Abstract
Different interoceptive systems must be integrated to ensure that multiple homeostatic insults evoke appropriate behavioral and physiological responses. Little is known about how this is achieved. Using C. elegans, we dissect cross-modulation between systems that monitor temperature, O2 and CO2. CO2 is less aversive to animals acclimated to 15°C than those grown at 22°C. This difference requires the AFD neurons, which respond to both temperature and CO2 changes. CO2 evokes distinct AFD Ca2+ responses in animals acclimated at 15°C or 22°C. Mutants defective in synaptic transmission can reprogram AFD CO2 responses according to temperature experience, suggesting reprogramming occurs cell autonomously. AFD is exquisitely sensitive to CO2. Surprisingly, gradients of 0.01% CO2/second evoke very different Ca2+ responses from gradients of 0.04% CO2/second. Ambient O2 provides further contextual modulation of CO2 avoidance. At 21% O2 tonic signalling from the O2-sensing neuron URX inhibits CO2 avoidance. This inhibition can be graded according to O2 levels. In a natural wild isolate, a switch from 21% to 19% O2 is sufficient to convert CO2 from a neutral to an aversive cue. This sharp tuning is conferred partly by the neuroglobin GLB-5. The modulatory effects of O2 on CO2 avoidance involve the RIA interneurons, which are post-synaptic to URX and exhibit CO2-evoked Ca2+ responses. Ambient O2 and acclimation temperature act combinatorially to modulate CO2 responsiveness. Our work highlights the integrated architecture of homeostatic responses in C. elegans.
Author Summary
Many animals are either attracted or repelled by carbon dioxide. We show that the way C. elegans responds to CO2 depends on the temperature it has acclimated to and the oxygen tensions it is experiencing. The effects of acclimation temperature are mediated by a temperature-sensing neuron called AFD that also responds to CO2. The responses evoked in AFD by a change in CO2 concentration are reprogrammed by acclimation temperature. This reprogramming does not appear to require synaptic input from other neurons. O2 modulates CO2 avoidance by setting the activity of the tonically signalling O2 sensor URX. A switch from 21% to 19% O2 is sufficient to convert CO2 from a neutral stimulus to an aversive one in a C. elegans wild strain. Modulation of CO2 responses by O2 cues requires the interneuron RIA which itself responds to changes in CO2 and is directly post-synaptic to URX. CO2 gradients are likely to be common in rotting fruit where Caenorhabditis live. Such gradients could be associated with food, pathogens, conspecifics or predators of C. elegans. The value of CO2 as a sensory cue thus depends crucially on context. This may explain the remarkable complexity of CO2 sensing in C. elegans.
Introduction
To maintain a constant internal milieu animals use internal sensory receptors to monitor cues such as CO2/pH [1], O2 [2], temperature [3], and osmolality [4]. These interoceptors counter changes in internal milieu by coordinating homeostatic responses that alter physiology and behavior [5]. Cross-talk between different interoceptive systems is likely to be important to ensure an integrated homeostatic response by the animal to multiple homeostatic insults. However, relatively little is known, at the molecular and circuitry levels, about how such cross-talk is encoded.
In vertebrates electrophysiological studies have identified cell populations and circuits that respond to homeostatic imbalance in O2, CO2/pH and temperature. The neurons comprising these circuits are only beginning to be resolved, and the molecular mechanisms controlling their responses are poorly understood. Nevertheless, studies in several animals suggest that cross-modulation of homeostatic responses is important for survival. In panting mammals, a rise in core body temperature elicits increased ventilation rate to help cooling, even though this causes temporary alkalosis of the blood due to excessive blowing off of CO2. This over-ride appears to be achieved by changing the set-point at which CO2 sensors inhibit ventilation when [CO2] decreases, but the mechanisms involved are unclear [6]. In the mouse, recent work has shown that suppressing the activity of serotonergic neurons impairs both respiratory and body temperature control, although whether the same or different sub-populations of neurons mediate these effects is unclear [7], [8]. In mammals, the drive to increase ventilation rate is stimulated more strongly when animals simultaneously experience a drop in O2 and a rise in CO2 [9].
In invertebrates, such as the free-living nematode C. elegans, behavioral mechanisms that counter homeostatic imbalance are particularly important, since the animal's buffering capacity is limited. C. elegans responds to variation in temperature, O2 and CO2 by mounting sophisticated behavioral responses. Exposure to temperatures above or below the range in which C. elegans can grow elicits strong avoidance responses [10]. When navigating thermal clines in which it can thrive, ∼15°C to 25°C, C. elegans migrates to the temperature at which it grew recently, as long as this was not associated with starvation [11], [12]. These responses require the animal to memorize its recent temperature experience and to change this memory when temperature or nutrient conditions change. A neural circuit that subserves these behaviors has been identified, and involves the thermosensory neurons AFD and AWC [13]–[16]. Temperature experience alters the thermosensing properties of AFD neurons: in animals acclimated to higher temperatures, the threshold at which a temperature rise evokes a Ca2+ response in AFD occurs at correspondingly higher temperatures [17], [18]. This plasticity allows animals to respond homeostatically to external temperature fluctuations, by seeking and remaining at temperatures they are acclimated to.
C. elegans also displays responses to variation in [O2], and avoids both high and low O2 [19]. Wild-caught C. elegans strongly avoids 21% O2, both on and off food, and burrow to escape from the surface [20]. This avoidance response is sculpted by O2-sensing neurons in the body cavity called AQR, PQR and URX [20], [21], [22]. When [O2] levels rise towards 21% the AQR, PQR and URX neurons become activated, by a mechanism involving the atypical soluble guanylate cyclases GCY-35/GCY-36. The tuning of the O2 response is sharpened by a neuroglobin expressed in AQR, PQR and URX neurons, called GLB-5, that suppresses neuronal activity when ambient [O2] falls just below 21% [20], [23]. The AQR, PQR and URX neurons are all tonic receptors: they show sustained signalling as long as [O2] is high [24]. This tonic activity stimulates sustained rapid movement until animals encounter a preferred lower [O2] environment.
C. elegans also avoids elevated CO2 [25], [26]. As in vertebrates, high [CO2] is harmful to C. elegans, reducing brood size and disrupting muscle structure [27]. An array of sensory neurons mediates CO2 avoidance behavior [28]. This network includes the temperature sensor AFD, the major gustatory neuron ASE, and the BAG neurons, which are also activated by decreasing O2 levels [22].
Here we investigate how the temperature and O2 sensing systems of C. elegans modulate the distributed circuit that mediates responses to CO2.
Results
Previous temperature experience sets CO2 avoidance in C. elegans
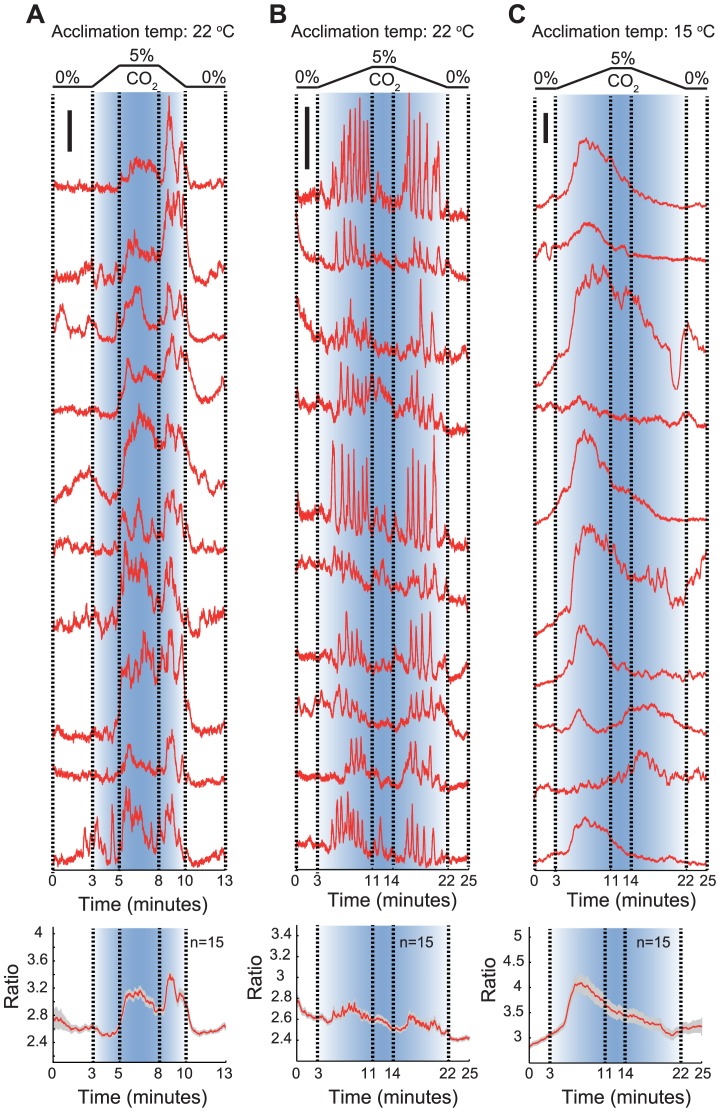
To examine if temperature can modify C. elegans' responses to CO2 we grew N2(Bristol) animals at 22°C and compared their behavior in CO2 gradients at 15°C and 22°C (Figure 1A, B) [25], [28]. CO2 avoidance at the two temperatures was similar when animals navigated 3%–0% and 5%–0% CO2 gradients. However, animals in a 1–0% CO2 gradient avoided the high CO2 half of the microfluidic device more strongly when assayed at 15°C compared to 22°C (Figure 1A).
