Abstract
Background
Cocaine is a major cause of acute coronary syndrome (ACS), especially in young adults; however, the mechanistic underpinning of cocaine-induced ACS remains limited. Previous studies in animals and in patients undergoing cardiac catheterization suggest that cocaine constricts coronary microvessels, yet direct evidence is lacking.
Methods and Results
We used myocardial contrast echocardiography (MCE) to test the hypothesis that cocaine causes vasoconstriction in the human coronary microcirculation. Measurements were performed at baseline and after a low non-intoxicating dose of intranasal cocaine (2 mg/kg) in 10 healthy cocaine-naïve young men (median age 32 years). Post-destruction time-intensity MCE kinetic data were fit to the equation: y = A(1-e- β t) to quantify functional capillary blood volume (A), microvascular flow velocity (β), and myocardial perfusion (A × β). Heart rate (HR), mean arterial pressure (MAP), and LV work (two-dimensional echocardiography) were measured before and 45 minutes after cocaine. Cocaine increased MAP (+14±2 mmHg; mean ± SE), HR (+8±3 beats/min), and LV work (+50±18 mmHg·mL-1·bpm-1). Despite increasing these determinants of myocardial oxygen demand, myocardial perfusion decreased by 30% (103.7±9.8 to 75.9±10.8 a.u/s, p<0.01) due mainly to decreased capillary blood volume (133.9±5.1 to 111.7±7.7 a.u., p<.05) with no significant change in microvascular flow velocity (0.8±0.1 to 0.7±0.1 a.u.).
Conclusions
In healthy cocaine-naïve young adults, a low-dose cocaine challenge evokes a sizeable decrease in myocardial perfusion. Moreover, the predominant effect is to decrease myocardial capillary blood volume rather than microvascular flow velocity, suggesting a specific action of cocaine to constrict terminal feed arteries.
Keywords: Cocaine, Myocardial Contrast Echocardiography, Myocardial Perfusion Imaging, Coronary Microcirculation
Introduction
Cocaine is the second most widely trafficked drug in the world (second only to marijuana) and constitutes a major cause of cardiovascular disease, especially acute coronary syndrome (ACS).1 The incidence of cocaine-induced ACS has increased steadily over the last two decades,2 as cocaine use has increased worldwide— especially in Europe, Africa, and Asia.3 Treatment of cocaine-induced ACS however remains largely empirical because the underlying pathogenesis is incompletely understood, and an efficient method to evaluate putative countermeasures is lacking.2
Cocaine is a potent sympathomimetic, stimulating adrenergic receptors to simultaneously increase: a) heart rate and blood pressure,4-8 and thus myocardial oxygen demand; and; b) coronary vascular resistance, which could limit oxygen delivery. Indeed, a low non-intoxicating dose of intranasal cocaine (2 mg/kg) has been shown to decrease both coronary artery diameter and coronary sinus blood flow—an indirect measure of coronary arterial flow— in middle-aged patients undergoing diagnostic cardiac catheterization for evaluation of chest pain.9-14 In those studies, the reduction in diameter of the epicardial coronary arteries averaged 8-12%, which, by itself, would be far too small to impair myocardial perfusion.15 Thus, the decrease in coronary sinus flow has been viewed as indirect evidence for constriction at the level of the coronary microcirculation. However, cocaine’s putative effect on coronary microvessels has not been tested directly.
Here we used echogenic gas-filled microspheres (i.e., myocardial contrast echocardiography, or “MCE”) to test the hypothesis that cocaine constricts human coronary microvessels. The data show a remarkable decrease in capillary blood volume with unchanged feed artery flow, indicating constriction of the most distal coronary microvessels, when cocaine-naïve healthy young adults are challenged with the non-intoxicating 2 mg/kg dose of intranasal cocaine.
Methods
The research protocol was approved by the Institutional Review Board at Cedars-Sinai Medical Center. Healthy volunteers, between the ages of 18 and 55 years of age, were recruited by advertisement in local media, and scheduled for an initial screening visit to determine eligibility. After obtaining informed written consent, subjects were screened with a physical examination, complete medical history, 12-lead electrocardiogram, two-dimensional transthoracic echocardiogram, and venous blood sampling to assess electrolytes, lipid profile, inflammatory markers (C-reactive protein), glucose levels, and liver function. Exclusion criteria were as follows: history of substance abuse; intracardiac shunt by echocardiogram; evidence of cardiopulmonary disease by history, physical examination, electrocardiogram, or echocardiogram; history of kidney or liver disease, diabetes mellitus, systemic illness or hypertension; blood pressure ≥140/90 mmHg at screening; hyperlipidemia, or elevated blood glucose at screening; and inadequate echocardiography image quality as determined by a senior echocardiography-board certified cardiologist.
