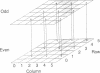
Abstract
A class of stochastic visual textures are used to analyze the components of the visual evoked potential (VEP). This procedure exploits the differential sensitivity of populations of visual neurons to aspects of contrast and pattern. A simple transformation of VEP responses elicited by these stimuli separates components that reflect complex aspects of visual processing from those that reflect elementary aspects. Simultaneous recordings of the VEP and cellular activity in the cat lateral geniculate nucleus are obtained. Responses to traditional VEP stimuli contain a mixture of intracortically generated and precortically generated components. A theoretical and experimental analysis demonstrates that the present approach cleanly separates intracortical generators of the VEP from precortical generators.
Full text
PDF
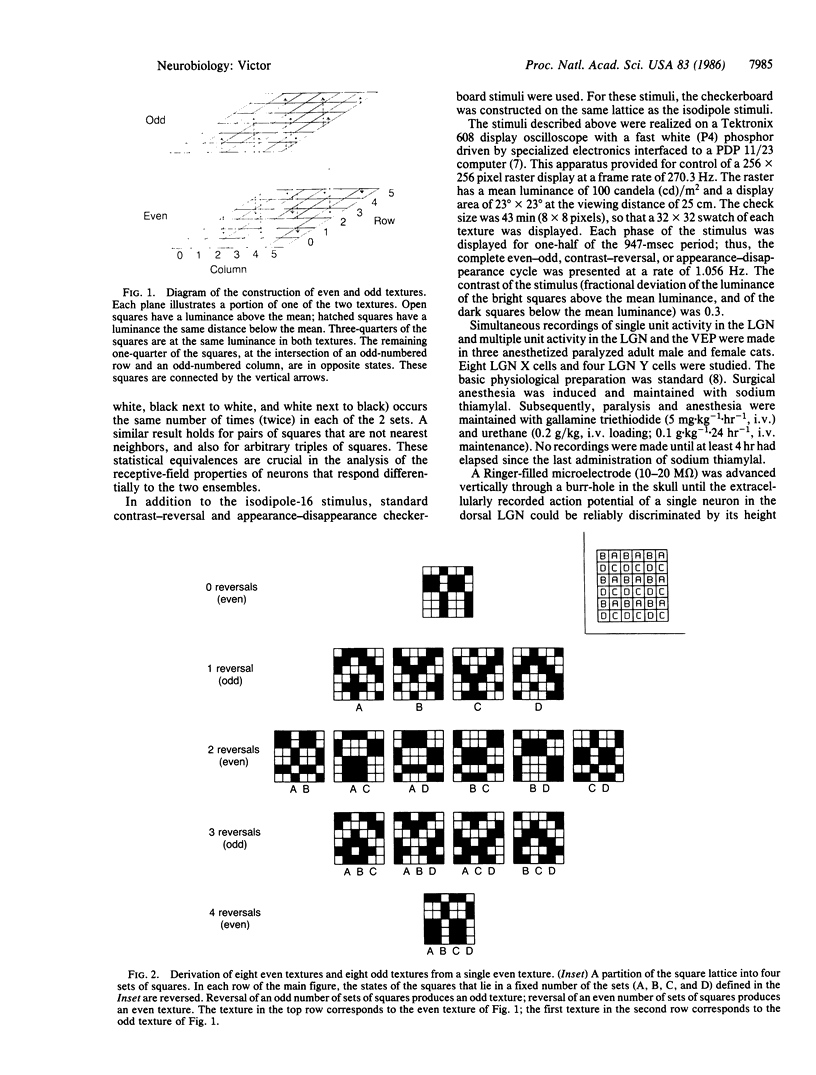
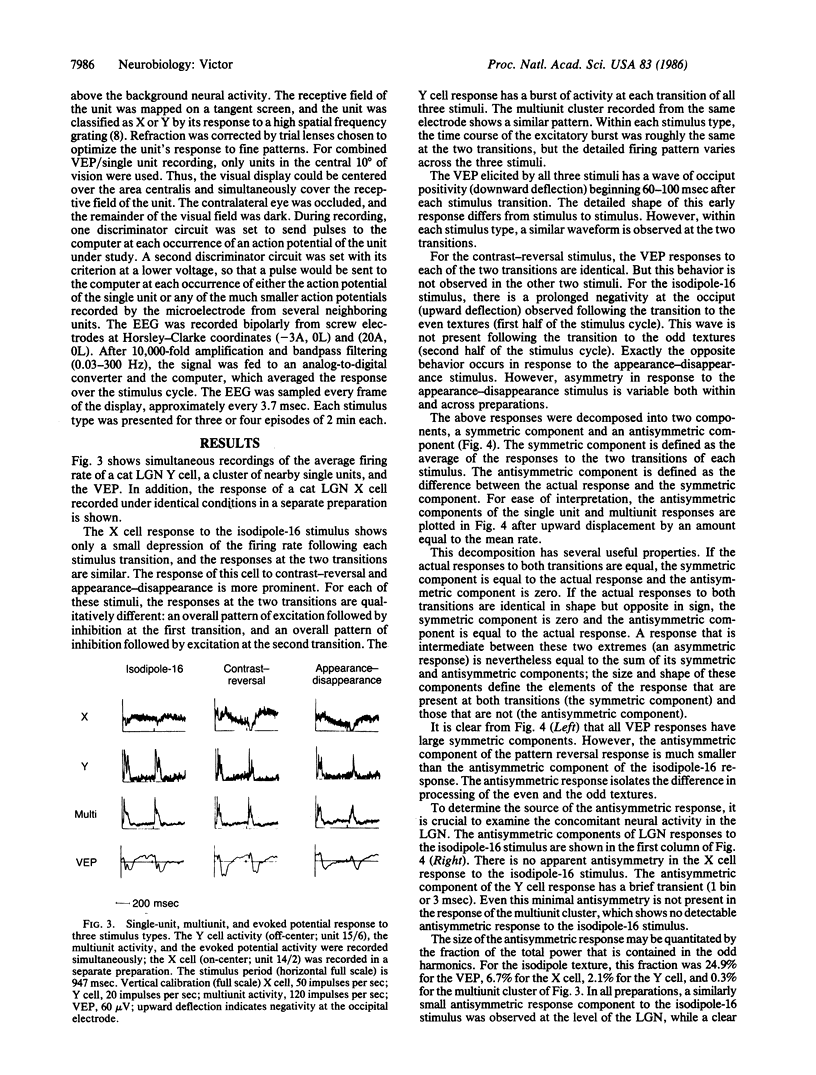
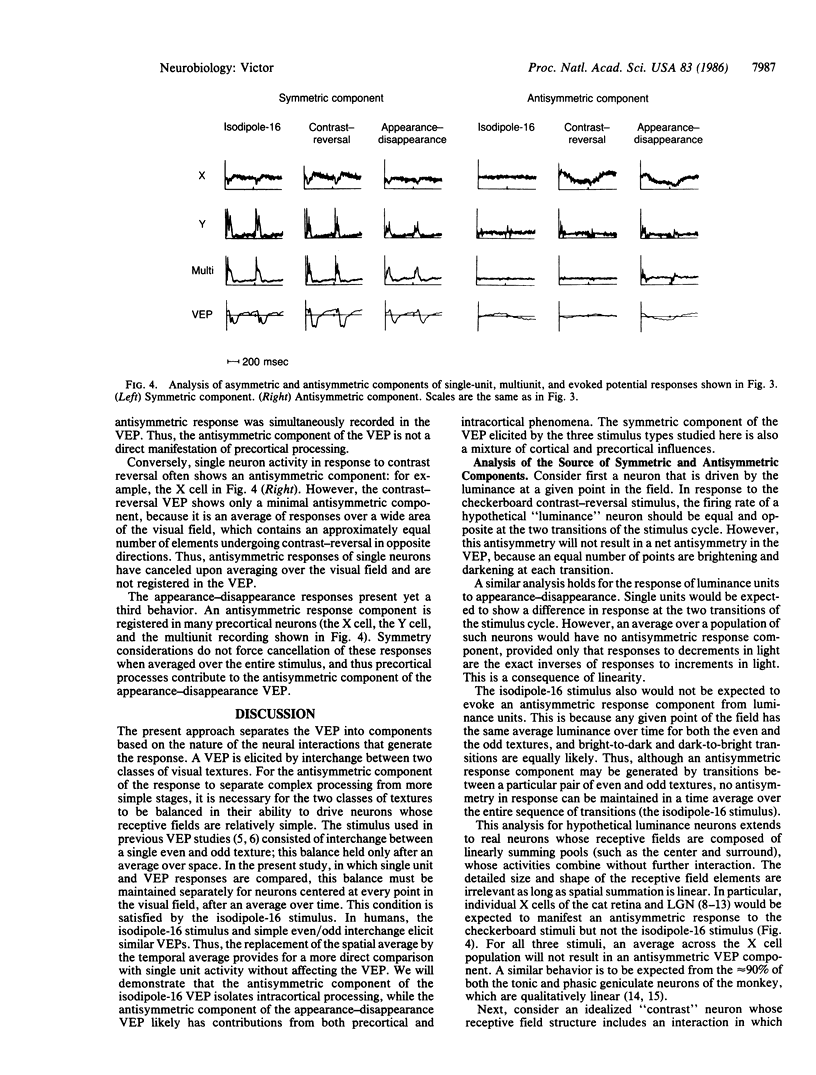

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Valois R. L., Morgan H. C., Polson M. C., Mead W. R., Hull E. M. Psychophysical studies of monkey vision. I. Macaque luminosity and color vision tests. Vision Res. 1974 Jan;14(1):53–67. doi: 10.1016/0042-6989(74)90116-3. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Morgan H., Snodderly D. M. Psychophysical studies of monkey vision. 3. Spatial luminance contrast sensitivity tests of macaque and human observers. Vision Res. 1974 Jan;14(1):75–81. doi: 10.1016/0042-6989(74)90118-7. [DOI] [PubMed] [Google Scholar]
