Abstract
Most intracellular pathogens avoid lysing their host cells during invasion by wrapping themselves in a vacuolar membrane. This parasitophorous vacuole membrane (PVM) is often retained, serving as a critical transport interface between the parasite and the host cell cytoplasm. To test whether the PVM formed by the parasite Toxoplasma gondii is derived from host cell membrane or from lipids secreted by the parasite, we used time-resolved capacitance measurements and video microscopy to assay host cell surface area during invasion. We observed no significant change in host cell surface area during PVM formation, demonstrating that the PVM consists primarily of invaginated host cell membrane. Pinching off of the PVM from the host cell membrane occurred after an unexpected delay (34-305 sec) and was seen as a 0.219 +/- 0.006 pF drop in capacitance, which corresponds well to the predicted surface area of the entire PVM (30-33 microns2). The formation and closure of a fission pore connecting the extracellular medium and the vacuolar space was detected as the PVM pinched off. This final stage of parasite entry was accomplished without any breach in cell membrane integrity.
Full text
PDF

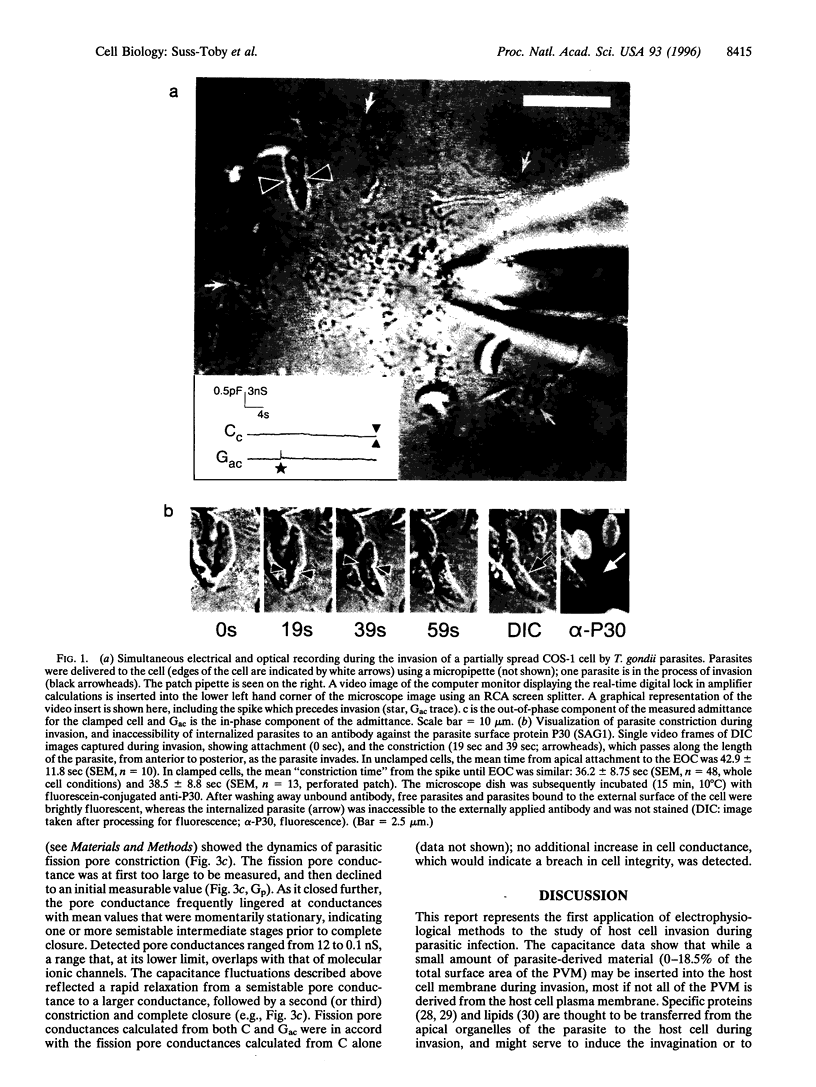
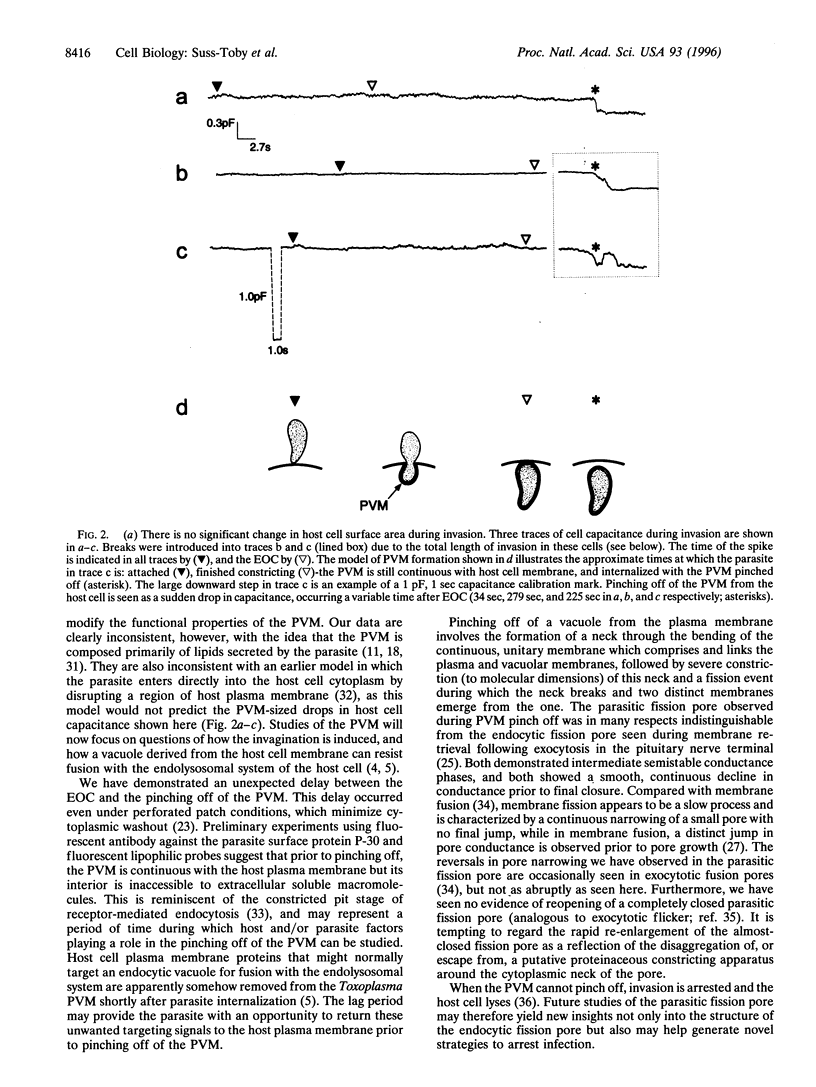


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Miller L. H., Rabbege J. R., Epstein N. Freeze-fracture study on the erythrocyte membrane during malarial parasite invasion. J Cell Biol. 1981 Oct;91(1):55–62. doi: 10.1083/jcb.91.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson C. T., Aikawa M., Perry G., Fujino T., Bennett V., Davidson E. A., Howard R. J. Ultrastructural localization of erythrocyte cytoskeletal and integral membrane proteins in Plasmodium falciparum-infected erythrocytes. Eur J Cell Biol. 1988 Feb;45(2):192–199. [PubMed] [Google Scholar]
- Bannister L. H., Mitchell G. H., Butcher G. A., Dennis E. D. Lamellar membranes associated with rhoptries in erythrocytic merozoites of Plasmodium knowlesi: a clue to the mechanism of invasion. Parasitology. 1986 Apr;92(Pt 2):291–303. doi: 10.1017/s0031182000064064. [DOI] [PubMed] [Google Scholar]
- Bannister L. H., Mitchell G. H. The fine structure of secretion by Plasmodium knowlesi merozoites during red cell invasion. J Protozool. 1989 Jul-Aug;36(4):362–367. doi: 10.1111/j.1550-7408.1989.tb05527.x. [DOI] [PubMed] [Google Scholar]