All experiments were performed under normothermic conditions (22°C), with subjects in the left lateral decubitus position. Heart rate and blood pressure were measured continuously. Blood pressure was measured by the oscillometric technique with a highly accurate validated oscillometric monitor (Datascope Mindray Passport V Monitor (Mindray North America, Mahwah, NJ).16 Heart rate was monitored by a cardiotachometer triggered by R wave of an ECG lead. Height was recorded by a standard clinical stadiometer, and body weight measured using a digital balance scale (Scale-Tronix 5002, White Plains, NY).
Measurement of left ventricular function
Contrast enhanced two-dimensional transthoracic echocardiography was performed by a single licensed sonographer, using a phased-array probe interfaced with an imaging system (iE33, Philips Medical Systems, Andover, MA), to evaluate left ventricular (LV) systolic function and estimate cardiac work as a reflection of myocardial oxygen demand. Parasternal short-axis images, at the level of the papillary muscles, were acquired to measure end-systolic cavity area and end-systolic myocardial area, with the endocardial and epicardial cavity areas recorded, respectively. Apical 4-chamber and 2-chamber images were acquired, with the endocardial surface manually traced at end-diastole and end-systole, for measurement of LV end-diastolic and end-systolic volumes, respectively, using a modified Simpson’s method.17 All data are reported as the average of at least three cardiac cycles, acquired with the breath held at end-expiration.
Stroke volume was calculated as end-diastolic volume minus end-systolic volume and cardiac output calculated as heart rate multiplied by stroke volume. Indices of LV systolic function included: 1) ejection fraction, calculated as stroke volume divided by end-diastolic volume; 2) LV end-systolic single point elastance, calculated as end-systolic pressure (0.9 × systolic blood pressure) divided by end-systolic volume; 3) LV stroke work, calculated as end-systolic pressure × stroke volume; and 4) LV total work, calculated as LV end-systolic elastance × heart rate. LV end-systolic wall stress was measured by:18 PRi/2h(1+h/2Ri); where P is equal to end-systolic pressure, Ri is internal radius (square root of end-systolic cavity area divided by π), and h is wall thickness (end-systolic myocardial area minus end-systolic cavity area). Myocardial oxygen demand was calculated according to a previously validated equation:19 (7.2 × 10-4) × (heart rate × systolic blood pressure) + 1.42.
Myocardial Contrast Echocardiography
One vial (1.3 mL) of lipid-shell octafluoropropane microbubbles (Definity; Lantheus Medical Imaging, North Billerica, USA) was diluted to a total volume of 30 mL in normal saline and administered intravenously using an infusion pump (Medfusion 3500 Syringe Pump, Smiths Medical ASD Inc. St. Paul, MN, USA) at a rate of 1.5mL/min. A multi-pulse contrast-specific imaging protocol (power modulation imaging) was performed at a low mechanical index (0.1). Gain, depth, transmit focus, and post-processing were optimized at the beginning of the study and held constant throughout. Imaging was performed in the apical four-chamber view, with the septum focused in the middle of the window. The replenishment of myocardial Definity at consecutive pulsing intervals after a destructive pulse with an MI of 0.8 was recorded; only end-systolic frames were used for analysis.
Image analysis was performed off-line by a single experienced operator [XT], according to established methods.20, 21 Briefly, a large region-of-interest was placed over the mid-ventricular septum of ten to fifteen consecutive end-systolic images beginning with the immediate post-destruction frame. Time vs. background-subtracted acoustic intensity plots were then generated and fit to the exponential function y=A(1-eβt), where y is the acoustic intensity at a time of t, A is the plateau where acoustic intensity represents myocardial blood volume, and β represents the mean microbubble flux rate through the microcirculation (referred to as microvascular flow velocity). Data are reported as the average of at least three separate microbubble destruction-refill time vs. acoustic intensity plots, each satisfying all the following criteria: 1) adequate destruction of microbubbles following high mechanical index pulse by visual inspection, 2) the septum was focused in the middle of the window throughout the entire destruction-refill observation period, and 3) consistent imaging plane. Myocardial perfusion was calculated as the product of myocardial blood volume (A) and microvascular flow velocity (β). Myocardial conductance was calculated as myocardial perfusion divided by mean arterial pressure.
