Abstract
Objectives
Bioactive glass (BAG) is known to possess antimicrobial properties and release ions needed for remineralization of tooth tissue, and therefore may be a strategic additive for dental restorative materials. The objective of this study was to develop BAG containing dental restorative composites with adequate mechanical properties comparable to successful commercially available composites, and to confirm the stability of these materials when exposed to a biologically challenging environment.
Methods
Composites with 72 wt.% total filler content were prepared while substituting 0–15% of the filler with ground BAG. Flexural strength, fracture toughness, and fatigue crack growth tests were performed after several different soaking treatments: 24 hours in DI water (all experiments), two months in brain-heart infusion (BHI) media+S. mutans bacteria (all experiments) and two months in BHI media (only for flexural strength). Mechanical properties of new BAG composites were compared along with the commercial composite Heliomolar by two-way ANOVA and Tukey’s multiple comparison test (p≤0.05).
Results
Flexural strength, fracture toughness, and fatigue crack growth resistance for the BAG containing composites were unaffected by increasing BAG content up to 15% and were superior to Heliomolar after all post cure treatments. The flexural strength of the BAG composites was unaffected by two months exposure to aqueous media and a bacterial challenge, while some decreases in fracture toughness and fatigue resistance were observed. The favorable mechanical properties compared to Heliomolar were attributed to higher filler content and a microstructure morphology that better promoted the toughening mechanisms of crack deflection and bridging.
Significance
Overall, the BAG containing composites developed in this study demonstrated adequate and stable mechanical properties relative to successful commercial composites.
Keywords: Resin Composite, Bioactive Glass, Strength, Fracture Toughness, Fatigue, Bacteria, Hydration
1 Introduction
While the use of dental restorative composites has increased dramatically for posterior teeth, annual failure rates up to 15% have been reported depending on restoration class [1], and a review of the literature has suggested the average lifetime of posterior dental composites is only six years [2]. Secondary caries at margins has been considered for over twenty years the most common reason for restoration replacement [3–7]. The second most common reason is partial or complete fracture of the composite restoration, while other significant causes are erosion and discoloration [8,9]. It has been reported that the replacement of posterior composites is primarily due to fracture of the restoration within the first five years, but as a response to secondary caries thereafter [10], although this has not been observed in all clinical studies [11]. A review of the numerous causes identified for restoration replacements based on multiple surveys may be found in Deligeorgi et al, 2001 [12].
One of the most common reasons for secondary caries is biofilm (plaque) formation on the margin of the tooth and restoration. Bacteria in the plaque (e.g., Streptococcus mutans) metabolize sucrose to lactic acid which can demineralize tooth tissue [13,14]. Resin based composites may ideally provide good sealing of the cavity with no marginal gaps; however, polymerization shrinkage during placement, combined with cyclic mechanical loading during function, may lead to local interface failure and gap development. These marginal gaps can serve as suitable anchorage sites for bacterial colonies [15]. A minimum gap size exceeding 0.4 mm has been suggested for significant bacterial colonization of dental amalgam [16], but a similar relationship has not been discerned for composites. Moreover, increased roughness of the restoration increases the ability of bacteria to colonize a given area, by affecting pellicle formation and causing a favorable environment, often resulting in secondary caries formation [17,18]. Microfloral analysis of marginal biofilms revealed that anaerobic bacteria are dominant with Streptococcus mutans, Actinomyces naeslundii and Lactobacillus casei being the most abundant bacterial species [19]. Svanberg et al. found significantly larger Streptococcus mutans colony counts at the tooth interface with composite restorations compared to interfaces with amalgam [20].
One possible approach to increasing the resistance of restorations to secondary caries formation is to add agents that 1) negatively influence the micro-organisms and/or 2) promote remineralization of tooth structure after damage has occurred. In this regard, there is a substantial amount of published literature demonstrating the antibacterial qualities of various bioactive glass (BAG) compositions against many different bacterial species [21–33]. However, to date there have been no published studies of dental restorative composites containing bioactive glasses. There are several concerns regarding the development of a successful bioactive glass dental restorative composite. First, there is a concern that BAG fillers not well adhered to the composite matrix will result in unsuitably low mechanical properties. Second, because the composite will leach ions there is a concern about the stability of the mechanical properties over time. Finally, it must be confirmed that sufficient antimicrobial and/or remineralization activity can be achieved in BAG containing composites to slow secondary caries at the marginal gaps of tooth restorations. The goal of this study is to address the former two issues, while the latter will be addressed in the future by ongoing studies. Accordingly, the objective of this paper was to test the hypotheses that new BAG-containing dental restorative composites can be developed with mechanical properties comparable to successful commercially available composites, and that the properties will remain adequately stable after aging in a bacterial environment.
2 Materials and methods
2.1 Materials
The bioactive glass (BAG) used in this study had the composition 65% SiO2, 31% CaO and 4% P2O5 (mol%) and was produced by a sol-gel process, as previously described [34]. In brief, the BAG was produced from high-purity metal alkoxides including tetraethyl orthosilicate (TEOS, Si(OC2H5)4), calcium methoxyethoxide (CMOE, C6H14O4Ca), and triethyl phosphate (TEP, (C2H5)3PO4). All reagents were purchased (Sigma Aldrich), except the CMOE was synthesized from pure Ca metal and methoxyethanol, to produce a 20% solution in methoxyethanol, and this alcohol served as a mutual solvent for all of the alkoxides as well as being the water source used to initiate hydrolysis and glass formation. The solutions were prepared in a dry nitrogen-environment glovebox, aged in distilled water, air-dried and stabilized in a dedicated furnace at 600°C to completely remove residual alcohols and alkoxide components, while retaining high surface area (between 200 to 300 m2/g). After rapidly cooling, the glass was ball milled in ethanol and sieved to a gross particle dimension of less than 38 μm. The particles were then further processed to a fine particle size (0.04–3.0 μm) using a Micronizer jet mill. (Sturtevant Inc., Hanover, MA).
Three BAG-containing composites were produced by mixing the glass into a 50:50 mixture of bisphenol A glycidyl methacrylate (BisGMA):triethylene glycoldimethacrylate (TEGDMA) monomers with 0.4 wt% of camphorquinone (CQ), 0.8 wt% of 4-dimethylaminobenzoic acid ethyl ether (EDMAB), and 0.05 wt% of 3, 5-di-tertbutyl-4-hydroxytoluene (BHT). Samples denoted as 5BAG, 10BAG, and 15BAG were produced by combining the resin with 3.0 μm average size silanated strontium glass (Bisco Inc.) and 5, 10, or 15 wt% unsilanated bioactive glass, respectively, to a total filler of 72 wt% and mixed in a DAC-150 speed mixer (FlackTek Inc., Landrum, SC) at 3000 rpm for 2 minutes. Control samples (denoted 0BAG) had the same formulation as 5BAG with 5 wt% silane treated aerosol-silica filler (OX-50, Degussa) substituted for the BAG.