Figure 1. CO2 avoidance is modulated by acclimation temperature.
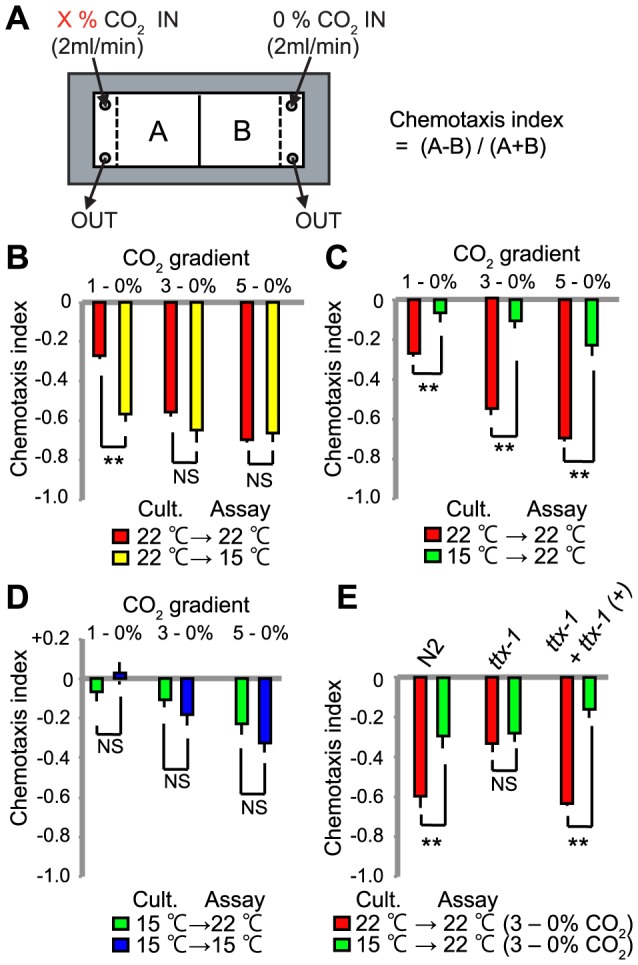
A. Assay for C. elegans CO2 responses. Animals navigate a defined CO2 gradient in a microfluidic device. The chemotaxis index is calculated by counting animals in two halves of the device, using the formula shown. B–D. Chemotaxis indices for animals cultivated at either 15°C or 22°C and assayed in different CO2 gradients at either 15°C or 22°C. **, p<0.01; n.s., not significant, Student's t-test. E. A mutation in ttx-1, which is specifically required to confer AFD neural identity, disrupts modulation of CO2 avoidance by acclimation temperature. Assays were performed in 3%–0% CO2 gradients. **, p<0.01; n.s., not significant, Student's t-test.
C. elegans can retune its temperature preference according to the temperature to which it is acclimated [13], [29]. This behavior is encoded in AFD [17], [18], a neuron that also responds to CO2 [28]. We therefore examined how previous temperature experience altered subsequent CO2 responses. We grew animals at 15°C or 22°C, and assayed their CO2 responses at each temperature. Strikingly, previous temperature experience altered CO2 avoidance. Animals grown at 15°C avoided CO2 less strongly than animals grown at 22°C, regardless of whether the assay temperature was 15°C or 22°C (Figure 1B–D). Animals grown at 15°C showed weaker CO2 avoidance even when exposed to relatively high CO2 levels, 5% (Figure 1B–D). Thus, the temperature to which C. elegans has acclimated helps determine the aversiveness of CO2.
Acclimation temperature does not reprogram CO2 responses in AFD-defective mutants
We investigated if the AFD neurons helped to reprogram CO2 avoidance behavior according to acclimation temperature. The ttx-1 (thermotaxis defective) gene encodes a member of the OTD/OTX subclass of homeodomain transcription factors [30]. Mutations in ttx-1 selectively disrupt AFD specification, and confer a thermotaxis-defective phenotype. Loss of ttx-1 also reduces CO2 avoidance in animals navigating CO2 spatial gradients [28]. If AFD neurons were important for temperature regulation of CO2 avoidance responses, then ttx-1 mutants would display similar CO2 avoidance regardless of cultivation temperature. As shown previously, ttx-1 mutants grown at 22°C only avoided CO2 weakly [28], resembling wild-type animals grown at 15°C (Figure 1E). This defect was rescued by a wild-type ttx-1 transgene (Figure 1E). By contrast, loss of ttx-1 did not alter the CO2-avoidance behavior of animals cultivated at 15°C. These data suggest AFD is required for acclimation temperature to modify CO2 aversive responses.
Acclimation temperature re-programs the CO2 responsiveness of AFD
Acclimation temperature sets the response threshold of AFD neurons to warming [17]. This prompted us to investigate whether acclimation temperature also alters the CO2 responsiveness of AFD. To measure CO2-evoked Ca2+ responses in AFD we expressed the genetically encoded Ca2+ sensor cameleon YC3.60 [31] from the gcy-8 promoter [32]. For our recordings we used animals acclimated to 15°C or 22°C, but maintained animals at 22°C while we imaged them. In animals acclimated to 22°C high CO2 evoked in AFD the complex Ca2+ response described previously (Figure 2A) [28]. This typically consisted of an initial slight drop in Ca2+ when CO2 levels rose, followed by a rise in Ca2+ to above pre-stimulus levels, and finally, when the CO2 stimulus was removed, a Ca2+ spike that rapidly decayed back to baseline. By contrast, animals acclimated to 15°C exhibited a simple response: a rise in Ca2+ when CO2 levels rose, and a fall when CO2 was removed (Figure 2B). These data suggest that the previous temperature experience of C. elegans reconfigures the CO2 response properties of AFD neurons.
Figure 2. Acclimation temperature alters CO2-evoked Ca2+ responses in AFD neurons.
In animals cultivated at 22°C a rise and fall in CO2 evokes a complex Ca2+ response in AFD neurons (A). Ca2+ initially falls when CO2 begins to increase, then rises. When CO2 levels fall, there is a Ca2+ spike. By contrast, animals cultivated at 15°C show a simple response to the same stimulus (B). C–D The effect of acclimation temperature on CO2-evoked Ca2+ responses in AFD neurons is unaltered in snb-1 mutants defective in synaptobrevin. E. Ca2+ responses evoked in AFD by a 0%–1%–3%–5%–3%–1%–0% CO2 stimulus train in animals acclimated to 22°C. Shading highlights switch times. Acclimation temperature is shown for each panel under genotype.
To investigate if this retuning was driven by the intrinsic temperature-sensing properties of AFD neurons, or required pre-synaptic input, we imaged the Ca2+ responses of AFD neurons to CO2 in snb-1 (synaptobrevin-1) mutants, which are defective in synaptic transmission [33]. CO2–evoked responses in AFD neurons were not altered in snb-1 animals compared to wild type, regardless of acclimation temperature (Figure 2C, D). These data suggest that the temperature experience can retune the CO2 response properties of AFD neurons when synaptic signalling is defective.