Cocaine metabolites and blood levels
Venous blood was collected 30 min after cocaine administration for detection of the cocaine metabolite benzoylecgonine. Blood samples were immediately centrifuged, with the serum frozen (-80°C), and later analyzed by gas chromatography-mass spectroscopy (Pacific Toxicology Laboratories, Chatsworth, CA) for the determination of cocaine blood levels.
Experimental Protocols
Protocol 1: Internal validation studies
First, we established within-subject test-retest reproducibility of MCE perfusion indices in our laboratory. In each subject, baseline measurements were performed twice separated by at least 15 minutes, which is the time needed to return the intravascular microbubble concentration to baseline levels.
Then, we assessed the ability of MCE to detect small changes in microvascular perfusion induced by dobutamine, used as an internal coronary vasodilator control. Measurements were performed before and during continuous intravenous infusion of low-dose (5 μg/kg/min) dobutamine (Harvard Apparatus, Holliston, MA, USA). Previous dog studies show that low-dose dobutamine increases microvascular flux rate with little change in capillary blood volume.20 Whether low-dose dobutamine evokes a similar pattern of microvascular coronary dilation in human subjects has not previously been tested.
Protocol 2: Effects of low-dose intra-nasal cocaine on microvascular perfusion
After baseline data were obtained, a low non-intoxicating dose of topical intranasal cocaine hydrochloride (2 mg/kg, 10% solution) was administered, followed by repeat measurements of MCE and LV function. This dose of intranasal cocaine is half the standard clinical dose for rhinolaryngologic procedures,22 and the same dose used in many previous studies to show that cocaine decreases epicardial coronary artery diameter by 8-12% as measured by quantitative coronary angiography in patients undergoing evaluation for chest pain.9-14
Statistical Methods
All data are expressed as a mean ± SEM, unless otherwise specified. Statistical analyses were performed with SigmaStat software (Systat, San Jose, CA). Protocol 1: Simple linear regressions, along with calculation of the coefficient of variation, and Bland-Altman analyses were performed to assess test-retest reproducibility. Differences between baseline and dobutamine were assessed using a paired sample t-test. Protocol 2: Differences between baseline and cocaine were assessed using a paired sample t-tests. Significance was set a priori at P < 0.05.
Results
Twenty four potential subjects responded to advertisement in local media and were screened for participation. Thirteen individuals were excluded from study for the following reasons: suboptimal echocardiographic image quality (n=9); elevated blood pressure at screening (n=2); hyperlipidemia on screening (n = 1); and medical history of chronic systemic illness (n=1). The remaining eleven individuals (7 non-Hispanic white males and 4 Hispanic white males) qualified to participate: age 33 ± 3 years (mean ± SE, range: 22 to 45 years); height 179.7 ± 2.5 cm; weight 83.7 ± 3.6 kg; body mass index 25.9 ± 1 kg/m2; systolic blood pressure 113 ± 4 mmHg, diastolic blood pressure 62 ± 4 mmHg, heart rate 71 ± 5 beats·min-1; LV ejection fraction 63 ± 0.5%; and Framingham risk scores ranging from <1% to 2%.
Protocol 1: Validation Studies
Test-retest reproducibility of myocardial perfusion indices under baseline conditions
The MCE indices were highly reproducible with coefficients of variation ranging from 4% to 14% (Table 1). No systematic bias was detected on Bland-Altman plots (Table 1 and Supplemental Figure S1).
Table 1.