Mechanical property values are compared to published literature values and also the full set of mechanical property experiments were conducted on the commercial composite Heliomolar (Ivoclar Vivadent AG, batch # 4432). Heliomolar has a composition of 19 wt% Bis-GMA + urethane dimethacrylate, 3 wt% decandiol dimethacrylate, 66.7 wt% total filler content (highly dispersed silicon dioxide + ytterbium trifluoride) along with prepolymer and <1wt% stabilizers, catalysts and pigments. Heliomolar is classified as an inhomogeneous microfilled composite (< 1μm filler size) and was chosen for this study because it is a clinically successful example of a composite for anterior and posterior restorations [35–37].
2.2 Specimen preparation for mechanical testing
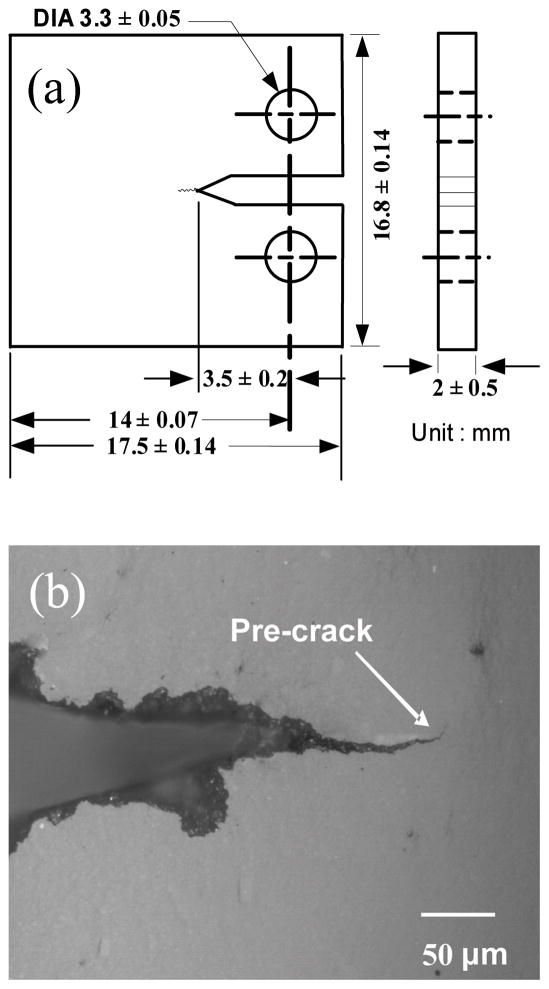
For flexural strength testing, three-point bend beams (N=10 for each composition) were prepared by dispensing the composite paste into 25 mm long quartz tubes (square 2 mm × 2 mm cross-section) followed by curing for 40 s on two opposite sides in a visible light curing unit (Triad II, Dentsply International, York Division, PA, USA). Compact-tension, C(T), specimens were made for fracture toughness (N=5 for each composition) and fatigue crack growth (N=3 for each composition) experiments. The composites were dispensed into a stainless-steel rectangular split mold, pressed flat, and cured for 40 s on each side as described above. The cured rectangular blanks were then machined into C(T) specimens as shown in Fig. 1a. To enable observation and measurement of cracks, samples were ground and polished using progressively finer SiC grinding papers and alumina oxide polishing compounds down to 0.05 μm and finally finished with MasterPolish (Buehler, Lake Bluff, IL, USA). A sharp pre-crack (Fig. 1b) was introduced by manually extending the starter notch using a razor blade until a pre-crack formed from the notch.
Fig. 1.
(a) Schematic and dimensions of C(T) specimen and (b) optical micrograph of a typical pre-crack profile.
2.3 Post-cure treatments
Specimens for all experiments were treated in two different ways: 24 hours aging in deionized (DI) water and 58 ± 3 days at 37°C in brain-heart infusion (BHI) media with Streptococcus mutans (strain ATCC25175) cultures growing in logarithmic phase with the media changed every other day. The longer of the two aging times was chosen since previous studies have estimated that is the amount of time needed for the specimens to become fully (>98%) saturated with water [38]. Lyophilized bacterial cultures were obtained from the American Type Culture Collection (ATTC, Manassas, VA, USA). All DI water aged samples were tested immediately after removal from the water. After the aging period in BHI media with Streptococcus mutans, composite samples were immersed in 50% bleach for 5 min and then rinsed with BHI three times. The specimens were then soaked in BHI without sucrose and stored in a refrigerator until mechanical testing. With the exception of the fatigue tests, all mechanical testing was performed within a day of removal from the test aging solution. Due to the time required for fatigue testing, some samples needed to be stored for days or weeks while waiting for testing, so these samples were continually stored in sterile BHI media at ~4°C. Finally, bending beams made from the experimental composites were also soaked in sterile BHI media without bacteria for ~60 days and used for strength testing.
2.4 Mechanical Testing
Flexure strength was tested in 3-point bending (20 mm span) on a universal testing machine at a cross-head speed of 0.254 mm/min, in general accord with ISO 4049 [39]. The steel supports had rollers of 2 mm diameter and the loading piston was a steel ball of 2 mm diameter. The flexure strength was determined using the maximum load.
Fracture toughness tests were conducted on wet samples immediately after removal from the storage solution using a computer controlled hydraulic testing machine (Instron 8872, Canton, MA, USA). Tests were conducted in load control with a 1.1 N/s loading rate until fracture occurred. KIC was calculated from the peak load at fracture according to the standard stress intensity factor equation for the C(T) sample geometry [40].
