Abstract
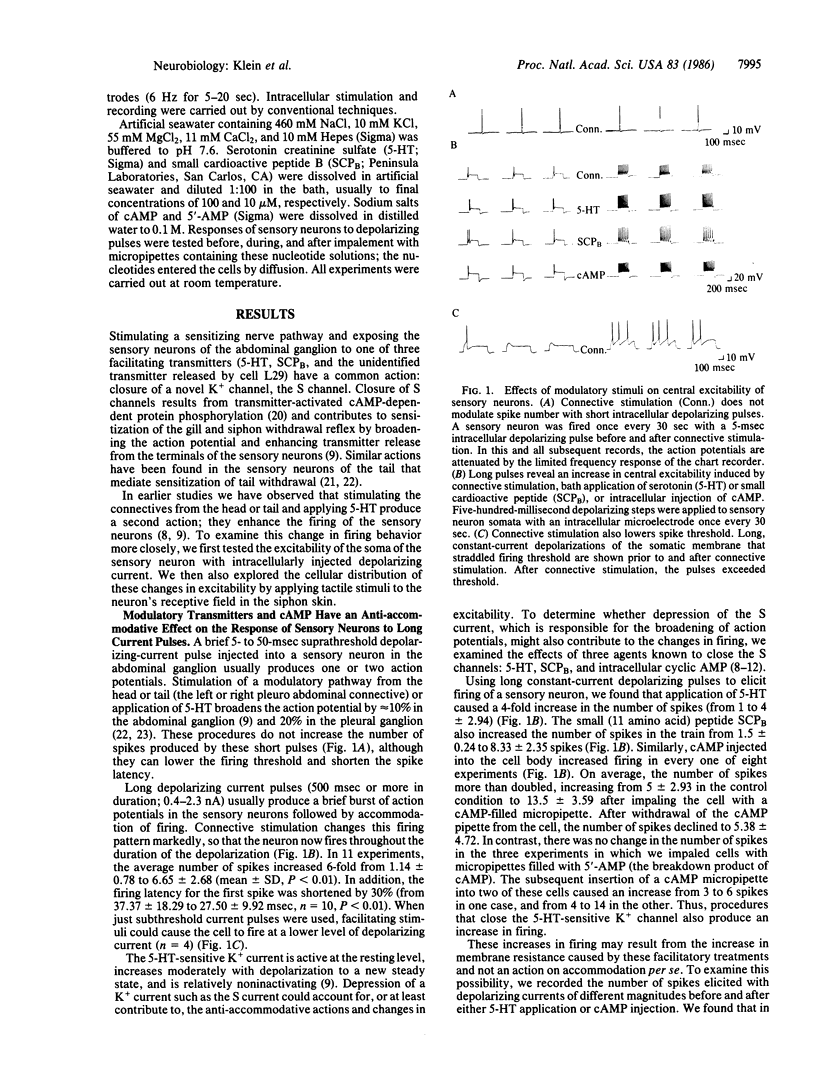
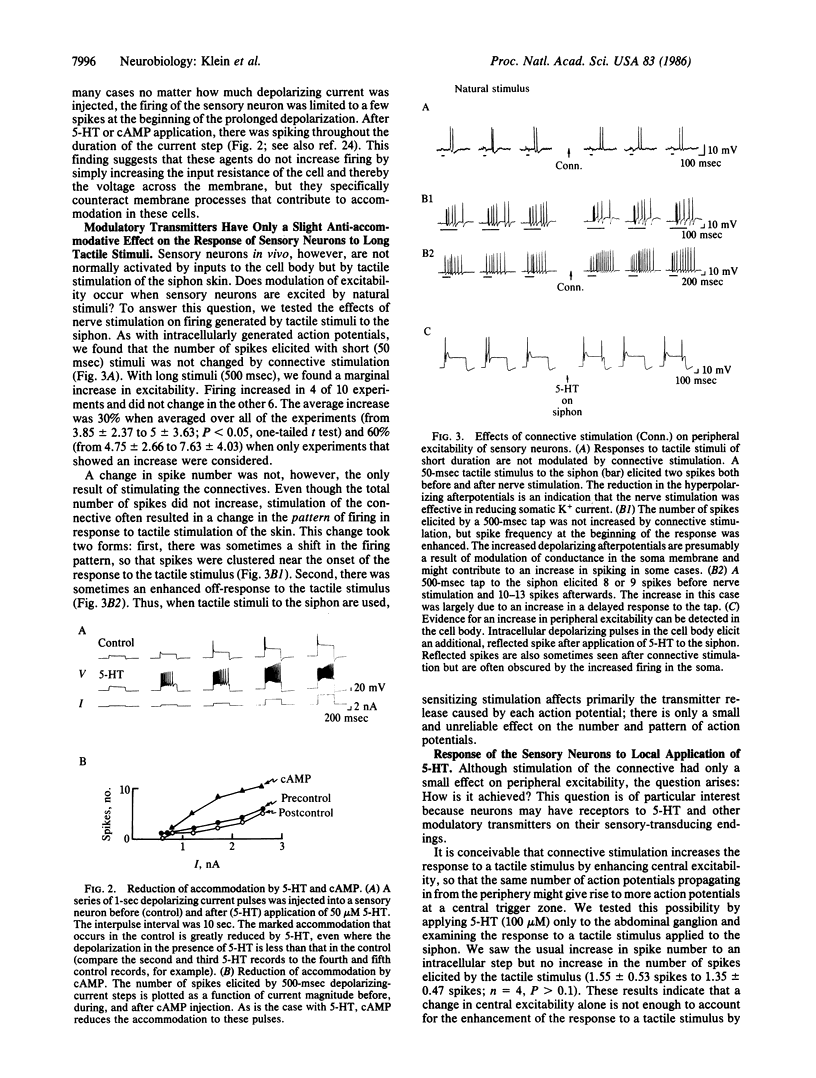
Presynaptic facilitation of transmission from sensory to motor neurons contributes significantly to behavioral sensitization of defensive withdrawal reflexes in Aplysia. Presynaptic facilitation is associated with a decrease in the serotonin-sensitive K+ conductance. This decrease broadens the presynaptic action potential. In addition, the procedures that cause facilitation—stimulation of the connective (the pathway from the tail and head), application of modulatory transmitters, or injection of cAMP—also increase the excitability of the sensory neurons as tested with intracellular depolarizing pulses injected into the cell body. The increased excitability is reflected in a decreased threshold for generating action potentials and a reduction in accommodation to prolonged constant current stimuli. By influencing the excitability of the peripheral processes of the sensory neurons, stimulation of the connectives or serotonin also produces a small enhancement of the response of the sensory neurons to a tactile stimulus applied to the siphon. The excitability changes appear to result, at least in part, from the same cellular mechanisms that lead to broadening of the action potential, a cAMP-mediated closure of K+ channels. Therefore, these findings indicate that the same class of mechanisms can, in principle, have a dual action and provide further evidence for parallel processing in the modulation of transmitter release from a single neuron.