We characterized the response properties of the AFD neurons further. Previously, we had only exposed animals to sharp changes in CO2 that occurred within 1–2 s, and we always returned animals to 0% CO2 between stimuli [29]. To examine AFD responses to rises in CO2 from non-zero levels, we subjected animals acclimated to 22°C to a stimulus train involving multiple CO2 switches, namely 0%–1%–3%–5%–3%–1%–0%. Whenever CO2 levels increased, we observed an initial drop in Ca2+ followed by a rise in Ca2+ (Figure 2E). Whenever CO2 levels decreased, we observed a spike of Ca2+ that rapidly returned to baseline. This pattern of CO2 evoked Ca2+ response suggests that AFD can encode whether an animal is moving towards higher or lower CO2.
Previous work has identified one potential molecular sensor for CO2, the transmembrane guanylate cyclase gcy-9 [34]. We compared CO2-evoked responses in AFD neurons in wild type and gcy-9 mutants. We observed no difference in the response, suggesting that molecules other than GCY-9 confer CO2-responsiveness to AFD neurons (Figure S1).
AFD responses to CO2 are reconfigured by the steepness of the CO2 gradient
The ubiquity of CO2 suggests that its value as a cue is likely to depend not only on context (such as temperature) but also on the shape of the CO2 stimulus. Very rapid change in CO2 levels may convey a different meaning from a very gradual change. In our behavioral experiments, animals navigated shallow CO2 gradients and encountered changes in the order of 0.01% CO2 per second (depending on speed and direction of travel in the gradient). To examine if AFD could respond to such shallow CO2 gradients, we exposed animals cultivated at 22°C to gradual linear increases and decreases in CO2 concentration at rates of 0.04% and 0.01% per second (Figure 3A,B). AFD responded to both these CO2 gradients, but with very different response patterns. Gradients of 0.04% CO2/second evoked AFD Ca2+ responses reminiscent of those elicited by sharp changes in CO2 (>1% CO2/second; see Figure 2): Ca2+ levels decreased while CO2 was slowly rising to 5%, then rose sharply as CO2 levels stabilized at 5%. When we gradually reduced CO2 levels back to 0%, Ca2+ levels spiked, returning to baseline when animals were in 0% CO2 (Figure 3A). By contrast, gradients of 0.01% CO2/second evoked a series of Ca2+ spikes while CO2 levels were rising (Figure 3B). Ca2+ levels tended to return to baseline when CO2 levels stopped rising, but spiking resumed when CO2 levels started falling. This spiking pattern disappeared when we imaged Ca2+ responses evoked by the same 0.01% CO2/second gradient in animals acclimated to 15°C (Figure 3C). In these animals responses were more similar to those evoked by steeper CO2 gradients in animals acclimated to 15°C (compare Figure 3C to Figure 2B). These data indicate that AFD neurons respond to both rapid and slow changes in CO2, but with different response patterns. The also highlight complexity in how AFD encodes CO2 stimuli.
Figure 3. Shallow and steep CO2 gradients evoke qualitatively different Ca2+ responses in AFD.
A. Ca2+ responses evoked in AFD by CO2 switches indicated at top, involving linear 0–5% and 5%–0% CO2 gradients occurring over 2 minutes. This corresponds to a rate of change of 0.04% CO2/second. The upper part of the panel shows traces obtained from 10 randomly selected individual AFD neurons; an average trace is plotted at the bottom. Animals imaged in this panel were acclimated to 22°C. B, C. Ca2+ responses evoked in AFD by CO2 switches indicated at top, involving linear switches from 0–5% and 5%–0% CO2 occurring over 8 minutes. This corresponds to a change of 0.01% CO2/second. The upper part of the panels shows traces obtained from 10 randomly selected individual AFD neurons; average traces are plotted at the bottom. Animals imaged in (B) were acclimated to 22°C; those in (C) were acclimated at 15°C. For each panel, individual and average traces are at the same scale. The scale bar in each panel represents 0.4 YFP/CFP ratio unit.
Ambient O2 levels regulate C. elegans CO2 avoidance behaviour
To investigate further how different homeostatic responses are integrated, we examined if CO2 avoidance behavior was modulated by different background ambient [O2]. In body fluids and many ecological niches low [O2] coincides with high [CO2], and, conversely, 21% O2 is associated with low CO2. Cross-talk between the two gas sensing circuits could enable C. elegans to recognize and respond appropriately to such environments.
To examine this possibility, we placed N2 animals in microfluidic chambers containing gradients of CO2 at different fixed concentrations of O2. As expected, increasing [CO2] elicited increasing avoidance behavior: C. elegans avoided 5% CO2 more strongly than 3% or 1% CO2 (Figure 4A) [25], [26]. Moreover, CO2 avoidance was influenced by the background ambient O2 concentration. N2 animals navigated down CO2 gradients more strongly when ambient O2 concentration was 11%, than when it was 21%. Increased avoidance was particularly striking when animals navigated shallow gradients of 1–0% CO2 (Figure 4A). Such shallow CO2 gradients are likely to be ecologically relevant in the rotting habitats where C. elegans thrives.
Figure 4. Ambient O2 levels set CO2 avoidance.

A. C. elegans avoids shallow gradients of CO2 more strongly when O2 levels are low. The CO2 gradients used are indicated above the graph. B. Artificially high O2 levels can reduce CO2 avoidance further. **, p<0.01; *, p<0.05, Student's t-test.
To test the dynamic range of O2 regulation, we asked if increasing [O2] to above 21% could further suppress CO2 avoidance. Although this is unphysiological, previous studies have shown that C. elegans can grow and reproduce in even 100% O2 without any apparent adverse effects [35]. Since C. elegans only weakly avoided 1% CO2 in 21% O2, we used a steeper 3–0% CO2 gradient, to improve our dynamic range. Increasing ambient [O2] to 50% significantly suppressed avoidance of 3% CO2 (Figure 4B). These data suggest that ambient O2 concentration provides a contextual cue to modulate C. elegans avoidance of CO2.
Tonically signalling O2 sensors inhibit CO2 avoidance at high ambient [O2]
Our results suggested that O2-sensing neurons or neuroendocrine cells persistently signal O2 concentration to modify the activity of CO2 transducing circuits. Previous studies have shown that the AQR, PQR and URX O2 sensors signal tonically when ambient [O2] is close to 21%, and become progressively less active as [O2] falls [24]. The O2–evoked Ca2+ responses of these neurons requires the atypical soluble guanylyl cyclases GCY-35 and GCY-36, which appear to be O2 sensors [20], [22], [36]. In gcy-35 or gcy-36 loss-of-function mutants the Ca2+ levels in the O2 sensing neurons reported by cameleon YC3.60, are low, resembling those found in wild type animals kept at low [O2] [24]. To test if tonic signalling by AQR, PQR and URX neurons persistently repressed CO2 avoidance in high [O2], we compared the CO2 avoidance of wild type, gcy-35, and gcy-36 mutants at 21% and 11% O2. In 11% O2 gcy-35 and gcy-36 mutants avoided CO2 like N2 controls. However, whereas increasing background O2 levels to 21% inhibited the CO2 avoidance behavior of wild type animals, it had no effect on gcy-35 or gcy-36 mutant animals (Figure 5A). These data suggest that tonic signalling from one or more of the AQR, PQR and URX O2 sensors represses CO2 avoidance at high O2 concentrations.
Figure 5. Disrupting gcy-35 or gcy-36 confers strong CO2 avoidance regardless of ambient O2.

A. gcy-35 or gcy-36 mutants strongly avoid the high CO2 half of a 1–0% CO2 gradient regardless of ambient O2. Statistics refer to comparisons to N2 at 21% O2. ***, p<0.001, Anova, Bonferroni corrected p-value. None of the strains apart from N2 show significant differences between assays carried out at 21% and 11% O2 (Student's t-test). B. The CO2-avoidance phenotype of gcy-36 mutants can be rescued by expressing gcy-36 cDNA in AQR, PQR and URX, using gcy-32 or gcy-36 promoters, or in URX alone, using the flp-8 promoter. **, p<0.01, ***, p<0.001, Anova, Bonferroni corrected p-value.