Fatigue crack growth testing was done in general accordance with ASTM standard E647 [41], using computer controlled hydraulic testing machine (Instron 8872, Canton, MA, USA) and a sine waveform with frequency ν = 1.5 Hz, which corresponds to a typical human chewing frequency [42]. A constant load ratio R=Pmin/Pmax=0.1 was used, where Pmax and Pmin are the maximum and minimum loads experienced during the loading cycle, respectively. Fatigue crack growth rates, da/dN, were characterized as a function of the stress intensity range, ΔK =Kmax − Kmin, where Kmax and Kmin are the stress intensity values calculated from Pmax and Pmin, respectively. After initial establishment of a high crack growth rate of 10−7–10−6 m/cycle, the test was conducted in decreasing ΔK control using a normalized K-gradient (1/ΔK[dΔK/da]) of −0.08 mm−1. Crack length was determined by measuring the load point compliance using a capacitance displacement gage (HPT150, Capacitec, Inc., Ayer, MA) attached to the clevises of the testing machine. The sample compliance was converted to crack length using published calibrations [43]. Data points were collected roughly every 10−5 m of crack extension. Samples were kept wet during the entire test using a sponge to surround the sample and a custom drip system to keep it wet. After the test, the final crack length was measured optically. When the final compliance and optically measured crack lengths differed, the crack length data was corrected by assuming the error accumulated linearly with crack extension. From the crack length data, the crack growth rates (da/dN) were determined as a function of ΔK by fitting over ranges of ~100 μm of crack length change.
For statistical comparisons of data, ANOVA followed by Tukey’s multiple comparison test was used with α < 0.05 considered statistically significant.
After fatigue crack growth and fracture toughness experiments, both crack profiles and fracture surfaces, respectively, were examined using a scanning electron microscope (Quanta 600 FEG SEM, FEI Company, Hillsboro, OR) in order to discern crack-microstructure interactions and toughening mechanisms.
3 Results
3.1 Flexural strength results
Flexural strength and statistical test results are shown in Table 1. BAG composites did not show a significant difference in flexural strength as a function of the various soaking treatments. However, Heliomolar composites did show a significant reduction in flexural strength between 24h water and 2 months in bacteria. The experimental BAG composites all had superior flexural strength when compared to Heliomolar.
Table 1.
Mean flexural strengths with standard deviations in parentheses
| 24h DI Water | 2 months bacteria | 2 months media | |
|---|---|---|---|
| Material | Flexural Strength (MPa) | Flexural Strength (MPa) | Flexural Strength (MPa) |
| Heliomolar | 73.2 (4.4)a | 61.7 (7.9)a | - |
| 0BAG | 123.5 (16.2) | 114.9 (12.3) | 107.4 (12.8) |
| 5BAG | 112.8 (12.9) | 108.4 (12.2) | 107.4 (11.2) |
| 10BAG | 116.4 (14.2) | 112.6 (13.0) | 95.7 (16.2) |
| 15BAG | 116.9 (10.7) | 105.6 (17.7) | 101.2 (10.8) |
denotes value has statistically significant difference from rest of column
3.2 Fracture toughness results
Fracture toughness data and statistical test results for both Heliomolar and BAG composites is shown in Table 2.
Table 2.
Mean fracture toughness results with standard deviations in parentheses
| 24h DI water | 2 months bacteria | ||
|---|---|---|---|
| Material | KIC(MPa√m) | KIC(MPa√m) | % decrease |
| Heliomolar | 0.98 (0.17)a | 0.77 (0.02)a | −21%b |
| 0BAG | 1.45 (0.13) | 1.25 (0.15) | −13% |
| 5 BAG | 1.52 (0.17) | 1.12 (0.13) | −26%b |
| 10BAG | 1.54 (0.17) | 1.40 (0.01)a | −9% |
| 15BAG | 1.31 (0.14) | 1.10 (0.05) | −16%b |
denotes value has statistically significant difference from rest of column
denotes statistically significant difference between the two aging conditions
Statistical analyses showed that the experimental BAG composites all have significantly higher fracture toughness compared to Heliomolar at 24 hours and after 2 months aging in bacteria (Table 2). Furthermore, the degradation in toughness of the experimental BAG composites after two months of aging was comparable to, or less severe (i.e., not significant) than Heliomolar (Table 2).
3.3 Fatigue crack growth results
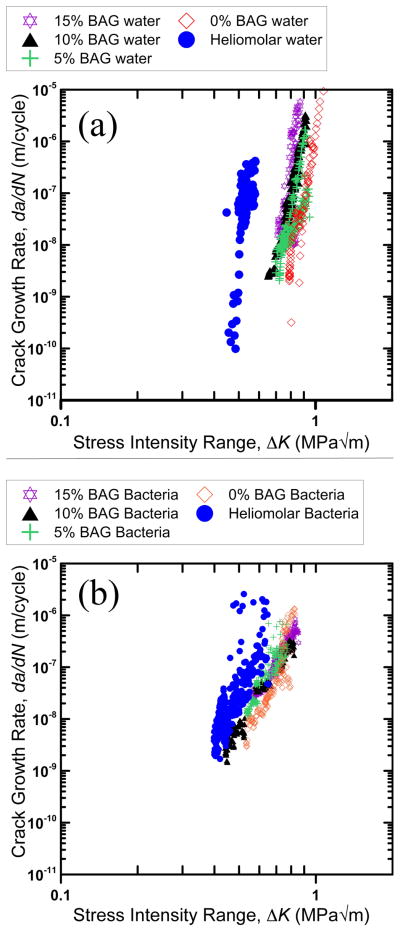
Fatigue crack growth rate, da/dN, results are shown in Fig. 2 plotted as a function of the stress intensity range, ΔK. Generally, the data for the 0 – 15BAG composites produced in this study overlapped considerably. Furthermore, the commercial composite Heliomolar showed inferior fatigue crack growth resistance for both aging conditions with the curves shifted to higher growth rates at a given stress intensity range. The difference between Heliomolar and the 0 – 15BAG composites was more pronounced for the 24 h water aging condition.
Fig. 2.
Fatigue crack growth rate data for (a) 24h water aging and (b) 2 months bacteria aging treatments.
Each curve in Fig. 2 was produced from a composite of N = 3 samples measured over different, overlapping, ranges of growth rates; thus, fatigue crack growth data for each material was pooled and fit to the Paris law [44]:
| [1] |
and mean values for the Paris exponent, m, are given in Table 3. All of the composites showed decrease in m after two months aging in bacteria. The fatigue thresholds, ΔKTH, below which cracks are presumed not to propagate was also assessed for each sample in Table 3. Due to practical time limitations of testing, ΔKTH was defined where growth slowed to a rate approaching 10−9 m/cycle. Since each curve in Fig. 2 was produced from multiple samples tested with different growth rates, the ΔKTH given in Table 3 were taken from the sample tested at the slowest growth rates.
Table 3.