Keywords: K+ currents, second messengers, presynaptic facilitation, excitability, sensitization
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W., Castellucci V. F., Camardo J. S., Kandel E. R., Lloyd P. E. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Galvan M. Voltage-dependent currents of vertebrate neurons and their role in membrane excitability. Adv Neurol. 1986;44:137–170. [PubMed] [Google Scholar]
- Bailey C. H., Castellucci V. F., Koester J., Kandel E. R. Cellular studies of peripheral neurons in siphon skin of Aplysia californica. J Neurophysiol. 1979 Mar;42(2):530–557. doi: 10.1152/jn.1979.42.2.530. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Hawkins R. D., Chen M. C., Kandel E. R. Interneurons involved in mediation and modulation of gill-withdrawal reflex in Aplysia. IV. Morphological basis of presynaptic facilitation. J Neurophysiol. 1981 Feb;45(2):340–360. doi: 10.1152/jn.1981.45.2.340. [DOI] [PubMed] [Google Scholar]
- Boyle M. B., Klein M., Smith S. J., Kandel E. R. Serotonin increases intracellular Ca2+ transients in voltage-clamped sensory neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7642–7646. doi: 10.1073/pnas.81.23.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J., Castellucci V., Kandel E. R. Receptive fields and response properties of mechanoreceptor neurons innervating siphon skin and mantle shelf in Aplysia. J Neurophysiol. 1974 Sep;37(5):1041–1064. doi: 10.1152/jn.1974.37.5.1041. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Castellucci V. F., Byrne J. H., Kandel E. R. Quantitative analysis of relative contribution of central and peripheral neurons to gill-withdrawal reflex in Aplysia californica. J Neurophysiol. 1979 Mar;42(2):497–509. doi: 10.1152/jn.1979.42.2.497. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V., Kandel E. R. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976 Dec 10;194(4270):1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Castellucci V., Pinsker H., Kupfermann I., Kandel E. R. Neuronal mechanisms of habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970 Mar 27;167(3926):1745–1748. doi: 10.1126/science.167.3926.1745. [DOI] [PubMed] [Google Scholar]
- Clark G. A., Kandel E. R. Branch-specific heterosynaptic facilitation in Aplysia siphon sensory cells. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2577–2581. doi: 10.1073/pnas.81.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Alkon D. L. Associative behavioral modification in hermissenda: cellular correlates. Science. 1980 Jul 18;209(4454):412–414. doi: 10.1126/science.209.4454.412. [DOI] [PubMed] [Google Scholar]
- Farley J., Auerbach S. Protein kinase C activation induces conductance changes in Hermissenda photoreceptors like those seen in associative learning. Nature. 1986 Jan 16;319(6050):220–223. doi: 10.1038/319220a0. [DOI] [PubMed] [Google Scholar]
- Grafe P., Mayer C. J., Wood J. D. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980 Aug;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Heterosynaptic facilitation in neurones of the abdominal ganglion of Aplysia depilans. J Physiol. 1965 Nov;181(1):1–27. doi: 10.1113/jphysiol.1965.sp007742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler H. B., Jr, Hawkins R. D., Koester J., Steinbusch H. W., Kandel E. R., Schwartz J. H. Distribution of serotonin-immunoreactive cell bodies and processes in the abdominal ganglion of mature Aplysia. J Neurosci. 1985 Jan;5(1):72–80. doi: 10.1523/JNEUROSCI.05-01-00072.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Pollock J. D., Bernier L., Camardo J. S. Serotonin and cyclic adenosine 3':5'-monophosphate modulate the potassium current in tail sensory neurons in the pleural ganglion of Aplysia. J Neurosci. 1985 Jul;5(7):1862–1871. doi: 10.1523/JNEUROSCI.05-07-01862.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci U S A. 1980 Jan;77(1):629–633. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- VanderMaelen C. P., Aghajanian G. K. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980 Sep 25;287(5780):346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H., Carew T. J., Kandel E. R. Mechanoafferent neurons innervating tail of Aplysia. II. Modulation by sensitizing stimulation. J Neurophysiol. 1983 Dec;50(6):1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- Woody C. D., Engel J., Jr Changes in unit activity and thresholds to electrical microstimulation at coronal-pericruciate cortex of cat with classical conditioning of different facial movements. J Neurophysiol. 1972 Mar;35(2):230–241. doi: 10.1152/jn.1972.35.2.230. [DOI] [PubMed] [Google Scholar]
- Woody C. D., Swartz B. E., Gruen E. Effects of acetylcholine and cyclic GMP on input resistance of cortical neurons in awake cats. Brain Res. 1978 Dec 15;158(2):373–395. doi: 10.1016/0006-8993(78)90682-0. [DOI] [PubMed] [Google Scholar]
- Woollacott M., Hoyle G. Neural events underlying learning in insects: changes in pacemaker. Proc R Soc Lond B Biol Sci. 1977 Jan 14;195(1120):395–415. doi: 10.1098/rspb.1977.0017. [DOI] [PubMed] [Google Scholar]