To confirm our results, we rescued the gcy-36 mutant phenotype using cell-specific promoters. Expressing gcy-36 cDNA from its own upstream sequence, which drives expression in AQR, PQR and URX, restored to gcy-36 mutants reduced CO2 avoidance at 21% O2 (Figure 5B). gcy-36 mutants expressing gcy-36 cDNA from the gcy-32 promoter, which also drives expression in AQR, PQR and URX, gave similar rescue (Figure 5B). Expressing gcy-36 cDNA from the flp-8 promoter, which drives expression in URX (and AUA and PVM) neurons but not in AQR and PQR also rescued the O2-regulated CO2 avoidance phenotype of gcy-36 mutants. These results suggest that tonic signalling by the URX O2-sensing neuron can persistently suppress CO2 avoidance while O2 levels are high.
To extend our results we also examined the consequence of deleting gcy-32 and gcy-34, atypical soluble guanylate cyclases expressed in AQR, PQR and URX neurons whose activities are also likely to be modulated by O2, but whose deletion only subtly alters O2-evoked behaviors. We observed no effects of these deletions on O2 regulation of CO2 avoidance (Figure S2). We did however observe a slight decrease in CO2 avoidance at 11% O2 in mutants defective in gcy-33, an atypical soluble guanylate cyclase required for the BAG sensory neurons to respond to decreases in O2 levels (Figure S2) [22]. BAG is also a major CO2 sensor [28] [34].
The npr-1 and glb-5 genes modulate CO2 avoidance by O2
O2 responses in the standard laboratory N2 strain differ from those of aggregating wild C. elegans, due to genetic differences that have evolved during domestication [19], [20], [23], [36], [37]. N2 animals harbor a gain-of-function allele of the npr-1 neuropeptide receptor that inhibits signalling output from O2-sensing circuits in feeding animals. N2 animals also carry a loss-of-function mutation in the neuroglobin glb-5 that increases the excitability of the AQR, PQR and URX O2 sensors.
We investigated if variation at npr-1 and glb-5 altered O2 modulation of CO2 avoidance. In N2 animals, stepwise increases in O2 from 11% to 21% caused stepwise decreases in CO2 avoidance (Figure 6A). Animals defective in both the npr-1 receptor and the glb-5 neuroglobin (i.e. npr-1 mutants) were attracted to CO2 at 21% O2, but became progressively more repelled by CO2 as O2 concentrations fell. A functional glb-5(Hawaii) allele made CO2 more aversive to npr-1 defective animals: decreasing [O2] still stimulated CO2 avoidance, but at each concentration tested glb-5; npr-1 animals avoided CO2 more strongly than npr-1 animals (Figure 6A). Adding the functional glb-5(Hawaii) allele to N2 animals bearing the npr-1 gain-of-function allele did not significantly change their CO2 avoidance behaviour at any O2 tensions. Thus, variation at the glb-5 and npr-1 genes, which alter O2 sensing circuits, changes the extent to which O2 levels modifies CO2 aversiveness.
Figure 6. Re-configuring O2 sensing circuits by altering the npr-1 and glb-5 genes alters CO2 avoidance behavior.
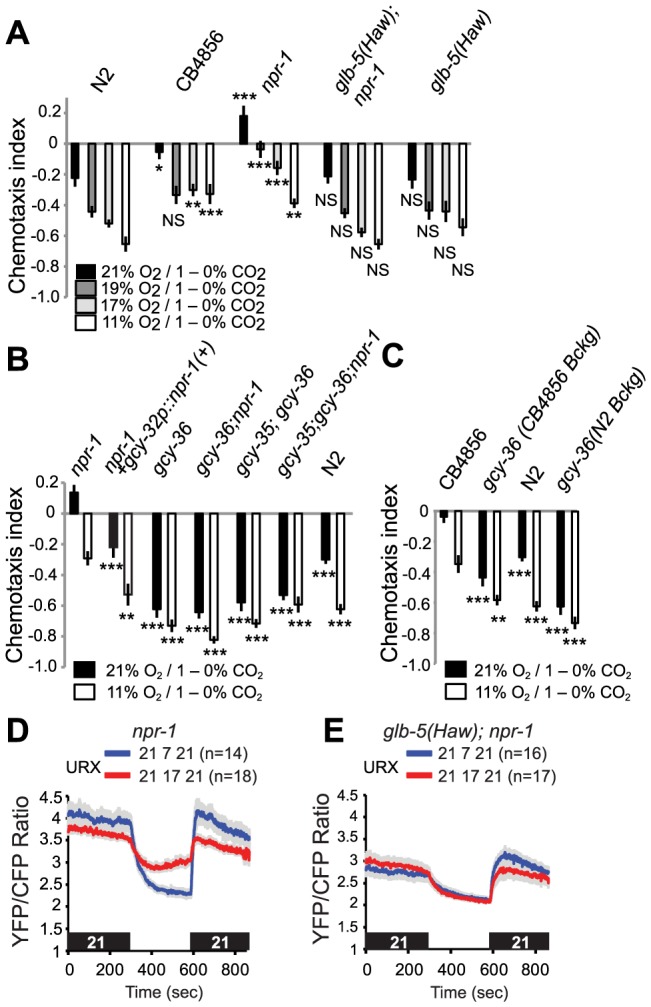
A. Tuning of CO2 avoidance behavior by different O2 concentrations in N2 (Bristol), CB4856 (Hawaiian), npr-1(ad609), glb-5(Haw); npr-1(ad609), and glb-5(Haw) animals. All assays used a 1–0% CO2 gradient. Statistical comparisons are to the N2 response at the corresponding O2 concentration, *** p<0.001; ** p<0.01; *p<0.05 (Anova, p value protected Fisher's LSD). B. gcy-35 and gcy-36 mutants strongly avoid CO2 regardless of genotype at the npr-1 locus. Statistical comparisons are to the npr-1 response at the corresponding O2 concentration. ***, p<0.001, **, p<0.01, Anova, Bonferroni corrected p value). C. CB4856 (Hawaii) animals defective in gcy-36 strongly avoid CO2 regardless of O2 levels. Statistical comparisons are to the CB4856 response at the corresponding O2 concentration. ***, p<0.001, **, p<0.01, Anova, Bonferroni corrected p value). D, E. Tonic Ca2+ levels in URX neurons of glb-5(Haw); npr-1 animals kept at 21% O2 and 17% O2 is lower than Ca2+ levels in URX in npr-1 animals kept at the corresponding O2 concentrations. Ca2+ measurements were made using cameleon YC2.60.
To investigate how O2 modified CO2 avoidance in a non-domesticated C. elegans strain, we examined the responses of animals from the Hawaiian CB4856 isolate. As reported previously [23], [25], [26], the Hawaiian strain showed weaker CO2 avoidance than N2 at 21% O2. Reducing O2 levels to 19% was sufficient to strongly stimulate CO2 avoidance in Hawaiian animals, and further decreases in [O2] had no significant effects (Figure 6A, C). Together, these data suggest that the Hawaiian animals do not avoid CO2 when O2 is at 21%, i.e. when animals are at the surface, and but that very small decreases in O2 are sufficient to increase CO2-avoidance behavior. The sharp tuning of CB4856 responses to CO2 by O2 levels appears to involve the natural alleles of npr-1, npr-1 215F, the glb-5(Haw) alleles.
To shed further light on the genetic control of this cross-talk of CO2 and O2 responses, we examined how knocking out the soluble guanylate cyclases gcy-35 and gcy-36 altered CO2 responses in different genetic backgrounds. Knocking out either soluble guanylate cyclase strongly stimulated CO2 avoidance in npr-1 animals: the avoidance behaviour of gcy-35; npr-1 or gcy-36; npr-1 animals resembled that of gcy-35 or gcy-36 mutants, and of N2 animals at 11% O2 (Figure 6B). We also examined the effect of disrupting gcy-36 in the Hawaiian genetic background (Figure 6C). CB4856 animals defective in gcy-36 avoided CO2 much more strongly than CB4856 controls, and changing ambient O2 had little effect on their CO2 responses (Figure 6C). Thus, the modulation we describe in domesticated N2 also occurs in wild aggregating C. elegans. Expressing cDNA encoding the npr-1 215V allele found in N2 animals in the AQR, PQR and URX neurons, using the gcy-32 promoter, restored N2-like behaviour to npr-1 mutants (Figure 6B). Thus, npr-1 acts in the O2-sensing neurons themselves to counter the inhibitory effect of high O2 on CO2 avoidance.