Measured fatigue parameters: ΔKTH values and Paris exponents
| 24h in DI water | 60 days in bacteria | ||||
|---|---|---|---|---|---|
| ΔKTH (MPa√m) | m | ΔKTH (MPa√m) | m | % decrease in m | |
| Heliom | 0.40 | 23.0 | 0.53 | 9.3 | −60% |
| 0BAG | 0.79 | 24.7 | 0.54 | 11.5 | −53% |
| 5BAG | 0.70 | 14.3 | 0.54 | 8.3 | −42% |
| 10BAG | 0.65 | 22.0 | 0.46 | 7.8 | −65% |
| 15BAG | 0.71 | 25.8 | 0.56 | 8.2 | −68% |
3.4 Crack Paths and Fractography
Due to similarities of the observed microstructures and crack-microstructure interactions for all of the BAG composites, only micrographs for the 0BAG and 15BAG composites will be shown as representative. Crack path observations were made on the fatigue crack growth specimens, while fracture surface observations were made on both the fracture toughness and fatigue samples.
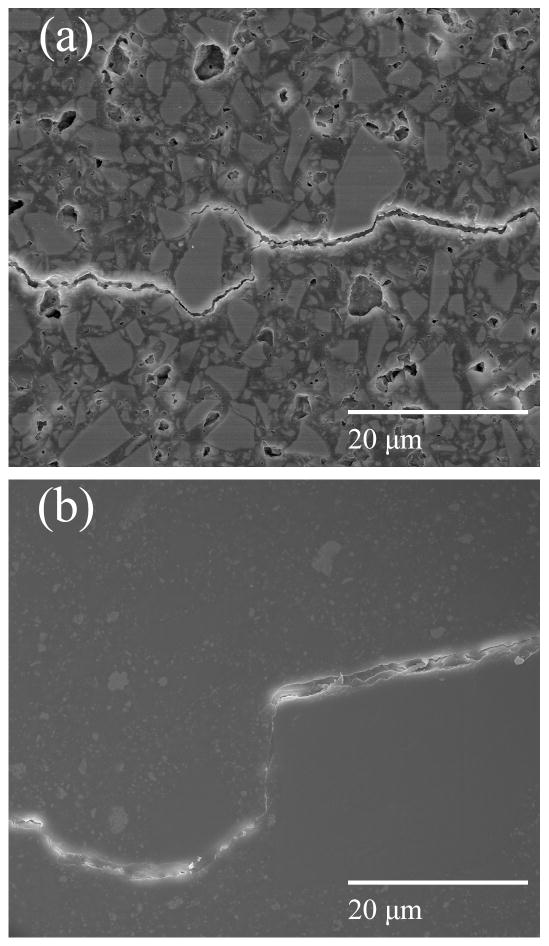
Figs. 3a & 3b show multiple crack deflections and crack bridging near the fatigue crack tip of a 15BAG composite. For the Heliomolar composite crack bridges were also observed at the crack tip (Fig. 3c). For all of the 0 – 15 BAG composites, but not Heliomolar, crack bridges were also found in the crack wake far behind the crack tip (Fig. 4a).
Fig. 3.
Micrographs of crack tips (2 months bacteria treatment). a–b) Crack deflection and bridging at a fatigue crack tip in the 15BAG composite. c) Crack bridging at a fatigue crack tip in the Heliomolar composite. Direction of crack propagation was left to right.
Fig. 4.
Micrographs of toughening mechanisms (2 months bacteria treatment). a) Crack bridge created by filler particle in the 15BAG composite. This bridge was located 0.052 mm behind the fatigue crack tip. b) A rare high angle crack deflection by a large prepolymerized agglomerate in the Heliomolar composite. Direction of crack propagation was left to right.
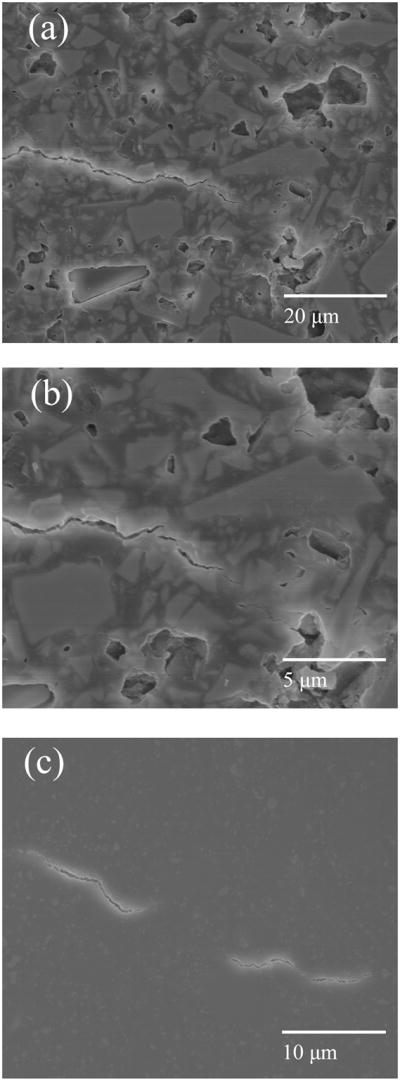
Significant crack deflections were rarely observed in the Heliomolar composite and were located only at the widely spaced, large prepolymerized agglomerates of composite that were often ~20 μm or larger in size (Fig. 4b). Fig. 5 shows an equal magnification comparison of the crack paths for the 15BAG and Heliomolar composites over a larger amount of crack extension. On the microscale, the crack in the 15BAG composite appears more tortuous due to the high frequency of deflection by particles (Fig. 5a), while for the Heliomolar composite there is some crack meandering over large extensions, but generally a less tortuous path on the microscale (Fig. 5b). Also, in Figs 3–5 there is evidence of BAG particle dissolution from the polished surfaces due to the 2 month aging treatment for the 15BAG composite (Fig. 3a, 3b, 4a, 5a) which was absent for the 0BAG samples (Fig. 5c).
Fig. 5.