- Dreher B., Fukada Y., Rodieck R. W. Identification, classification and anatomical segregation of cells with X-like and Y-like properties in the lateral geniculate nucleus of old-world primates. J Physiol. 1976 Jun;258(2):433–452. doi: 10.1113/jphysiol.1976.sp011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. Algebraic summation of centre and surround inputs to retinal ganglion cells of the cat. Nature. 1970 May 2;226(5244):458–459. doi: 10.1038/226458a0. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julesz B., Gilbert E. N., Victor J. D. Visual discrimination of textures with identical third-order statistics. Biol Cybern. 1978 Dec 5;31(3):137–140. doi: 10.1007/BF00336998. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Shapley R. M. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982 Sep;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H., Bishop P. O., Orban G. A. Hypercomplex and simple/complex cell classifications in cat striate cortex. J Neurophysiol. 1978 Sep;41(5):1071–1095. doi: 10.1152/jn.1978.41.5.1071. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Receptive field organization of complex cells in the cat's striate cortex. J Physiol. 1978 Oct;283:79–99. doi: 10.1113/jphysiol.1978.sp012489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol. 1978 Oct;283:53–77. doi: 10.1113/jphysiol.1978.sp012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Mackeben M. Steady state visual evoked potentials in the alert primate. Vision Res. 1982;22(10):1261–1271. doi: 10.1016/0042-6989(82)90138-9. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Response of cat retinal ganglion cells to moving visual patterns. J Neurophysiol. 1965 Sep;28(5):819–832. doi: 10.1152/jn.1965.28.5.819. [DOI] [PubMed] [Google Scholar]