To provide a neural explanation for why npr-1 animals avoided CO2 less than glb-5(Haw); npr-1 animals at 17%, 19% and 21% O2 (Figure 6A, p<0.0001, Anova, Bonferroni-corrected p value at all three O2 values), we compared tonic Ca2+ signalling in URX at different O2 concentrations. While URX Ca2+ levels were similar in npr-1 and glb-5; npr-1 animals at 7% O2, Ca2+ was higher in npr-1 than in glb-5; npr-1 animals at 21% and 17% O2, consistent with greater inhibition of CO2 avoidance by URX signalling at these O2 concentrations (Figure 6D, E).
O2 can modulate CO2 avoidance in animals defective in AFD and BAG CO2 sensors
CO2 avoidance in C. elegans is mediated by a distributed set of sensory neurons that includes the BAG O2 sensor, the AFD temperature sensor, and the ASE gustatory neuron [28], [34]. To examine if O2 levels modified CO2-evoked Ca2+ responses in any of these neurons we imaged their responses at 11% and 21% O2 concentrations using the YC3.60 sensor (Figure S3A–C). We did not observe any differences between CO2-evoked responses at the two O2 concentrations in any of the three neurons under our imaging conditions. This suggests either that O2 modulation occurs downstream of these sensory neurons, or that our imaging conditions limit our ability to observe modulation by O2.
O2 input could selectively modulate the CO2 responses mediated by one CO2-sensing neuron, or it could modulate circuits involving multiple CO2 sensors. To examine these possibilities, we specifically disrupted AFD and/or BAG function in N2 animals, and measured CO2 avoidance at 21% and 11% O2. Genetically abating BAG neurons or disrupting AFD specification by mutating the ttx-1 transcription factor, or doing both, reduced CO2 avoidance at 11% O2, but did not abolish modulation by ambient O2 levels (Figure 7). These data suggest that O2 levels either modulate the output from several CO2 sensors, or exert their effects on unidentified CO2 sensors, or both.
Figure 7. Ambient O2 can modulate CO2 avoidance in animals lacking BAG and AFD CO2 sensors.

Animals in which BAG neurons are ablated by specific expression of egl-1 caspase, and AFD neurons are defective due to loss of ttx-1, retain O2-modulation of CO2 avoidance. egl-1 expression in BAG neurons is driven by the flp-17 promoter. ∧∧∧, p<0.001, Student's t test, comparing a strain's responses at 21% and 11% O2. *** p<0.001; ** p<0.01; * p<0.05, Anova, Bonferroni corrected p value, comparing responses to that of N2 at the same O2 concentration.
RIA interneurons are part of the circuit mediating O2-modulated CO2 avoidance
To dissect further how O2-sensing neurons modulated CO2 responses, we sought mutations that disrupted O2 modulation without abrogating CO2 responsiveness. One such mutation we identified was ttx-7, which disrupts a myo-inositol-1-monophosphatase [38]. ttx-7 mutants showed only mild defects in CO2 avoidance when assayed at 21% O2 (Figure 8A–C). The chemotaxis index of ttx-7 mutants was not significantly different from that of N2 controls when animals were assayed in 1–0% and 5–0% CO2 gradients; we only observed a small but significant decrease in CO2 avoidance when ttx-7 mutants were assayed in 3–0% CO2 gradients. However, ttx-7 mutant animals did not increase their CO2 avoidance when assayed at 11% O2, regardless of the CO2 gradient we used (Figure 8A–C). ttx-7 mutants behaved indistinguishably from N2 animals when assayed in O2 gradients (Figure S4), suggesting they were not generally defective in O2-evoked responses.
Figure 8. TTX-7 acts in RIA interneurons to promote CO2 avoidance when ambient O2 levels are low.
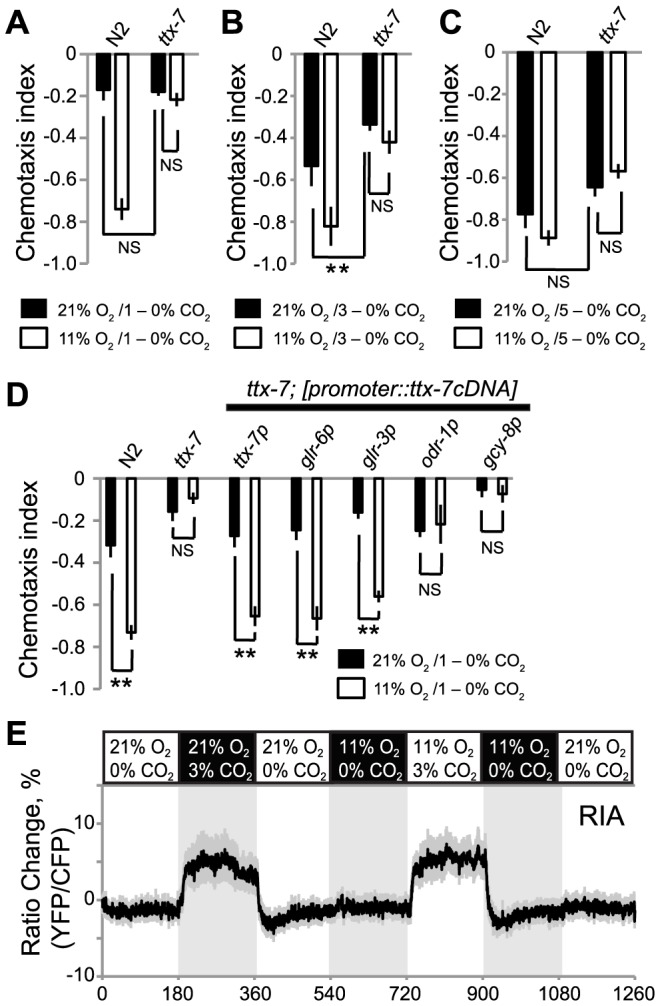
A–C. Mutations in ttx-7 strongly reduce CO2 avoidance at 11% O2 but have relatively weak effects on CO2 avoidance at 21% O2. ns, not significant, ** p<0.01, Student's t test. D. Expressing ttx-7 specifically in RIA neurons, using the glr-3 or glr-6 promoters, restores strong CO2 avoidance to ttx-7 mutants assayed at 11% O2. Expressing ttx-7 specifically in AFD, using the gcy-8 promoter, or in AWB and AWC, using the odr-1 promoter does not rescue the ttx-7 phenotype. ns, not significant, ** p<0.01, Student's t test. E. CO2 evokes a Ca2+ response in RIA neurons. Ca2+ responses were measured in immobilized animals cultivated at 22°C using a pglr-6::YC3.60 Ca2+ reporter. Shading highlights gas switch times. The CO2/O2 stimulus train used is indicated above the plot.
To confirm that the defect in O2-dependent modulation of CO2 avoidance was due to the ttx-7 mutation, we showed we could restore strong CO2 avoidance at 11% O2 to ttx-7 mutants by expressing ttx-7 cDNA from the ttx-7 promoter (Figure 8D). Together, these data suggest that ttx-7 mutants can sense and respond to O2 but cannot communicate information about ambient [O2] to the appropriate circuits that mediate CO2 responses.
To identify neurons where ttx-7 acts to promote CO2 avoidance at low [O2] we rescued the ttx-7 CO2 avoidance phenotype by driving ttx-7 cDNA in small subsets of neurons. We focussed on neurons that receive synaptic input from the URX O2 sensors, since our gcy-36 rescue experiments implied that URX was sufficient for O2 to modulate CO2 avoidance (Figure 5B). URX neurons make several synapses onto the RIA interneurons [39]. In turn, RIA neurons receive direct or indirect inputs from many sensory neurons, and are connected to numerous downstream interneurons, making them good candidates for transmitting information about ambient O2 to CO2 circuits. Previous work has shown that ttx-7 is required in the RIA neurons to promote appropriate synapse formation and to enable C. elegans to navigate temperature gradients [38]. Expressing ttx-7 cDNA from the glr-3 or glr-6 promoters, which drive expression exclusively in RIA [40], restored strong CO2 avoidance at 11% O2 (Figure 8D). By contrast, ttx-7 expression in AFD, using the gcy-8 promoter, or in AWC and AWB olfactory neurons, using the odr-1 promoter, did not. These data suggest that RIA interneurons are involved in communicating information from O2-sensing neurons and/or CO2-responsive circuits, to enable its integration.
