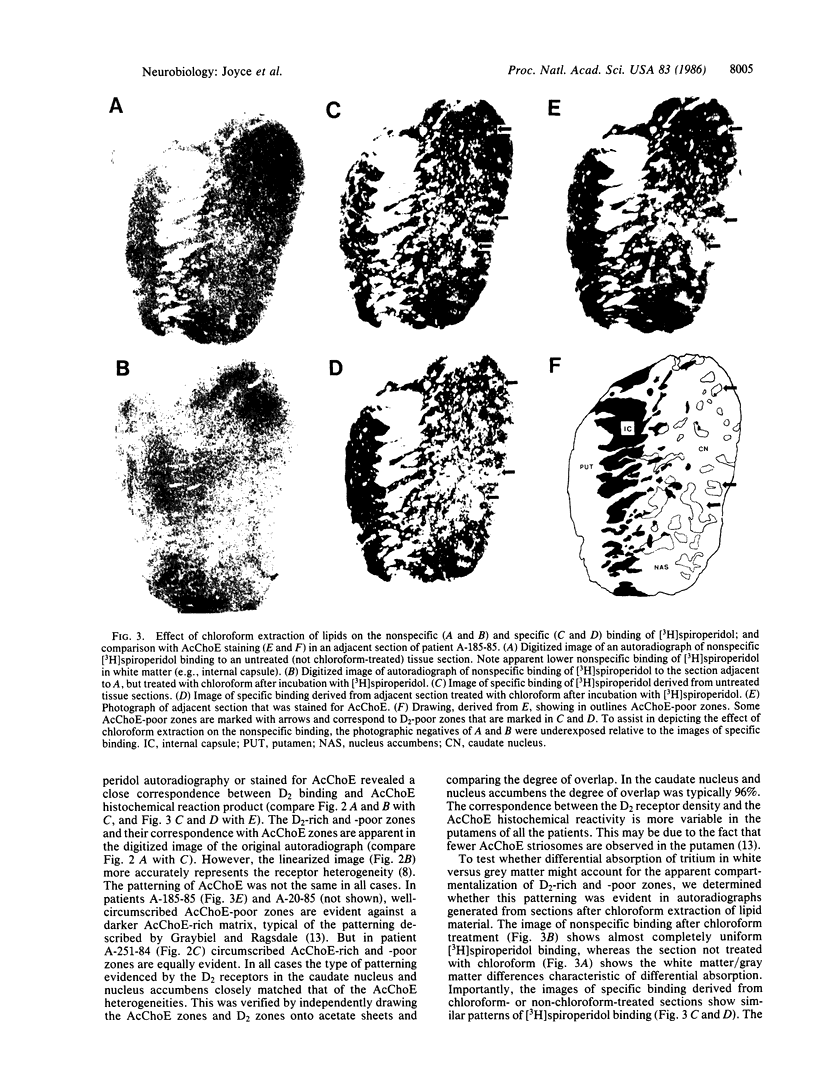
Abstract
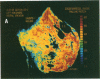
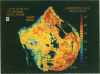
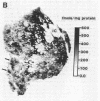
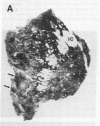
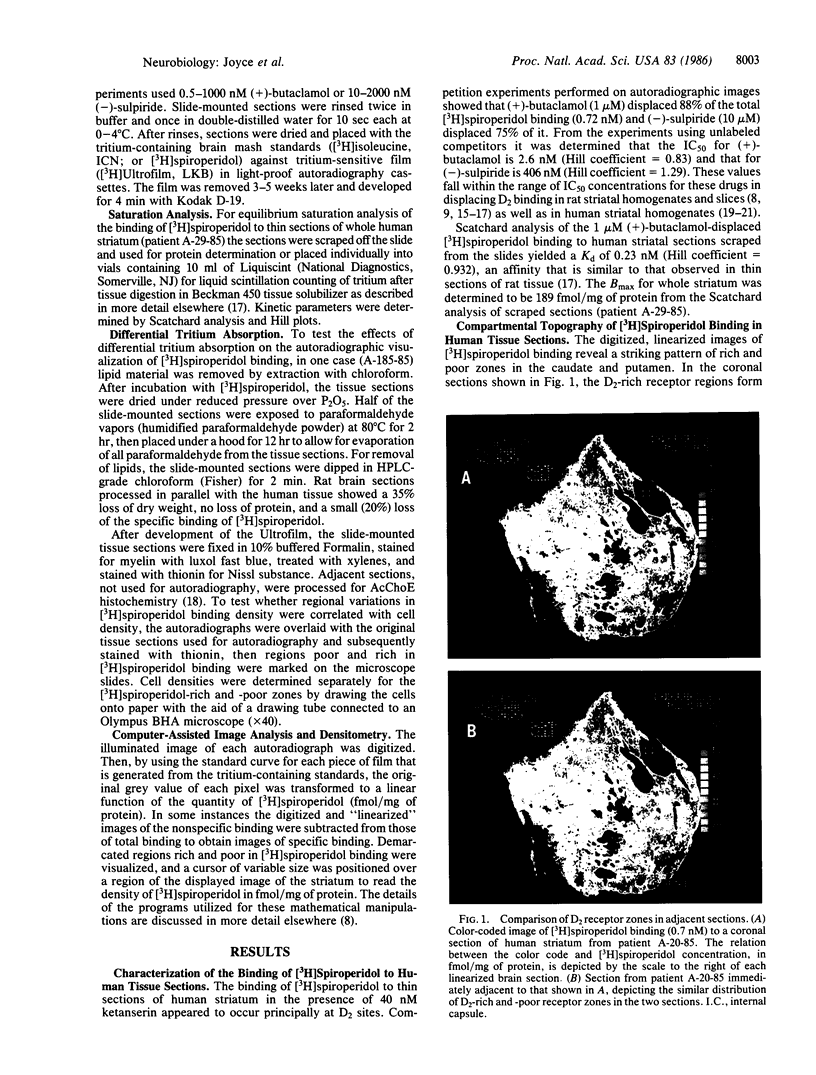
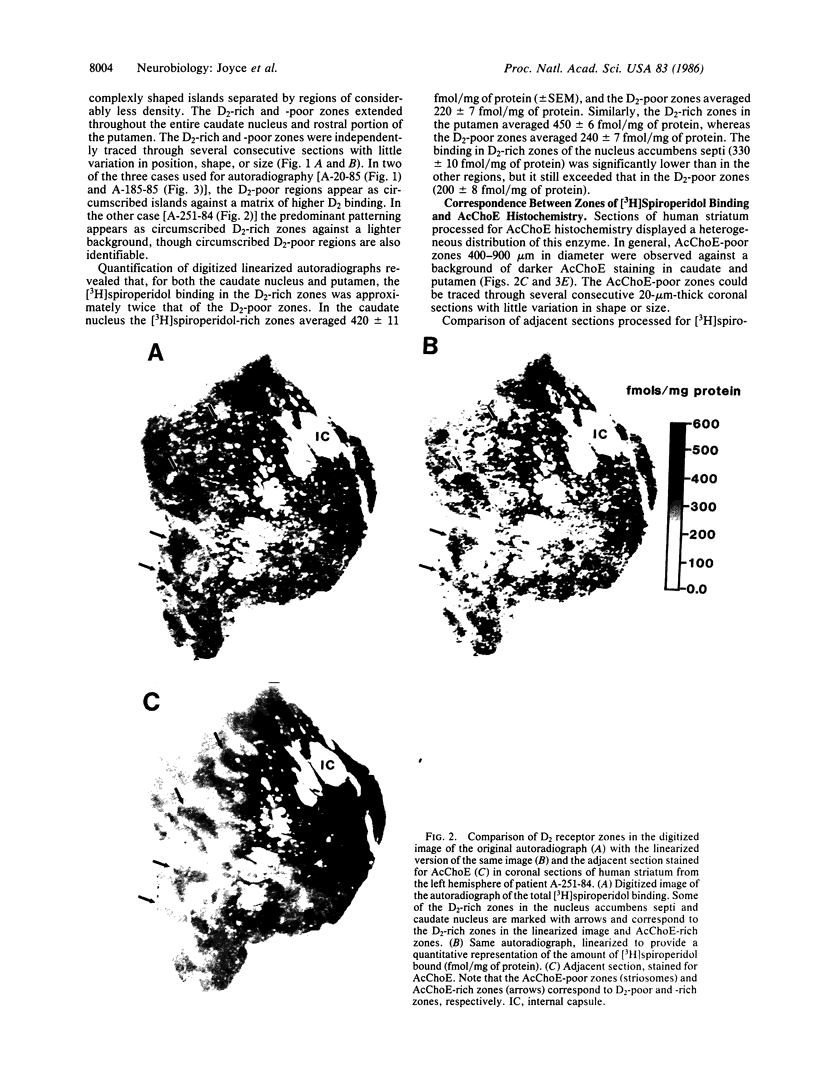
Dopamine (D2) receptors visualized in postmortem human striatum by quantitative autoradiography of [3H]spiroperidol binding are organized into circumscribed zones of low receptor density separated from other such zones by regions of higher D2 density. The D2-rich zones of the caudate nucleus and putamen contain twice the binding of D2-poor zones. The Hill coefficient, obtained from saturation analysis of [3H]spiroperidol binding to thin sections of human striatum, gave a value near unity, indicating the binding was occurring to a single type of site. The patchiness of [3H]spiroperidol binding was unaltered by postincubation removal of lipid from the tissue sections, indicating that a differential absorption of tritium in white and grey matter does not account for the heterogeneous distribution. The D2-rich and D2-poor regions appear to form labyrinths oriented in the anterior-posterior axis and are typically aligned with, respectively, acetylcholinesterase-rich and -poor compartments as visualized on stained adjacent sections. Thus, the distribution of dopamine D2 receptors conforms to the "striosomal" organization of the human caudate-putamen, a finding that suggests that this receptor subtype may mediate the influence of dopamine on distinct neurochemical compartments within the structure.