- Beckers C. J., Dubremetz J. F., Mercereau-Puijalon O., Joiner K. A. The Toxoplasma gondii rhoptry protein ROP 2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994 Nov;127(4):947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge L. J., Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. 1987 Aug 27-Sep 2Nature. 328(6133):814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Curran M. J., Cohen F. S., Chandler D. E., Munson P. J., Zimmerberg J. Exocytotic fusion pores exhibit semi-stable states. J Membr Biol. 1993 Apr;133(1):61–75. doi: 10.1007/BF00231878. [DOI] [PubMed] [Google Scholar]
- Dluzewski A. R., Fryer P. R., Griffiths S., Wilson R. J., Gratzer W. B. Red cell membrane protein distribution during malarial invasion. J Cell Sci. 1989 Apr;92(Pt 4):691–699. doi: 10.1242/jcs.92.4.691. [DOI] [PubMed] [Google Scholar]
- Dluzewski A. R., Mitchell G. H., Fryer P. R., Griffiths S., Wilson R. J., Gratzer W. B. Origins of the parasitophorous vacuole membrane of the malaria parasite, Plasmodium falciparum, in human red blood cells. J Cell Sci. 1992 Jul;102(Pt 3):527–532. doi: 10.1242/jcs.102.3.527. [DOI] [PubMed] [Google Scholar]
- Dvorak J. A., Miller L. H., Whitehouse W. C., Shiroishi T. Invasion of erythrocytes by malaria merozoites. Science. 1975 Feb 28;187(4178):748–750. doi: 10.1126/science.803712. [DOI] [PubMed] [Google Scholar]
- Elford B. C., Cowan G. M., Ferguson D. J. Parasite-regulated membrane transport processes and metabolic control in malaria-infected erythrocytes. Biochem J. 1995 Jun 1;308(Pt 2):361–374. doi: 10.1042/bj3080361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf H. G., Haldar K. Secretory transport in Plasmodium. Parasitol Today. 1993 Mar;9(3):98–102. doi: 10.1016/0169-4758(93)90216-3. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A. Rhoptry lipids and parasitophorous vacuole formation: a slippery issue. Parasitol Today. 1991 Sep;7(9):226–227. doi: 10.1016/0169-4758(91)90232-d. [DOI] [PubMed] [Google Scholar]
- Joiner K. A. Vacuolar membranes surrounding intracellular pathogens: where do they come from and what do they do? Infect Agents Dis. 1993 Aug;2(4):215–219. [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992 Aug;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- McLaren D. J., Bannister L. H., Trigg P. I., Butcher G. A. Freeze fracture studies on the interaction between the malaria parasite and the host erythrocyte in Plasmodium knowlesi infections. Parasitology. 1979 Aug;79(1):125–139. doi: 10.1017/s0031182000052021. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. B., Kamber M., Wadwa K. S., Lin P. S., Schmidt-Ullrich R. The role of lipids in Plasmodium falciparum invasion of erythrocytes: a coordinated biochemical and microscopic analysis. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5956–5960. doi: 10.1073/pnas.85.16.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. H., Bannister L. H. Malaria parasite invasion: interactions with the red cell membrane. Crit Rev Oncol Hematol. 1988;8(4):225–310. doi: 10.1016/s1040-8428(88)80011-8. [DOI] [PubMed] [Google Scholar]