We examined if CO2 elicited a Ca2+ response in RIA interneurons, and if this response was modulated by O2 context. We exposed animals expressing cameleon YC3.60 in RIA to a stimulus train in which we sequentially altered O2 and CO2 levels, and measured fluorescence changes in the cell body. 3% CO2 evoked a Ca2+ response in RIA neurons that was not significantly altered by background O2 (Figure 8E). These data suggest that RIA interneurons form part of a CO2 responsive circuit. Our inability to detect modulation of CO2-evoked Ca2+ responses in RIA by O2 levels could reflect a limitation of our imaging conditions. Alternatively, O2 could regulate RIA independently of Ca2+ entry, or could act on neurons downstream of RIA.
Acclimation temperature and ambient O2 act combinatorially to regulate CO2 responsiveness
Both acclimation temperature and acute ambient O2 concentrations altered C. elegans' responsiveness to CO2. We investigated how animals integrated information from all three homeostatic systems – temperature, O2 and CO2. We grew animals at either 15°C or 22°C, and then assayed CO2 responses at 15°C or 22°C in the presence of either 21% or 11% O2. Our results suggest that the temperature and O2 sensing systems act additively to set CO2 responsiveness. Decreasing O2 from 21% to 11% enhanced avoidance of 1% CO2 regardless of acclimation temperature and assay temperature (Figure 9A–C). Similarly, acclimating animals to 15°C decreased avoidance of 1% CO2 at both 21% and 11% O2 (Figure 9A–C). As described previously (Figure 1A), animals acclimated to 22°C avoided a 1%–0% CO2 gradient more strongly when assayed at 15°C rather than 22°C. Changing O2 from 21% to 11% further stimulated CO2 avoidance in these animals. These data highlight how C. elegans homeostatic responses are intertwined with each other.
Figure 9. Acclimation temperature and ambient O2 levels have additive effects on CO2 avoidance.
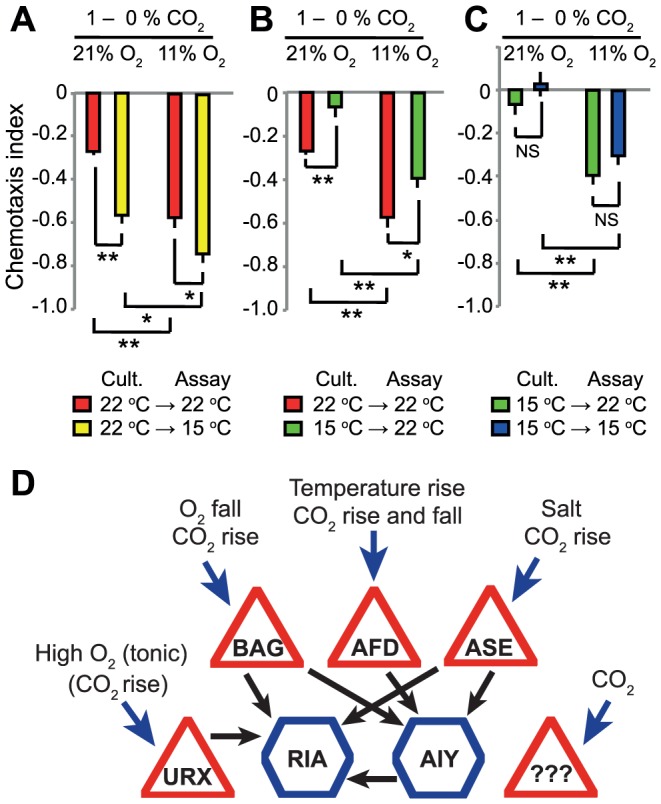
A. Animals cultivated at 22°C but assayed at 15°C avoid CO2 more strongly when ambient O2 is low. B–C. Reducing O2 levels from 21% to 11% increases CO2 avoidance regardless of acclimation temperature or assay temperature. In A–C, ** p<0.01, * p<0.05, ns, not significant, Student's t test. D. Coalitions of CO2 sensors elicit CO2 escape responses according to O2 environment, temperature experience, and CO2 stimulus dynamics. Triangles represent sensory neurons and hexagons interneurons. Black arrows indicate synapses. Several neurons respond to CO2 (blue arrows), each with distinct kinetics. Each of these neurons also responds to other sensory cues, as indicated. Three of the four identified CO2 sensors synapse directly onto the RIA interneuron. The fourth, AFD, synapses onto AIY which in turn synapses on RIA. The URX O2 sensor also synapses onto RIA. Note each neuron makes additional connections besides the ones highlighted here.
Discussion
Previous acclimation temperature and current ambient O2 levels set the aversiveness of CO2 to C. elegans. The temperature animals have experienced previously appears to modify CO2 responsiveness by changing the CO2 receptive properties of AFD. Acute ambient O2 controls CO2 preference by regulating tonic signaling from the O2 sensing neuron URX. Changes in CO2 responsiveness can be observed in shallow gradients with peak CO2 levels of 1%. Such gradients are likely to be ecologically relevant for C. elegans in the rotting fruit habitats where they are commonly found [41].
C. elegans can thrive at temperatures that span ∼15°C–25°C. Within this range, well-fed animals migrate to temperatures at which they were previously growing [13], [29]. Temperature preference appears to be encoded in the AFD neurons: acclimation temperature changes the threshold at which rising temperature evokes Ca2+ responses in this neuron [17], [18]. We find that AFD neurons are required for temperature experience to change C. elegans' CO2 responsiveness. Acclimation temperature qualitatively reconfigures CO2-evoked Ca2+ responses of AFD neurons. This re-configuration is retained in mutants defective in synaptic release, suggesting it can occur cell-autonomously. A speculative explanation of our observations is that AFD harbors multiple CO2 sensors whose contribution to the CO2-evoked Ca2+ response varies according to acclimation temperature.
AFD neurons are exquisitely sensitive to CO2. They respond robustly to changes in CO2 that range from <0.01% CO2/sec to >1% CO2/sec. Remarkably, in animals acclimated to 22°C, the Ca2+ responses evoked in AFD by slow (0.01% CO2/second) and faster (0.04% CO2/second) changes in CO2 are qualitatively different. This may explain previous observations that AFD promotes CO2 avoidance in shallow CO2 gradients, but can inhibit CO2 avoidance in steep ones [28].
C. elegans avoid CO2 less strongly at high O2 than at low O2. Ambient O2 levels provide a contextual cue that modulates the aversiveness of CO2. We use the term ‘contextual’ because modulation can occur when O2 levels are constant, and is sustained over many minutes. Contextual modulation by O2 levels can be graded: as O2 decreases from 21% to 11%, CO2 avoidance rises. Modulation of CO2 avoidance by O2 requires the gcy-35 and gcy-36 soluble guanylate cyclases, which act in the O2 sensing neurons AQR, PQR and URX to transduce O2 levels. gcy-35 or gcy-36 mutants behave like animals kept at low O2, regardless of actual O2 levels. The activity of the URX neurons alone appears sufficient to inhibit CO2 avoidance at 21% O2. Previous work has shown that URX neurons are tonically activated by high O2 [24], explaining the ability of these neurons to convey O2 context persistently to CO2 sensing circuits.
