Abstract
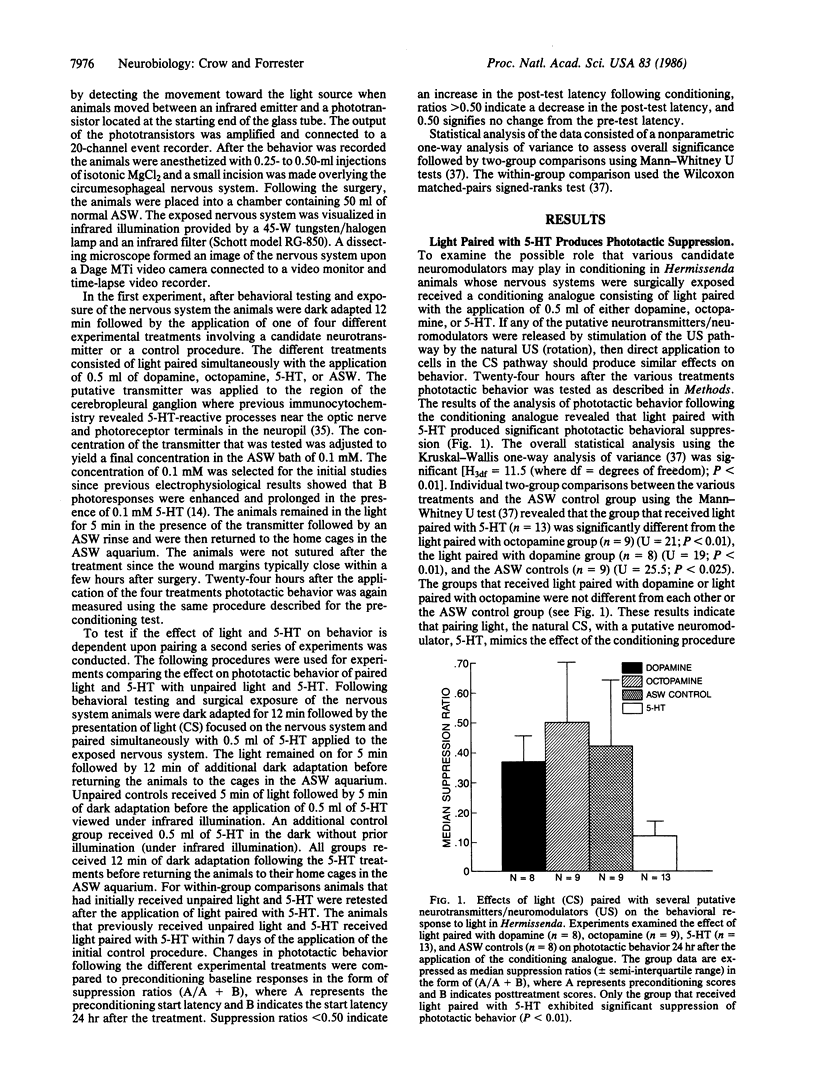
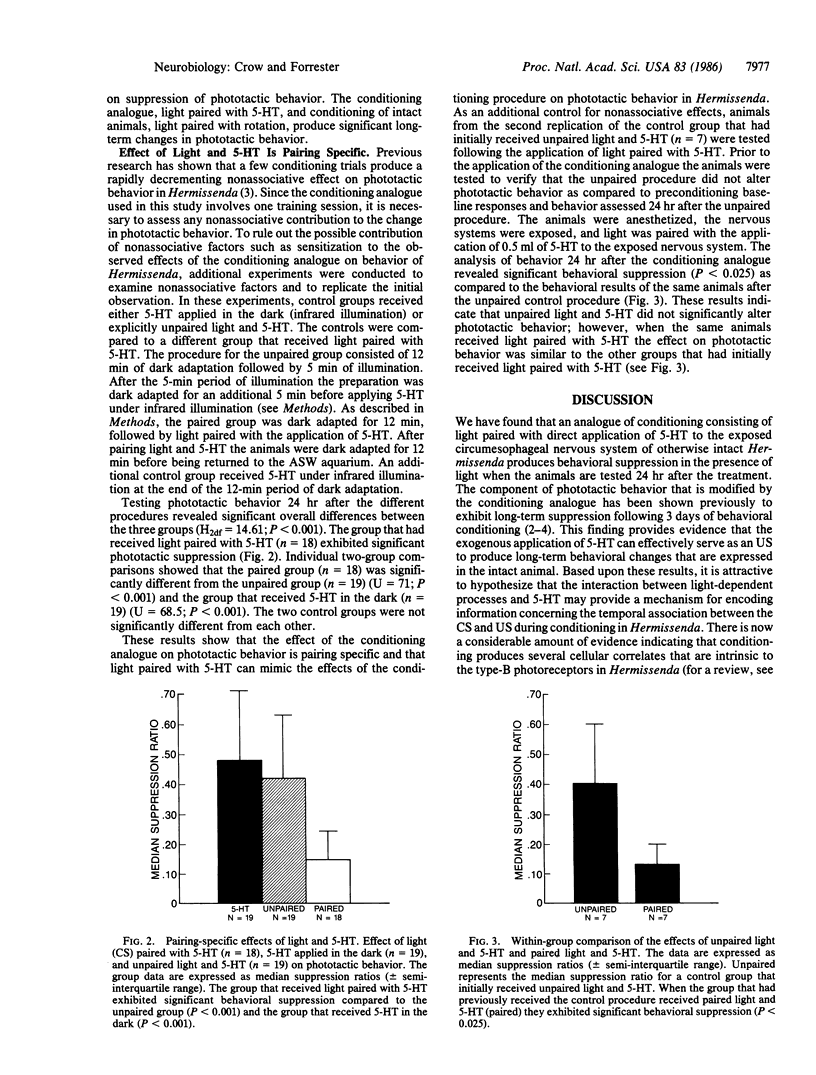
A conditioning procedure consisting of pairing-specific stimulation of the eyes and gravity-detecting statocysts in Hermissenda results in a long-term modification of normal positive phototactic behavior. The learning is expressed by a significant suppression of the initiation of locomotion in the presence of light. We now report that an analogue of the classical conditioning procedure, consisting of light paired with serotonin (5-HT) applied directly to the exposed circumesophageal nervous system of otherwise intact animals, mimics the effect of conditioning on long-term changes in phototactic behavior. The effect of the conditioning analogue on behavior shows some specificity with 5-HT since light paired with dopamine or octopamine does not significantly affect phototactic behavior. The conditioning analogue exhibits pairing specificity since unpaired light and 5-HT and 5-HT applied in the dark do not produce behavioral suppression. Animals that initially received unpaired light and 5-HT do show behavioral suppression after receiving paired light and 5-HT. These results indicate that light (the conditioned stimulus) paired with the putative transmitter of the unconditioned stimulus pathway (5-HT) is sufficient to produce long-term phototactic suppression.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W., Castellucci V. F., Camardo J. S., Kandel E. R., Lloyd P. E. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph A. R., Tuan F. J. Serotonin and inhibition in Limulus lateral eye. J Gen Physiol. 1972 Dec;60(6):679–697. doi: 10.1085/jgp.60.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike T., Alkon D. L. Sensory convergence on central visual neurons in Hermissenda. J Neurophysiol. 1980 Sep;44(3):501–513. doi: 10.1152/jn.1980.44.3.501. [DOI] [PubMed] [Google Scholar]
- Akon D. L., Fuortes M. G. Responses of photoreceptors in Hermissenda. J Gen Physiol. 1972 Dec;60(6):631–649. doi: 10.1085/jgp.60.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Alkon D. L. Intersensory interactions in Hermissenda. J Gen Physiol. 1973 Aug;62(2):185–202. doi: 10.1085/jgp.62.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon D. L. Neural organization of a molluscan visual system. J Gen Physiol. 1973 Apr;61(4):444–461. doi: 10.1085/jgp.61.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. A., Levitan I. B. Serotonin increases an anomalously rectifying K+ current in the Aplysia neuron R15. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3522–3525. doi: 10.1073/pnas.80.11.