ, et al. (2007) Fluorodeoxyglucose uptake of primary non-small cell lung cancer at positron emission tomography: new contrary data on prognostic role. Clin Cancer Res 13: 3105–3106. [DOI] [PubMed] [Google Scholar]
- 8. Taylor MD, Smith PW, Brix WK, Wick MR, Theodosakis N, et al. (2009) Fluorodeoxyglucose positron emission tomography and tumor marker expression in non-small cell lung cancer. J Thorac Cardiovasc Surg 137: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maiko K, Hayato K, Akihiko K, Satoshi H, Seiji K, et al. (2012) The relationship between GLUT-1 and Vascular Endothelial Growth Factor expression and 18F-FDG uptake in Esophageal Squamous Cell Cancer patients. Clinical Nuclear Medicine 37: 447–452. [DOI] [PubMed] [Google Scholar]
- 10. Li H, Jogl G (2009) Structural and biochemical studies of TIGAR (TP53-induced glycolysis and apoptosis regulator). J Biol Chem 284: 1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corcoran CA, Huang Y, Sheikh MS (2006) The regulation of energy generating metabolic pathways by p53. Cancer Biology and Therapy 5: 1610–1613. [DOI] [PubMed] [Google Scholar]
- 12. Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, et al. (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126: 107–120. [DOI] [PubMed] [Google Scholar]
- 13. Watanabe Y (2009) TNM classification for lung cancer. Ann Thorac Cardiovasc Surg 9: 343–350. [PubMed] [Google Scholar]
- 14. Bensinger SJ, Christofk HR (2012) New aspects of the Warburg effect in cancer cell biology. Seminars in cells & Developmental Biology 23: 352–361. [DOI] [PubMed] [Google Scholar]
- 15.Lemarie A, Grimm S (2011) Mitochondrial respiratory chain complexes: apoptosis sensors mutated in cancer. Oncogene 30, 3985–4003. [DOI] [PubMed]
- 16. Herling A, König M, Bulik S, Holzhütter HG (2011) Enzymatic features of the glucose metabolism in tumor cells. FEBS J 278: 2436–2459. [DOI] [PubMed] [Google Scholar]
- 17. Nguyen XC, So Y, Chung JH, Lee WW, Park SY, et al. (2008) High correlations between primary tumours and loco-regional metastatic lymph nodes in non-small cell lung cancer with respect to glucose transporter type 1 mediated 2-deoxy-2-F18-fluro-D-glucose uptake. Eur J Cancer 44: 692–698. [DOI] [PubMed] [Google Scholar]
- 18. Tateishi U, Yamaguchi U, Seki K, Terauchi T, Arai Y, et al. (2006) Glut-1 expression and enhanced glucose metabolism are associated with tumor grade in bone and soft tissue sarcomas: a prospective evaluation by 18F-fluorodeoxyglucose positron emission tomography. Eur J Nucl Med Mol Imaging 33: 683–691. [DOI] [PubMed] [Google Scholar]
- 19. Tohma T, Okazumi S, Makino H, Cho A, Mochiduki R, et al. (2005) Relationship between glucose transporter hexokinase and FDG-PET in esophageal cancer. Hepatogastroenterolggy 52: 486–490. [PubMed] [Google Scholar]
- 20. Wu C, Okar DA, Stoeckman AK, Peng LJ, Herrera AH, et al. (2004) Apotential role for fructose-2,6-bisphosphate in the stimulation of hepatic glucokinase gene expression. Endocrinology 145: 650–658. [DOI] [PubMed] [Google Scholar]
- 21. Zhao X, Feng D, Wang Q, Abdulla A, Xie XJ, et al. (2012) Regulation of lipogenesis by cyclin-dependent kinase 8-mediated control of SREBP-1. J Clin Invest 122: 2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. López-Guerra M, Trigueros-Motos L, Molina-Arcas M, Villamor N, Casado FJ, et al. (2008) Identification of TIGAR in the equilibrative nucleoside transporter 2-mediated response to fludarabine in chronic lymphocytic leukemia cells. Haematologica 93: 1843–1851. [DOI] [PubMed] [Google Scholar]
- 23. Madan E, Gogna R, Kuppusamy P, Bhatt M, Pati U, et al. (2012) TIGAR induces p53-mediated cell-cycle arrest by regulation of RB-E2F1 complex. British Journal of Cancer 107: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hasegawa H, Yamada Y, Iha H, Tsukasaki K, Nagai K, et al. (2009) Activation of p53 by Nutlin-3a, an antagonist of MDM2, induces apoptosis and cellular senescence in adult T-cell leukemia cells. Leukemia 23: 2090–2101. [DOI] [PubMed] [Google Scholar]