Test-retest reproducibility of myocardial contrast echocardiography and left ventricular volumes
| Measurement | Linear Regression | Coefficient of variation | Bland-Altman | |||
|---|---|---|---|---|---|---|
| R | P Value | Bias ± SD | 95% Limits of Agreement | |||
| Myocardial Contrast Echocardiography | ||||||
| Myocardial A | 0.925 | <0.001 | 3% | -1.4 ± 8.3 | -15.0 | 17.8 |
| Myocardial β | 0.824 | 0.002 | 13% | 0.04 ± 0.18 | -0.38 | 0.31 |
| Myocardial Perfusion (A·β) | 0.896 | <0.001 | 13% | -2.1 ± 24.1 | -49.4 | 45.2 |
| Left ventricular volumetric assessment | ||||||
| End-diastolic volume, mL | 0.874 | <0.001 | 4% | 1.5 ± 12.1 | -22.2 | 25.1 |
| End-systolic volume, mL | 0.860 | <0.001 | 6% | 0.33 ± 5.7 | -12.6 | 9.9 |
Effects of low-dose dobutamine
Low-dose intravenous dobutamine increased systolic blood pressure (111 ± 3 to 144 ± 4 mmHg, mean ±SE; P<0.01), diastolic blood pressure (55 ± 2 to 63 ± 2 mmHg, P<0.01), and mean arterial pressure (74 ± 2 to 90 ± 2 mmHg, P<0.01), with no change in heart rate (66 ± 3 to 68 ± 4 beats·min-1, P=0.522). Multiple indices of LV work increased as expected (Supplemental Table S1).
An illustrative experiment in one subject and group mean data for myocardial perfusion are shown in Figure 1. Microvascular flow velocity (β) increased significantly (from 0.7 ± 0.1 to 1.1 ± 0.1 s-1, from baseline to dobutamine, P < 0.01), while capillary blood volume remained unchanged (130 ± 7 vs. 124 ± 6 a.u., baseline vs. dobutamine, p=ns). As a result, myocardial perfusion increased by 72% (from 95 ± 16 to 145 ±19 a.u.·s-1, P < 0.05, Figure 1B), and myocardial conductance increased by 40% (from 1.3 ± 0.2 to 1.6 ± 0.2 a.u.·s-1·mmHg-1, P < 0.05). The ratio of myocardial perfusion to MVO2 was unaffected by dobutamine (Figure 1C), indicating proportionate increases in oxygen delivery and demand. The ratio of myocardial conductance to MVO2 was also unaffected by dobutamine (0.2 ± 0.03 vs. 0.2 ± 0.02, baseline vs. dobutamine).
Figure 1.
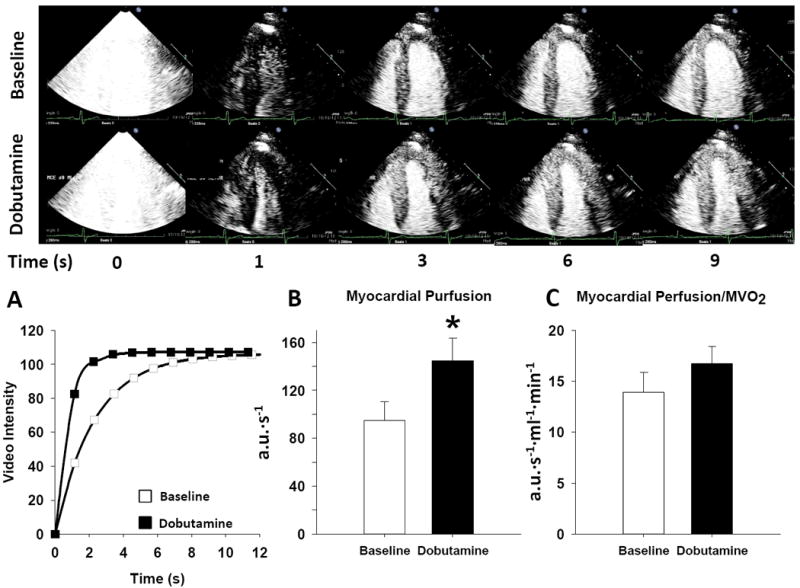
The myocardial contrast echocardiography response at baseline and following low-dose dobutamine (5 μg/kg/min). Top panel shows two-dimensional MCE images of the left ventricle at various time intervals after the destructive pulse sequence (denoted as T0). Note that with dobutamine, the rate of bubble replenishment (β) is much faster than baseline, reaching steady-state (A) within 3 seconds, compared to 6 seconds at baseline. Bottom panel illustrates: A) a typical time-intensity plot obtained at baseline and during dobutamine, B) summary data showing myocardial perfusion at baseline and in response to dobutamine, C) myocardial perfusion normalized to myocardial oxygen consumption (MVO2) at baseline and following dobutamine stress. Summary data reported as mean ± SE. *P < 0.05.