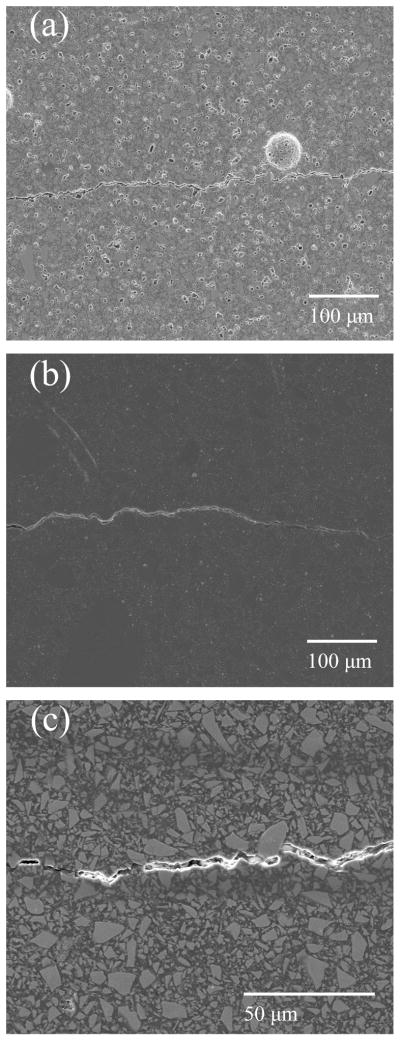
Micrographs of fatigue crack paths (2 months bacteria treatment). Panel a) shows the 15BAG composite, b) the Heliomolar composite and c) the 0BAG composite. Direction of crack propagation was left to right.
Overall, the 0 – 15BAG composites show a rougher fracture surface while the fracture surface of Heliomolar is smoother and entirely covered with resin (Fig. 6). It is seen in Figs. 6a & 6c that some of the particles of the 15BAG and 0BAG composites debonded cleanly from the matrix, giving a mixed matrix/interface crack path. In contrast, for Heliomolar (Fig. 6e) the crack always moved through the resin matrix leaving a polymer coating on the fracture surface. The resin coated fracture surface is seen more clearly in Fig. 6f at higher magnification where, in contrast, the smooth surface of debonded glass particles can be seen for the 0BAG and 15BAG composites at higher magnifications (Fig. 6b & 6d). Figs. 6a & 6c also show that the 0BAG composite has fewer debonded particles than the 15BAG composite. Approximately 94 debonded particles/mm2 and 159 debonded particles/mm2 were observed on the fracture surfaces for 0BAG and 15BAG respectively.
Fig. 6.
Fracture surfaces of fracture toughness samples for (a, b) 15BAG, (c, d) 0BAG and (e, f) Heliomolar composites after 2 months bacteria treatment.
4 Discussion
4.1 Mechanical behavior
Overall, BAG composites exhibited significantly better mechanical properties than Heliomolar both after aging in DI water for 24 h as well as after two months soaking in bacteria containing media. These differences in mechanical properties may be attributed primarily to differences in the material composition and microstructures, as will be discussed below. Also, Table 4 shows mechanical properties for two other commercial composites after 60 days soaking in H2O. Generally, the strength, fracture toughness, and fatigue threshold properties of the BAG containing composites after soaking for two months in bacteria containing aqueous media are comparable to, or better than, these two popular commercial composites.
Table 4.
Published mechanical properties of some commercial composites with standard deviations in parentheses [38,54]
| Flexural Strength (MPa) | Fracture Toughness* (MPa√m) | Fatigue Threshold (MPa√m) | |
|---|---|---|---|
| Filtek Z250 (60 days H2O) | 91.4 (14.8) | 1.26 (0.05) | 0.54 |
| Filtek Supreme Plus (60 days H2O) | 52.7 (12.9) | 0.81 (0.06) | 0.41 |
These toughness values are the maximum point of the fracture resistance curve, which gives an upper bound for the critical value of toughness, KIC.
4.1.1 Flexural strength
The first notable difference between the microstructures is that the 0 – 15BAG composites have significantly higher filler particle concentration than Heliomolar (72 wt% versus 66.7 wt%, respectively). The higher measured strength values for the BAG composites is thus consistent with published observations for resin based dental composites that increased filler content leads to increased flexural strength [45,46] and that filler content is the dominant factor even with different filler morphologies [47].
It is interesting to note that the BAG composites showed no degradation in strength after soaking approximately two months in both sterile and bacteria containing media. Many commercial dental restorative composites demonstrate a loss in strength after similar long term aging in aqueous media [38,48–51]. This degradation in strength is attributed to water uptake causing plasticization of the resin matrix and/or a degradation of the matrix-filler interface [38,48–51]. Since the BAG-containing composites are designed to have ions leaching out of the BAG filler, there is a legitimate concern that such ion leaching could lead to degradation of the filler, and thus decreased mechanical properties. While the SEM images show evidence for particle dissolution from the composite surface (Figs. 3–5), the mechanical testing results reflect that the flexural strengths of the present BAG composites are quite stable after aging two months in aqueous media.
4.1.2 Fracture toughness and fatigue crack growth resistance
While some correlations have been observed over a specific groups of composites [52], the fracture toughness and fatigue properties of resin based dental restorative composites often do not scale simply with factors like filler volume fraction or filler size and, rather, are highly dependent on the morphology of the composite microstructure [38,45,53–61]. Previous studies have suggested that microstructures that maintain good matrix/particle adhesion while promoting toughening mechanisms such as crack deflection and crack bridging are advantageous for achieving good fracture and fatigue properties [38,53–55,58,59,61].
Generally, it was observed that crack deflection and bridging was more pronounced in the BAG composites than in Heliomolar. Heliomolar is categorized as a microfill composite with filler size <1 μm; however, the microstructure is quite inhomogeneous and contains widely spaced large agglomerates composed of prepolymerized resin fillers added to increase overall filler volume and reduce curing shrinkage. Based on crack path (Fig. 5b) and fracture surface (Figs. 6e–6f) observations, cracks moved through the homogeneous microfilled regions with few deflections, with significant high angle deflections only rarely observed at the occasional large agglomerate (Fig. 4b). Furthermore, the only detectable crack bridges were very small and only observed very near the crack tip (Fig. 3c). In contrast, the BAG composites contained significantly more medium to large sized particles capable of 1) deflecting the crack at the microscale and 2) creating large crack bridges that sustain load far behind the crack tip (Fig. 3–5).
Crack deflection and bridging are two toughening mechanisms that often act in concert; indeed, crack deflection commonly leads to crack bridging. Furthermore, natural tooth enamel and dentin are also toughened by those same mechanisms [62–65]. Both mechanisms increase the toughness by lowering the mode I stress intensity at the crack tip. Since both the fracture toughness and fatigue crack growth resistance are determined by the mode I stress intensity, both are generally improved by these mechanisms. With crack deflection, the microstructure forces the crack to deviate from the mode I path, causing a decrease in the mode I stress intensity at the crack tip. Crack bridging often results where the crack locally arrests or is deflected by microstructural inhomogeneities. As a result, load bearing bridges may be left in the crack wake due to either imperfect connection of the three-dimensional crack front, or new microcracks forming ahead of the crack tip. Overall, these mechanisms were observed to be more active in the BAG composites resulting in higher fracture toughness and fatigue crack growth resistance.