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., Kim H., Marshall J. F. Computer imaging and analysis of dopamine (D2) and serotonin (S2) binding sites in rat basal ganglia or neocortex labeled by [3H]spiroperidol. J Pharmacol Exp Ther. 1985 May;233(2):527–538. [PubMed] [Google Scholar]
- Altar C. A., O'Neil S., Walter R. J., Jr, Marshall J. F. Brain dopamine and serotonin receptor sites revealed by digital subtraction autoradiography. Science. 1985 May 3;228(4699):597–600. doi: 10.1126/science.2580352. [DOI] [PubMed] [Google Scholar]
- Altar C. A., Walter R. J., Jr, Neve K. A., Marshall J. F. Computer-assisted video analysis of [3H]spiroperidol binding autoradiographs. J Neurosci Methods. 1984 Mar;10(3):173–188. doi: 10.1016/0165-0270(84)90054-2. [DOI] [PubMed] [Google Scholar]
- Bernheimer H., Birkmayer W., Hornykiewicz O., Jellinger K., Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973 Dec;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bowers M. B., Jr Biochemical processes in schizophrenia: an update. Schizophr Bull. 1980;6(3):393–403. doi: 10.1093/schbul/6.3.393. [DOI] [PubMed] [Google Scholar]
- Donoghue J. P., Herkenham M. Neostriatal projections from individual cortical fields conform to histochemically distinct striatal compartments in the rat. Brain Res. 1986 Feb 19;365(2):397–403. doi: 10.1016/0006-8993(86)91658-6. [DOI] [PubMed] [Google Scholar]