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle M. B., Klein M., Smith S. J., Kandel E. R. Serotonin increases intracellular Ca2+ transients in voltage-clamped sensory neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7642–7646. doi: 10.1073/pnas.81.23.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976 Dec 10;194(4270):1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Corrent G., McAdoo D. J., Eskin A. Serotonin shifts the phase of the circadian rhythm from the Aplysia eye. Science. 1978 Dec 1;202(4371):977–979. doi: 10.1126/science.309655. [DOI] [PubMed] [Google Scholar]
- Crow T. J., Alkon D. L. Retention of an associative behavioral change in Hermissenda. Science. 1978 Sep 29;201(4362):1239–1241. doi: 10.1126/science.694512. [DOI] [PubMed] [Google Scholar]
- Crow T., Bridge M. S. Serotonin modulates photoresponses in Hermissenda type-B photoreceptors. Neurosci Lett. 1985 Sep 16;60(1):83–88. doi: 10.1016/0304-3940(85)90385-4. [DOI] [PubMed] [Google Scholar]
- Crow T. Conditioned modification of locomotion in Hermissenda crassicornis: analysis of time-dependent associative and nonassociative components. J Neurosci. 1983 Dec;3(12):2621–2628. doi: 10.1523/JNEUROSCI.03-12-02621.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. Conditioned modification of phototactic behavior in Hermissenda. I. Analysis of light intensity. J Neurosci. 1985 Jan;5(1):209–214. doi: 10.1523/JNEUROSCI.05-01-00209.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T., Offenbach N. Modification of the initiation of locomotion in Hermissenda: behavioral analysis. Brain Res. 1983 Jul 25;271(2):301–310. doi: 10.1016/0006-8993(83)90292-5. [DOI] [PubMed] [Google Scholar]
- Dennis M. J. Electrophysiology of the visual system in a nudibranch mollusc. J Neurophysiol. 1967 Nov;30(6):1439–1465. doi: 10.1152/jn.1967.30.6.1439. [DOI] [PubMed] [Google Scholar]
- Detwiler P. B., Alkon D. L. Hair cell interactions in the statocyst of Hermissenda. J Gen Physiol. 1973 Nov;62(5):618–642. doi: 10.1085/jgp.62.5.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldman E., Alkon D. L. Neurotransmitter synthesis in the nervous system of the mollusc Hermissenda. Comp Biochem Physiol C. 1978;59(2):117–125. doi: 10.1016/0306-4492(78)90042-4. [DOI] [PubMed] [Google Scholar]