Modulation of CO2 avoidance by O2 levels can be observed when N2 (Bristol), npr-1, glb-5(Haw); npr-1, or CB4856 (Haw) animals navigate 1%–0% CO2 gradients. However, the degree of inhibition varies across these genotypes. In N2 animals, the inhibitory effect of O2 is limited by the action of the NPR-1 215V isoform in O2-sensing neurons. npr-1 215V does not appear to alter the excitability of O2 sensors, since N2 and npr-1 mutants show similar O2-evoked Ca2+ responses in URX, AQR or PQR ([22] and data not shown). Instead, we speculate that NPR-1 215V inhibits neurotransmission from URX, for example through Go signaling [42], [43], thus limiting the ability of URX to inhibit CO2 responsiveness. Previous work has highlighted coupling of NPR-1 215V to Go in heterologous systems [44]. The potent O2-dependent inhibition of CO2 avoidance found in npr-1 mutants is suppressed by the glb-5(Haw) allele. This suppression appears to reflect a reduction in the excitability of URX. Tonic Ca2+ levels in URX in glb-5; npr-1 animals kept at 21% O2 was only as high as that found in npr-1 animals at 17% O2. In the CB4856 (Haw) strain the combination of the npr-1 215F and glb-5(Haw) alleles (potentially modified by other loci) enables a switch from 21% to 19% O2 to convert CO2 from a neutral to a strongly aversive stimulus. While this paper was in preparation independent work also highlighted modulation of CO2 avoidance by O2 in npr-1 animals [45]. The assays used are different. Notably, in most of our work we used 1–0% CO2 gradients, whereas Carrillo et al. used 10%–0% gradients.
CO2 sensing in C. elegans is distributed across multiple sensory neurons, including the AFD and BAG neurons [28] (Figure 9D). Disrupting AFD and BAG abolishes CO2 avoidance at 21% O2, but CO2 avoidance at 11% O2 is only partly reduced. Thus, CO2 sensing neurons other than BAG and AFD can promote CO2 avoidance at low O2. O2 modulation of CO2 responsiveness involves the RIA interneurons. ttx-7 mutants disrupt O2 modulation of CO2 responsiveness, and expressing ttx-7 cDNA selectively in RIA neurons rescues this phenotype. ttx-7 encodes myo-inositol monophosphatase. In ttx-7 mutants RIA neurons exhibit defects in localization of both pre- and post-synaptic components, including synaptobrevin, SYD-2 Liprin, and the glutamate receptor GLR-1 [38]. Synaptic communication via RIA is thus likely to be compromised in ttx-7 mutants, and may explain the O2/CO2 integration phenotype.
Previous studies of context-dependent changes in behavior in C. elegans have focused mainly on the effects of food or of food deprivation. C. elegans' migration in salt and odor gradients can switch from attraction to repulsion if animals are deprived of food in the presence of the chemical cue [46]–[49]. Food and food deprivation have also been shown to modulate C. elegans response to temperature gradients [50]. It remains to be seen if acclimation temperature and ambient O2 levels have effects on other sensory modalities besides CO2 sensing. Whether CO2 itself can act as a contextual cue regulating other C. elegans sensory responses, including thermotaxis and O2 sensing, is also unknown.
The shallow CO2 gradients we study are likely to be common in the rotting fruit environments where C. elegans is frequently found. However, the ubiquitous production of CO2 by aerobically respiring organisms means its value as a sensory cue likely depends crucially on context. Bacterial food, bacterial pathogens, predators, mates and conspecifics may all generate CO2 gradients. Context-dependence of CO2 responses has been observed previously. C. elegans CO2 responses are modulated by food, exposure to hypoxia, and starvation [25]. Moreover, not only context, but also the rate of change in CO2 concentration (whether it is slow or rapid), appears to modify the contribution of different CO2-sensing neurons to C. elegans CO2 avoidance behaviors [28]. This complexity is mirrored in insects. For example in Drosophila airborne CO2 is aversive [51], whereas dissolved CO2 is attractive [52]. These properties are encoded by separate chemosensory neurons in the antenna (avoidance of gaseous CO2) and taste peg neurons (attraction to carbonation). Avoidance of airborne CO2 is inhibited by olfactory odors, presumably to enable flies to approach fermenting fruit [53]. Together, these data suggest CO2 sensing is remarkably sophisticated in both worms and flies. CO2 has been implicated in ageing in Drosophila [54], whereas O2-sensing neurons modulate longevity in Caenorhabditis [55], consistent with neurons sensing these gases also modulating physiology.
Materials and Methods
Strains
Strains were maintained at 22°C with plentiful food using standard methods [56]. Strains used in this work are listed in Supplementary methods.
Behavioral assays and analysis
Spatial carbon dioxide gradient assays were performed as described, with slight modifications [25], [28]. Briefly, rectangular PDMS chambers with a 33×15×0.2 mm space connected to gas syringes were placed over 100–200 worms on a 9 cm NGM agar plate. Assays ran for 20 minutes and the distribution of worms recorded by counting the number of animals in each of nine equal area divisions as well as in the two spaces at either end of the chamber. Animals were washed three times in a watch glass then transferred to the agar. A chemotaxis index was calculated by subtracting the number of animals in the low carbon dioxide half of the chamber from the number in the high carbon dioxide half and dividing by the total number of animals e.g. (A−B)/(A+B), as shown in Figure 1A. In chemotaxis assays, each data point represents the average of at least eight independent assays performed over three experimental days. Certified gases with indicated concentrations of O2 and CO2 were obtained from BOC UK Ltd. Assays marked 22°C were carried out at room temperature in a room in which temperature varied 22+/−1°C. Assays marked 15°C were carried out in a small thermostat-controlled room set to 15°C.
Statistical comparisons were carried out using the Student's t test or ANOVA, as indicated.
Molecular biology and germline transformation
Standard methods for molecular biology were used [57]. Cosmid and cDNA subcloning were performed using the Invitrogen Multisite Gateway Three-Fragment Vector Construction Kit.
Germline transformation was by microinjection [58] using 2–20 ng/µl for the DNA to be tested, along with 50 ng/µl pJMZ-lin-15 (+) construct and carrier DNA, pBluescriptII SK (+).
Ca2+ imaging
Ca2+ imaging was carried out as described previously [24], [28], using an inverted microscope (Axiovert, Zeiss), a 40× C-Apochromat lens, and MetaVue acquisition software (Molecular Devices).
Supporting Information
CO2-evoked responses in AFD do not require the GCY-9 transmembrane guanylate cyclase (A, B), whereas BAG responses do (C, D). For all experiments animals were grown at 22°C.
(EPS)
Disrupting gcy-33 reduces CO2 avoidance at 11% O2, whereas disrupting gcy-32 or gcy-34 has no effect on CO2 avoidance either at low or high O2. *, p<0.05, **, p<0.01, ns, not significant, Student's t-test.
(EPS)
CO2-evoked Ca2+ responses in ASE (A), BAG (B) and AFD (C) neurons are not altered by background O2 levels under our imaging conditions. CO2 and O2 stimuli are indicated above each plot.
(EPS)
ttx-7 mutants behave like N2 animals in 21%–0% O2 gradients.
(EPS)
Strain list.
(DOCX)
Acknowledgments
We thank the Caenorhabditis Genetics Centre, Ikue Mori, and Piali Sengupta for strains, and de Bono and Schafer lab members for comments and advice.