- Geary W. A., 2nd, Toga A. W., Wooten G. F. Quantitative film autoradiography for tritium: methodological considerations. Brain Res. 1985 Jun 24;337(1):99–108. doi: 10.1016/0006-8993(85)91613-0. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985 Jun 22;236(4):454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984 Oct 4;311(5985):461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr, Moon Edley S. Compartments in the striatum of the cat observed by retrograde cell labeling. Exp Brain Res. 1979 Jan 2;34(1):189–195. doi: 10.1007/BF00238352. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Sokoloff L. Quantitative receptor autoradiography: tissue defatting eliminates differential self-absorption of tritium radiation in gray and white matter of brain. Brain Res. 1984 Nov 12;321(2):363–368. doi: 10.1016/0006-8993(84)90194-x. [DOI] [PubMed] [Google Scholar]
- Joyce J. N., Loeschen S. K., Marshall J. F. Dopamine D-2 receptors in rat caudate-putamen: the lateral to medial gradient does not correspond to dopaminergic innervation. Brain Res. 1985 Jul 15;338(2):209–218. doi: 10.1016/0006-8993(85)90149-0. [DOI] [PubMed] [Google Scholar]
- Joyce J. N., Marshall J. F. Striatal topography of D-2 receptors correlates with indexes of cholinergic neuron localization. Neurosci Lett. 1985 Jan 7;53(1):127–131. doi: 10.1016/0304-3940(85)90108-9. [DOI] [PubMed] [Google Scholar]
- Joyce J. N. Multiple dopamine receptors and behavior. Neurosci Biobehav Rev. 1983 Summer;7(2):227–256. doi: 10.1016/0149-7634(83)90017-9. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Luabeya M. K., Maloteaux J. M., Laduron P. M. Regional and cortical laminar distributions of serotonin S2, benzodiazepine, muscarinic, and dopamine D2 receptors in human brain. J Neurochem. 1984 Oct;43(4):1068–1071. doi: 10.1111/j.1471-4159.1984.tb12845.x. [DOI] [PubMed] [Google Scholar]