- Heldman E., Grossman Y., Jerussi T. P., Alkon D. L. Cholinergic features of photoreceptor synapses in Hermissenda. J Neurophysiol. 1979 Jan;42(1 Pt 1):153–165. doi: 10.1152/jn.1979.42.1.153. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W., Acosta-Urquidi J. Serotonin decreases a background current and increases calcium and calcium-activated current in pedal neurons of Hermissenda. Cell Mol Neurobiol. 1985 Dec;5(4):407–412. doi: 10.1007/BF00755404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Abrams T., Bernier L., Carew T. J., Hawkins R. D., Schwartz J. H. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- Kistler H. B., Jr, Hawkins R. D., Koester J., Steinbusch H. W., Kandel E. R., Schwartz J. H. Distribution of serotonin-immunoreactive cell bodies and processes in the abdominal ganglion of mature Aplysia. J Neurosci. 1985 Jan;5(1):72–80. doi: 10.1523/JNEUROSCI.05-01-00072.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I. Modulatory actions of neurotransmitters. Annu Rev Neurosci. 1979;2:447–465. doi: 10.1146/annurev.ne.02.030179.002311. [DOI] [PubMed] [Google Scholar]
- Land P. W., Crow T. Serotonin immunoreactivity in the circumesophageal nervous system of Hermissenda crassicornis. Neurosci Lett. 1985 Dec 4;62(2):199–205. doi: 10.1016/0304-3940(85)90355-6. [DOI] [PubMed] [Google Scholar]
- Lloyd P. E., Kupfermann I., Weiss K. R. Evidence for parallel actions of a molluscan neuropeptide and serotonin in mediating arousal in Aplysia. Proc Natl Acad Sci U S A. 1984 May;81(9):2934–2937. doi: 10.1073/pnas.81.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S., Carew T. J. Locomotion in Aplysia: triggering by serotonin and modulation by bag cell extract. J Neurosci. 1983 Jul;3(7):1469–1477. doi: 10.1523/JNEUROSCI.03-07-01469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocorr K. A., Byrne J. H. Membrane responses and changes in cAMP levels in Aplysia sensory neurons produced by serotonin, tryptamine, FMRFamide and small cardioactive peptideB (SCPB). Neurosci Lett. 1985 Apr 9;55(2):113–118. doi: 10.1016/0304-3940(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Walters E. T., Byrne J. H. Associative conditioning analog selectively increases cAMP levels of tail sensory neurons in Aplysia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellmar T. C., Carpenter D. O. Serotonin induces a voltage-sensitive calcium current in neurons of Aplysia californica. J Neurophysiol. 1980 Sep;44(3):423–439. doi: 10.1152/jn.1980.44.3.423. [DOI] [PubMed] [Google Scholar]
- Tabata M., Alkon D. L. Positive synaptic feedback in visual system of nudibranch mollusk Hermissenda crassicornis. J Neurophysiol. 1982 Jul;48(1):174–191. doi: 10.1152/jn.1982.48.1.174. [DOI] [PubMed] [Google Scholar]
- Tyndale C. L., Crow T. J. An IC control unit for generating random and nonrandom events. IEEE Trans Biomed Eng. 1979 Dec;26(12):649–655. doi: 10.1109/tbme.1979.326454. [DOI] [PubMed] [Google Scholar]