Funding Statement
This study was funded by the Medical Research Council, Fellowships from the The Royal Society and the European Molecular Biology Organization (EMBO ALTF 123-2007) to EKN and an Advanced European Research Council grant to MdB (ERC project no. 269058 - Acronym ACMO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Guyenet PG, Stornetta RL, Bayliss DA (2010) Central respiratory chemoreception. J Comp Neurol 518: 3883–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lahiri S, Prabhakar NR, Forster RE (2000) Oxygen sensing: molecule to man. New York: Kluwer Academic/Plenum. [Google Scholar]
- 3. Morrison SF, Nakamura K (2011) Central neural pathways for thermoregulation. Front Biosci 16: 74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bourque CW (2008) Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9: 519–531. [DOI] [PubMed] [Google Scholar]
- 5. Morrison SF, Nakamura K, Madden CJ (2008) Central control of thermogenesis in mammals. Exp Physiol 93: 773–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poon CS (2010) Homeostatic competition: evidence of a serotonin-gated spinoparabrachial pathway for respiratory and thermoregulatory interaction. Adv Exp Med Biol 669: 61–65. [DOI] [PubMed] [Google Scholar]
- 7. Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, et al. (2011) Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333: 637–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hodges MR, Richerson GB (2010) The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol 108: 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spyer KM, Gourine AV (2009) Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond B Biol Sci 364: 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wittenburg N, Baumeister R (1999) Thermal avoidance in Caenorhabditis elegans: an approach to the study of nociception. Proc Natl Acad Sci U S A 96: 10477–10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garrity PA, Goodman MB, Samuel AD, Sengupta P (2010) Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev 24: 2365–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mori I, Sasakura H, Kuhara A (2007) Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol 17: 712–719. [DOI] [PubMed] [Google Scholar]
- 13. Mori I, Ohshima Y (1995) Neural regulation of thermotaxis in Caenorhabditis elegans . Nature 376: 344–348. [DOI] [PubMed] [Google Scholar]
- 14. Kuhara A, Okumura M, Kimata T, Tanizawa Y, Takano R, et al. (2008) Temperature sensing by an olfactory neuron in a circuit controlling behavior of C. elegans. Science 320: 803–807. [DOI] [PubMed] [Google Scholar]
- 15. Ramot D, MacInnis BL, Goodman MB (2008) Bidirectional temperature-sensing by a single thermosensory neuron in C. elegans. Nat Neurosci 11: 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wasserman SM, Beverly M, Bell HW, Sengupta P (2011) Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr Biol 21: 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kimura KD, Miyawaki A, Matsumoto K, Mori I (2004) The C. elegans thermosensory neuron AFD responds to warming. Curr Biol 14: 1291–1295. [DOI] [PubMed] [Google Scholar]
- 18. Clark DA, Biron D, Sengupta P, Samuel AD (2006) The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci 26: 7444–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gray JM, Karow DS, Lu H, Chang AJ, Chang JS, et al. (2004) Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 430: 317–322. [DOI] [PubMed] [Google Scholar]
- 20. Persson A, Gross E, Laurent P, Busch KE, Bretes H, et al. (2009) Natural variation in a neural globin tunes oxygen sensing in wild Caenorhabditis elegans. Nature 458: 1030–1033. [DOI] [PubMed] [Google Scholar]
- 21. Cheung BH, Arellano-Carbajal F, Rybicki I, De Bono M (2004) Soluble Guanylate Cyclases Act in Neurons Exposed to the Body Fluid to Promote C. elegans Aggregation Behavior. Curr Biol 14: 1105–1111. [DOI] [PubMed] [Google Scholar]
- 22. Zimmer M, Gray JM, Pokala N, Chang AJ, Karow DS, et al. (2009) Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 61: 865–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGrath PT, Rockman MV, Zimmer M, Jang H, Macosko EZ, et al. (2009) Quantitative mapping of a digenic behavioral trait implicates globin variation in C. elegans sensory behaviors. Neuron 61: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Busch KE, Laurent P, Soltesz Z, Murphy RJ, Faivre O, et al. (2012) Tonic signaling from O(2) sensors sets neural circuit activity and behavioral state. Nat Neurosci 15: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bretscher AJ, Busch KE, de Bono M (2008) A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci U S A 105: 8044–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hallem EA, Sternberg PW (2008) Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci U S A 105: 8038–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sharabi K, Hurwitz A, Simon AJ, Beitel GJ, Morimoto RI, et al. (2009) Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc Natl Acad Sci U S A 106: 4024–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, et al. (2011) Temperature, Oxygen, and Salt-Sensing Neurons in C. elegans Are Carbon Dioxide Sensors that Control Avoidance Behavior. Neuron 69: 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hedgecock EM, Russell RL (1975) Normal and mutant thermotaxis in the nematode Caenorhabditis elegans . Proc Natl Acad Sci USA 72: 4061–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Satterlee JS, Sasakura H, Kuhara A, Berkeley M, Mori I, et al. (2001) Specification of Thermosensory Neuron Fate in C. elegans Requires ttx-1, a Homolog of otd/Otx. Neuron 31: 943–956. [DOI] [PubMed] [Google Scholar]
- 31. Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A (2004) Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A 101: 10554–10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, et al. (2006) Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics 172: 2239–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nonet ML, Saifee O, Zhao H, Rand JB, Wei L (1998) Synaptic transmission deficits in Caenorhabditis elegans synaptobrevin mutants. J Neurosci 18: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hallem EA, Spencer WC, McWhirter RD, Zeller G, Henz SR, et al. (2011) Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc Natl Acad Sci U S A 108: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Voorhies WA, Ward S (2000) Broad oxygen tolerance in the nematode Caenorhabditis elegans . J Exp Biol 203 Pt 16: 2467–2478. [DOI] [PubMed] [Google Scholar]
- 36. Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M (2005) Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol 15: 905–917. [DOI] [PubMed] [Google Scholar]
- 37. Rockman MV, Kruglyak L (2009) Recombinational landscape and population genomics of Caenorhabditis elegans. PLoS Genet 5: e1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanizawa Y, Kuhara A, Inada H, Kodama E, Mizuno T, et al. (2006) Inositol monophosphatase regulates localization of synaptic components and behavior in the mature nervous system of C. elegans. Genes Dev 20: 3296–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode Caenorhabditis elegans . Philosophical Transactions of the Royal Society of London B 314: 1–340. [DOI] [PubMed] [Google Scholar]
- 40. Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV (2001) Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci 21: 1510–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barriere A, Felix MA (2005) High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol 15: 1176–1184. [DOI] [PubMed] [Google Scholar]
- 42. Nurrish S, Segalat L, Kaplan JM (1999) Serotonin inhibition of synaptic transmission: Gao decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242. [DOI] [PubMed] [Google Scholar]
- 43. Miller KG, Emerson MD, Rand JB (1999) Goalpha and diacylglycerol kinase negatively regulate the Gqalpha pathway in C. elegans. Neuron 24: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rogers C, Reale V, Kim K, Chatwin H, Li C, et al. (2003) Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat Neurosci 6: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 45. Carrillo MA, Guillermin ML, Rengarajan S, Okubo RP, Hallem EA (2013) O2-Sensing Neurons Control CO2 Response in C. elegans. J Neurosci 33: 9675–9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saeki S, Yamamoto M, Iino Y (2001) Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol 204: 1757–1764. [DOI] [PubMed] [Google Scholar]
- 47. Tomioka M, Adachi T, Suzuki H, Kunitomo H, Schafer WR, et al. (2006) The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron 51: 613–625. [DOI] [PubMed] [Google Scholar]
- 48. Shinkai Y, Yamamoto Y, Fujiwara M, Tabata T, Murayama T, et al. (2011) Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J Neurosci 31: 3007–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsunozaki M, Chalasani SH, Bargmann CI (2008) A behavioral switch: cGMP and PKC signaling in olfactory neurons reverses odor preference in C. elegans. Neuron 59: 959–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, et al. (2005) Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, et al. (2004) A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature 431: 854–859. [DOI] [PubMed] [Google Scholar]
- 52. Fischler W, Kong P, Marella S, Scott K (2007) The detection of carbonation by the Drosophila gustatory system. Nature 448: 1054–1057. [DOI] [PubMed] [Google Scholar]
- 53. Turner SL, Ray A (2009) Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 461: 277–281. [DOI] [PubMed] [Google Scholar]
- 54. Poon PC, Kuo TH, Linford NJ, Roman G, Pletcher SD (2010) Carbon dioxide sensing modulates lifespan and physiology in Drosophila. PLoS Biol 8: e1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu T, Cai D (2013) Counterbalance between BAG and URX neurons via guanylate cyclases controls lifespan homeostasis in C. elegans. EMBO J 32: 1529–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sulston J, Hodgkin J (1988) Methods. In: Wood WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor: CSHL Press. pp. 587–606. [Google Scholar]
- 57.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, New York: Cold Spring Harbor Press. [Google Scholar]
- 58. Mello CC, Kramer JM, Stinchcomb D, Ambros V (1991) Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CO2-evoked responses in AFD do not require the GCY-9 transmembrane guanylate cyclase (A, B), whereas BAG responses do (C, D). For all experiments animals were grown at 22°C.
(EPS)
Disrupting gcy-33 reduces CO2 avoidance at 11% O2, whereas disrupting gcy-32 or gcy-34 has no effect on CO2 avoidance either at low or high O2. *, p<0.05, **, p<0.01, ns, not significant, Student's t-test.
(EPS)
CO2-evoked Ca2+ responses in ASE (A), BAG (B) and AFD (C) neurons are not altered by background O2 levels under our imaging conditions. CO2 and O2 stimuli are indicated above each plot.
(EPS)
ttx-7 mutants behave like N2 animals in 21%–0% O2 gradients.
(EPS)
Strain list.
(DOCX)