- Neve K. A., Altar C. A., Wong C. A., Marshall J. F. Quantitative analysis of [3H]spiroperidol binding to rat forebrain sections: plasticity of neostriatal dopamine receptors after nigrostriatal injury. Brain Res. 1984 Jun 4;302(1):9–18. doi: 10.1016/0006-8993(84)91280-0. [DOI] [PubMed] [Google Scholar]
- Palacios J. M., Niehoff D. L., Kuhar M. J. [3H]Spiperone binding sites in brain: autoradiographic localization of multiple receptors. Brain Res. 1981 Jun 1;213(2):277–289. doi: 10.1016/0006-8993(81)90234-1. [DOI] [PubMed] [Google Scholar]
- Richelson E., Nelson A. Antagonism by neuroleptics of neurotransmitter receptors of normal human brain in vitro. Eur J Pharmacol. 1984 Aug 17;103(3-4):197–204. doi: 10.1016/0014-2999(84)90478-3. [DOI] [PubMed] [Google Scholar]
- Ruberg M., Bokobza B., Javoy-Agid F., Montfort J. C., Agid Y. [3H]spiperone binding in the nigrostriatal system in human brain. Eur J Pharmacol. 1984 Mar 23;99(2-3):159–165. doi: 10.1016/0014-2999(84)90237-1. [DOI] [PubMed] [Google Scholar]
- Seeman P. Brain dopamine receptors. Pharmacol Rev. 1980 Sep;32(3):229–313. [PubMed] [Google Scholar]
- Severson J. A., Marcusson J., Winblad B., Finch C. E. Age-correlated loss of dopaminergic binding sites in human basal ganglia. J Neurochem. 1982 Dec;39(6):1623–1631. doi: 10.1111/j.1471-4159.1982.tb07996.x. [DOI] [PubMed] [Google Scholar]