- Morisaki J. H., Heuser J. E., Sibley L. D. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995 Jun;108(Pt 6):2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B. A., O'Connor G. R. Penetration of mouse peritoneal macrophages by the protozoon Toxoplasma gondii. New evidence for active invasion and phagocytosis. Lab Invest. 1981 Apr;44(4):324–335. [PubMed] [Google Scholar]
- Niles W. D., Levis R. A., Cohen F. S. Planar bilayer membranes made from phospholipid monolayers form by a thinning process. Biophys J. 1988 Mar;53(3):327–335. doi: 10.1016/S0006-3495(88)83110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenboom H., Lindau M. Exo-endocytosis and closing of the fission pore during endocytosis in single pituitary nerve terminals internally perfused with high calcium concentrations. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5267–5271. doi: 10.1073/pnas.91.12.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sam-Yellowe T. Y., Shio H., Perkins M. E. Secretion of Plasmodium falciparum rhoptry protein into the plasma membrane of host erythrocytes. J Cell Biol. 1988 May;106(5):1507–1513. doi: 10.1083/jcb.106.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G., Kleinklaus A. K., Marrion N. V., Trimmer J. S. Properties of Kv2.1 K+ channels expressed in transfected mammalian cells. J Biol Chem. 1994 Sep 16;269(37):23204–23211. [PubMed] [Google Scholar]
- Stewart M. J., Schulman S., Vanderberg J. P. Rhoptry secretion of membranous whorls by Plasmodium falciparum merozoites. Am J Trop Med Hyg. 1986 Jan;35(1):37–44. doi: 10.4269/ajtmh.1986.35.37. [DOI] [PubMed] [Google Scholar]
- Takei K., McPherson P. S., Schmid S. L., De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-gamma S in nerve terminals. Nature. 1995 Mar 9;374(6518):186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Miller L. H., Dvorak J. A. The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J Cell Sci. 1993 Sep;106(Pt 1):237–248. doi: 10.1242/jcs.106.1.237. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Curran M., Cohen F. S., Brodwick M. Simultaneous electrical and optical measurements show that membrane fusion precedes secretory granule swelling during exocytosis of beige mouse mast cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1585–1589. doi: 10.1073/pnas.84.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]