- Spekreijse H., van der Twell L. H., Zuidema T. Contrast evoked responses in man. Vision Res. 1973 Aug;13(8):1577–1601. doi: 10.1016/0042-6989(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Spitzer H., Hochstein S. A complex-cell receptive-field model. J Neurophysiol. 1985 May;53(5):1266–1286. doi: 10.1152/jn.1985.53.5.1266. [DOI] [PubMed] [Google Scholar]
- Spitzer H., Hochstein S. Simple- and complex-cell response dependences on stimulation parameters. J Neurophysiol. 1985 May;53(5):1244–1265. doi: 10.1152/jn.1985.53.5.1244. [DOI] [PubMed] [Google Scholar]
- Tyler C. W., Apkarian P., Nakayama K. Multiple spatial-frequency tuning of electrical responses from human visual cortex. Exp Brain Res. 1978 Nov 15;33(3-4):535–550. doi: 10.1007/BF00235573. [DOI] [PubMed] [Google Scholar]
- Victor J. D. Complex visual textures as a tool for studying the VEP. Vision Res. 1985;25(12):1811–1827. doi: 10.1016/0042-6989(85)90005-7. [DOI] [PubMed] [Google Scholar]
- Victor J. D., Shapley R. M. Receptive field mechanisms of cat X and Y retinal ganglion cells. J Gen Physiol. 1979 Aug;74(2):275–298. doi: 10.1085/jgp.74.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J. D., Shapley R. M. The nonlinear pathway of Y ganglion cells in the cat retina. J Gen Physiol. 1979 Dec;74(6):671–689. doi: 10.1085/jgp.74.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor J. D., Zemon V. The human visual evoked potential: analysis of components due to elementary and complex aspects of form. Vision Res. 1985;25(12):1829–1842. doi: 10.1016/0042-6989(85)90006-9. [DOI] [PubMed] [Google Scholar]
- Zemon V., Ratliff F. Visual evoked potentials: evidence for lateral interactions. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5723–5726. doi: 10.1073/pnas.79.18.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]