Some studies have shown particle/matrix debonding can be detrimental to the fracture and fatigue performance of resin based dental composites [38,54]. In the present BAG composites, some fraction of the particles were observed to cleanly debond from the matrix (Fig. 6); however, overall adequate properties were achieved nonetheless when compared to the commercially successful Heliomolar. Also, the fraction of debonded particles was higher for the 15BAG composition than the 0BAG composition, suggesting the lack of silane treatment of the BAG particles led to less particle-matrix adhesion. However, overall it may be concluded that since the majority of particles were well bonded even for the 15BAG case (Fig. 6a) adequate mechanical properties were achieved.
Although the fracture toughness and fatigue crack growth resistance for the BAG composites degraded somewhat after long term aging in aqueous media, the properties remained better than Heliomolar in all cases. A degradation in fracture toughness is not unusual for other commercial resin based dental composites after long term exposure to water [38]. Moreover, the fracture toughness and fatigue threshold properties of the BAG containing composites are comparable to, or better than, the popular commercial composites (Table 4) that were tested after two months soaking in water [38,54]. Overall, it is concluded that the presence of the BAG particles does not raise immediate concerns regarding long term stability of the mechanical reliability of these composites.
4.2 Limitations of this study
Although crack bridging was identified as a toughening mechanism for the BAG composites, in the present study the contribution of crack bridging was not quantified. Such quantification would require the measurement of the fracture resistance curves (R-curves) for the materials to separate out the contribution of bridging [38,53,61], and such studies are left for future work. Another limitation is the aging time was limited to roughly two months. Although previous work suggests this is long enough to reach nearly full water saturation of the composite [38], it is unclear if degradation may occur at longer time scales and such studies are also left for future work.
5 Conclusions
Based on a study of the mechanical properties of a series of bioactive glass (BAG) containing composites, the following conclusions can be made:
All BAG containing composites exhibited superior mechanical properties over the commercial Heliomolar composite both after 24 hours in water and after 2 months in a bacteria containing aqueous media. Properties were also found to be comparable to, or better then, published values for two other commercial composites, Filtek Z250 and Filtek Supreme Plus.
The superior mechanical properties of the BAG composites compared to Heliomolar were attributed to: 1) a higher filler content; and 2) a microstructure morphology that better promoted the toughening mechanisms of crack deflection and bridging.
Overall, the BAG containing composites developed in this study demonstrated adequate mechanical properties relative to successful commercial composites.
Acknowledgments
This work was supported in part by NIH/NIDCR GRANT DE021372. The authors thank Dr. Mansen Wang and Dr. Fernanda Gwinner for their help in the flexure testing experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hickel R, Kaaden C, Paschos E, Buerkle V, Garcia-Godoy F, Manhart J. Longevity of occlusally-stressed restorations in posterior primary teeth. Am J Dent. 2005;18:198–211. [PubMed] [Google Scholar]
- 2.Downer MC, Azli NA, Bedi R, Moles DR, Setchell DJ. How long do routine dental restorations last? A systematic review. Br Dent J. 1999;187:432–439. doi: 10.1038/sj.bdj.4800298a1. [DOI] [PubMed] [Google Scholar]
- 3.Marks LAM, Weerheijm KL, van Amerongen WE, Groen HJ, Martens LC. Dyract versus Tytin class II restorations in primary molars: 36 months evaluation. Caries Research. 1999;33:387–392. doi: 10.1159/000016538. [DOI] [PubMed] [Google Scholar]
- 4.Mjör IA, Dahl JE, Moorhead JE. Placement and replacement of restorations in primary teeth. Acta Odontologica Scandinavica. 2002;60:25–28. doi: 10.1080/000163502753471961. [DOI] [PubMed] [Google Scholar]
- 5.Ostlund J, Möller K, Koch G. Amalgam, Composite Resin and Glass Ionomer Cement in Class-Ii Restorations in Primary Molars - a 3-Year Clinical-Evaluation. Swedish Dental Journal. 1992;16:81–86. [PubMed] [Google Scholar]
- 6.Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitao J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J Am Dent Assoc. 2007;138:775–783. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- 7.Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth - Findings from the new England children’s amalgam trial. J Am Dent Assoc. 2007;138:763–772. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- 8.Al Kayed MAS. Clinical Study of Placement and Replacement of Composite restoration in Jordan. The Saudi Dental Journal. 1999;11:53–59. [Google Scholar]
- 9.Attin T, Opatowski A, Meyer C, Zingg-Meyer B, Buchalla W, Monting JS. Three-year follow up assessment of Class II restorations In primary molars with a polyacid-modified composite resin and a hybrid composite. Am J Dent. 2001;14:148–152. [PubMed] [Google Scholar]
- 10.Brunthaler A, Konig F, Lucas T, Sperr W, Schedle A. Longevity of direct resin composite restorations in posterior teeth. Clin Oral Investig. 2003;7:63–70. doi: 10.1007/s00784-003-0206-7. [DOI] [PubMed] [Google Scholar]
- 11.Da Rosa Rodolpho PA, Donassollo TA, Cenci MS, Loguercio AD, Moraes RR, Bronkhorst EM, Opdam NJM, Demarco FF. 22-Year clinical evaluation of the performance of two posterior composites with different filler characteristics. Dent Mater. 2011;27:955–963. doi: 10.1016/j.dental.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 13.Loesche WJ. Microbiology of Dental Decay and Periodontal Disease. In: Baron S, editor. Medical Microbiology. 4. University of Texas Medical Branch at Galveston; Galveston, TX: 1996. [PubMed] [Google Scholar]
- 14.Mjör IA, Toffenetti OF. Secondary caries: A literature review with case reports. Quintessence Int. 2000;31:165–179. [PubMed] [Google Scholar]
- 15.Choi KK, Condon JR, Ferracane JL. The effects of adhesive thickness on polymerization contraction stress of composite. J Dent Res. 2000;79:812–817. doi: 10.1177/00220345000790030501. [DOI] [PubMed] [Google Scholar]
- 16.Kidd EAM, Joystonbechal S, Beighton D. Marginal Ditching and Staining as a Predictor of Secondary Caries around Amalgam Restorations - a Clinical and Microbiological Study. J Dent Res. 1995;74:1206–1211. doi: 10.1177/00220345950740051001. [DOI] [PubMed] [Google Scholar]
- 17.Marsh PD. The Role of Microbiology in Models of Dental Caries. Advances in Dental Research. 1995;9:244–254. doi: 10.1177/08959374950090030901. [DOI] [PubMed] [Google Scholar]
- 18.Reis AF, Giannini M, Lovadino JR, Dias CTD. The effect of six polishing systems on the surface roughness of two packable resin-based composites. Am J Dent. 2002;15:193–197. [PubMed] [Google Scholar]
- 19.Kidd EAM, Joystonbechal S, Beighton D. Microbiological Validation of Assessments of Caries Activity during Cavity Preparation. Caries Research. 1993;27:402–408. doi: 10.1159/000261571. [DOI] [PubMed] [Google Scholar]
- 20.Svanberg M, Mjör IA, Orstavik D. Mutans Streptococci in Plaque from Margins of Amalgam, Composite, and Glass-Ionomer Restorations. J Dent Res. 1990;69:861–864. doi: 10.1177/00220345900690030601. [DOI] [PubMed] [Google Scholar]
- 21.Martins CHG, Carvalho TC, Souza MGM, Ravagnani C, Peitl O, Zanotto ED, Panzeri H, Casemiro LA. Assessment of antimicrobial effect of Biosilicate (R) against anaerobic, microaerophilic and facultative anaerobic microorganisms. J Mater Sci-Mater Med. 2011;22:1439–1446. doi: 10.1007/s10856-011-4330-7. [DOI] [PubMed] [Google Scholar]
- 22.Hu S, Chang J, Liu MQ, Ning CQ. Study on antibacterial effect of 45S5 Bioglass(A (R)) J Mater Sci-Mater Med. 2009;20:281–286. doi: 10.1007/s10856-008-3564-5. [DOI] [PubMed] [Google Scholar]
- 23.Mortazavi V, Nahrkhalaji MM, Fathi MH, Mousavi SB, Esfahani BN. Antibacterial effects of sol-gel-derived bioactive glass nanoparticle on aerobic bacteria. J Biomed Mater Res Part A. 2010;94A:160–168. doi: 10.1002/jbm.a.32678. [DOI] [PubMed] [Google Scholar]
- 24.Lepparanta O, Vaahtio M, Peltola T, Zhang D, Hupa L, Hupa M, Ylanen H, Jukka IS, Matti KV, Eerola E. Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J Mater Sci-Mater Med. 2008;19:547–551. doi: 10.1007/s10856-007-3018-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Lepparanta O, Munukka E, Ylanen H, Viljanen MK, Eerola E, Hupa M, Hupa L. Antibacterial effects and dissolution behavior of six bioactive glasses. J Biomed Mater Res Part A. 2010;93A:475–483. doi: 10.1002/jbm.a.32564. [DOI] [PubMed] [Google Scholar]
- 26.Munukka E, Lepparanta O, Korkeamaki M, Vaahtio M, Peltola T, Zhang D, Hupa L, Ylanen H, Salonen JI, Viljanen MK, Eerola E. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J Mater Sci-Mater Med. 2008;19:27–32. doi: 10.1007/s10856-007-3143-1. [DOI] [PubMed] [Google Scholar]
- 27.Fooladi AAI, Hosseini HM, Hafezi F, Hosseinnejad F, Nourani MR. Sol-gel-derived bioactive glass containing SiO2-MgO-CaO-P2O5 as an antibacterial scaffold. J Biomed Mater Res Part A. 2013;101A:1582–1587. doi: 10.1002/jbm.a.34464. [DOI] [PubMed] [Google Scholar]
- 28.Gubler M, Brunner TJ, Zehnder M, Waltimo T, Sener B, Stark WJ. Do bioactive glasses convey a disinfecting mechanism beyond a mere increase in pH? Int Endod J. 2008;41:670–678. doi: 10.1111/j.1365-2591.2008.01413.x. [DOI] [PubMed] [Google Scholar]
- 29.Waltimo T, Brunner TJ, Vollenweider M, Stark WJ, Zehnder M. Antimicrobial effect of nanometric bioactive glass 45S5. J Dent Res. 2007;86:754–757. doi: 10.1177/154405910708600813. [DOI] [PubMed] [Google Scholar]
- 30.Stoor P, Soderling E, Salonen JI. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontologica Scandinavica. 1998;56:161–165. doi: 10.1080/000163598422901. [DOI] [PubMed] [Google Scholar]
- 31.Allan I, Newman H, Wilson M. Particulate Bioglass (R) reduces the viability of bacterial biofilms formed on its surface in an in vitro model. Clin Oral Implant Res. 2002;13:53–58. doi: 10.1034/j.1600-0501.2002.130106.x. [DOI] [PubMed] [Google Scholar]
- 32.Yli-Urpo H, Närhi T, Söderling E. Antimicrobial effects of glass ionomer cements containing bioactive glass (S53P4) on oral micro-organisms in vitro. Acta Odontologica Scandinavica. 2003;61:241–246. doi: 10.1080/00016350310004719. [DOI] [PubMed] [Google Scholar]
- 33.Yarborough L, Engle J, Wang M, Ferracane JL, Mitchell JC. Inhibition of Bacteria on a Bioactive Glass-containing, Anti-microbial Sealant Material. AADR/CADR Annual Meeting and Exhibition; Tampa, Florida. 2012. [Google Scholar]
- 34.Mitchell JC, Musanje L, Ferracane JL. Biomimetic dentin desensitizer based on nano-structured bioactive glass. Dent Mater. 2011;27:386–393. doi: 10.1016/j.dental.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Abdalla AI, Alhadainy HA. 2-year clinical evaluation of Class I posterior composites. Am J Dent. 1996;9:150–152. [PubMed] [Google Scholar]
- 36.Knibbs PJ, Smart ER. The clinical performance of a posterior composite resin restorative material, Heliomolar R.O: 3-year report. Journal Of Oral Rehabilitation. 1992;19:231–237. doi: 10.1111/j.1365-2842.1992.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 37.Rasmusson CG, Lundin SA. Class II restorations in six different posterior composite resins: five-year results. Swedish dental journal. 1995;19:173–182. [PubMed] [Google Scholar]
- 38.Shah M, Ferracane JL, Kruzic JJ. R-curve behavior and toughening mechanisms of resin based dental composites: Effects of hydration and post-cure heat treatment. Dent Mater. 2009;25:760–770. doi: 10.1016/j.dental.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 39.ISO Standard 4049. Dentistry -- Polymer-based restorative materials. Vol. 2009. International Organization for Standardization; Geneva, Switzerland: 2009. http://www.iso.org. [Google Scholar]
- 40.ASTM Standard E399. Standard Test Method for Linear-Elastic Plane-Strain Fracture Toughness KIc of Metallic Materials. ASTM International; West Conshohocken, Pennsylvania, USA: 2009. 2009. http://www.astm.org. [DOI] [Google Scholar]
- 41.ASTM Standard E647, 2008e1, Standard Test Method for Measurement of Fatigue Crack Growth Rates. ASTM International; West Conshohocken, Pennsylvania, USA: 2008. http://www.astm.org. [DOI] [Google Scholar]
- 42.Braem M, Lambrechts P, Vanherle G. Clinical relevance of laboratory fatigue studies. J Dent. 1994;22:97–102. doi: 10.1016/0300-5712(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 43.Newman JC., Jr Stress-intensity factors and crack-opening displacements for round compact specimens. Int J Fract. 1981;17:567–578. [Google Scholar]
- 44.Paris PC, Erdogan F. A critical analysis of crack propagation laws. J Basic Eng. 1963;85:528–534. [Google Scholar]
- 45.Kim KH, Ong JL, Okuno O. The effect of filler loading and morphology on the mechanical properties of contemporary composites. Journal of Prosthetic Dentistry. 2002;87:642–649. doi: 10.1067/mpr.2002.125179. [DOI] [PubMed] [Google Scholar]
- 46.Braem M, Finger W, Vandoren VE, Lambrechts P, Vanherle G. Mechanical-Properties and Filler Fraction of Dental Composites. Dent Mater. 1989;5:346–349. doi: 10.1016/0109-5641(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigues SA, Jr, Ferracane JL, Della Bona A. Flexural strength and Weibull analysis of a microhybrid and a nanofill composite evaluated by 3-and 4-point bending tests. Dent Mater. 2008;24:426–431. doi: 10.1016/j.dental.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Calais JG, Söderholm KJM. Influence of filler type and water exposure on flexural strength of experimental composite resins. J Dent Res. 1988;67:836–840. doi: 10.1177/00220345880670050801. [DOI] [PubMed] [Google Scholar]
- 49.Söderholm KJM, Roberts MJ. Influence of water exposure on the tensile strength of composites. J Dent Res. 1990;69:1812–1816. doi: 10.1177/00220345900690120501. [DOI] [PubMed] [Google Scholar]
- 50.Curtis AR, Shortall AC, Marquis PM, Palin WM. Water uptake and strength characteristics of a nanofilled resin-based composite. J Dent. 2008;36:186–193. doi: 10.1016/j.jdent.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 51.Lohbauer U, Frankenberger R, Kramer N, Petschelt A. Time-dependent strength and fatigue resistance of dental direct restorative materials. J Mater Sci-Mater Med. 2003;14:1047–1053. doi: 10.1023/b:jmsm.0000004001.73640.4c. [DOI] [PubMed] [Google Scholar]
- 52.Ferracane JL, Antonio RC, Matsumoto H. Variables Affecting the Fracture-Toughness of Dental Composites. J Dent Res. 1987;66:1140–1145. doi: 10.1177/00220345870660060901. [DOI] [PubMed] [Google Scholar]
- 53.Shah MB, Ferracane JL, Kruzic JJ. R-curve behavior and micromechanisms of fracture in resin based dental restorative composites. Journal of the Mechanical Behavior of Biomedical Materials. 2009;2:502–511. doi: 10.1016/j.jmbbm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Shah MB, Ferracane JL, Kruzic JJ. Mechanistic aspects of fatigue crack growth behavior in resin based dental restorative composites. Dent Mater. 2009;25:909–916. doi: 10.1016/j.dental.2009.01.097. [DOI] [PubMed] [Google Scholar]
- 55.Manhart J, Kunzelmann KH, Chen HY, Hickel R. Mechanical properties of new composite restorative materials. J Biomed Mater Res. 2000;53:353–361. doi: 10.1002/1097-4636(2000)53:4<353::aid-jbm9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 56.Htang A, Ohsawa M, Matsumoto H. Fatigue Resistance of Composite Restorations - Effect of Filler Content. Dent Mater. 1995;11:7–13. doi: 10.1016/0109-5641(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 57.Lohbauer U, Frankenberger R, Kramer N, Petschelt A. Strength and fatigue performance versus filler fraction of different types of direct dental restoratives. J Biomed Mater Res Part B. 2006;76B:114–120. doi: 10.1002/jbm.b.30338. [DOI] [PubMed] [Google Scholar]
- 58.Ornaghi BP, Meier MM, Rosa V, Cesar PF, Lohbauer U, Braga RR. Subcritical crack growth and in vitro lifetime prediction of resin composites with different filler distributions. Dent Mater. 2012;28:985–995. doi: 10.1016/j.dental.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Drummond JL, Lin LH, Al-Turki LA, Hurley RK. Fatigue behaviour of dental composite materials. J Dent. 2009;37:321–330. doi: 10.1016/j.jdent.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Elbishari H, Silikas N, Satterthwaite J. Filler size of resin-composites, percentage of voids and fracture toughness: is there a correlation? Dent Mater J. 2012;31:523–527. doi: 10.4012/dmj.2011-256. [DOI] [PubMed] [Google Scholar]
- 61.De Souza JA, Goutianos S, Skovgaard M, Sorensen BF. Fracture resistance curves and toughening mechanisms in polymer based dental composites. Journal of the Mechanical Behavior of Biomedical Materials. 2011;4:558–571. doi: 10.1016/j.jmbbm.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Imbeni V, Kruzic JJ, Marshall GW, Marshall SJ, Ritchie RO. The dentin-enamel junction and the fracture of human teeth. Nature Mater. 2005;4:229–232. doi: 10.1038/nmat1323. [DOI] [PubMed] [Google Scholar]
- 63.Bechtle S, Habelitz S, Klocke A, Fett T, Schneider GA. The fracture behaviour of dental enamel. Biomaterials. 2010;31:375–384. doi: 10.1016/j.biomaterials.2009.09.050. [DOI] [PubMed] [Google Scholar]
- 64.Bajaj D, Arola DD. On the R-curve behavior of human tooth enamel. Biomaterials. 2009;30:4037–4046. doi: 10.1016/j.biomaterials.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kruzic JJ, Nalla RK, Kinney JH, Ritchie RO. Crack blunting, crack bridging and resistance-curve fracture mechanics in dentin: Effect of hydration. Biomaterials. 2003;24:5209–5221. doi: 10.1016/s0142-9612(03)00458-7. [DOI] [PubMed] [Google Scholar]