Protocol 2. Effects of non-intoxicating low-dose topical intra-nasal cocaine on myocardial perfusion
As expected, none of the subjects developed chest pain, electrocardiographic evidence of ischemia or arrhythmias, or other complications from low-dose cocaine. In one subject, destruction-refill kinetic data were not of sufficient quality to meet inclusion criteria; thus data are presented on 10 subjects. Neither cocaine nor the cocaine metabolite benzoylecgonine were detected in plasma of any subject at baseline. After intranasal cocaine, the mean cocaine blood level was 64.5 ± 13.7 ng/mL; benzoylecgonine was detected in all subjects. Consistent with previous reports,4-8, 23, 24 low-dose topical intranasal cocaine increased systolic blood pressure (111 ± 3 to 125± 4 mmHg, mean ±SE; P<0.01), diastolic blood pressure (53 ± 2 to 67 ± 2 mmHg, P<0.01), mean arterial pressure (72 ± 2 to 86 ± 3 mmHg, P<0.01), heart rate (65 ± 2 to 73 ± 3 beats·min-1, P<0.01) and multiple indices of LV work (Table 2).
Table 2.
Myocardial responses to intranasal cocaine
| Baseline | Cocaine | |
|---|---|---|
| Myocardial contrast echocardiography | ||
| Myocardial A, a.u | 134 ± 5 | 112 ± 8* |
| Myocardial β, s-1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| Myocardial perfusion (A·β), a.u.·s-1 | 104 ± 10 | 76 ± 11* |
| Myocardial conductance, a.u.·mmHg-1 | 1.5 ± 0.2 | 0.9 ± 0.2* |
| Hemodynamics | ||
| Heart rate, beats·min-1 | 65 ± 2 | 73 ± 3* |
| Systolic Blood Pressure, mmHg | 111 ± 3 | 125 ± 4* |
| Diastolic Blood Pressure, mmHg | 53 ± 2 | 67 ± 2* |
| Mean Blood Pressure, mmHg | 72 ± 2 | 86 ± 3* |
| LV End-diastolic volume, mL | 152 ± 7 | 158.3 ± 7.4 |
| LV End-systolic volume, mL | 56 ± 4 | 53 ± 3 |
| LV Stroke volume, mL | 96 ± 5 | 105 ± 7* |
| Indices of LV work, function and oxygen demand | ||
| LV Ejection Fraction, % | 64 ± 1 | 66 ± 2 |
| LV end-systolic elastance, mmHg·mL-1 | 1.9 ± 0.2 | 2.5 ± 0.3* |
| LV end-systolic wall stress, 103 dyn/cm2 | 0.7 ± 0.1 | 0.8 ± 0.1 |
| LV stroke work (×10-3), mmHg·mL-1 | 9.6 ± 0.4 | 11.9 ± 0.8* |
| LV total work, mmHg·mL-1·bpm-1 | 125.6 ± 14.3 | 176.9 ± 17.2* |
| MVO2, ml·min-1 | 6.7 ± 0.3 | 8.1 ± 0.4* |
Data reported as mean ± SEM.
P < 0.05.
RBC, red blood cell; LV, left ventricle; MVO2, myocardial oxygen consumption
An illustrative experiment in one subject is shown in Figure 2 and group mean data are shown in Figure 2 and Table 2. The major new finding of this study is that with acute low-dose cocaine challenge myocardial capillary blood volume fell by 16% (from 134 ± 5 to 112 ± 8 a.u., P < 0.01), while microvascular flow velocity remained unchanged (Figure 2 and Table 2). As a result, myocardial perfusion fell by 23% (from 104 ± 10 to 76 ± 11 a.u.·s-1, P<0.01) and myocardial conductance fell by 35% (from to 1.5 + 0.2 to 0.9 ± 0.2 a.u.·s-1·mmHg-1, P<0.01; Figure 2B). Moreover, the ratio of myocardial perfusion to MVO2 decreased by 35% (from 16 ± 2 to 10 ± 1, P<0.01, Figure 2C) and the ratio of myocardial conductance to MV02 decreased by 44% (from 0.2 ± 0.03 to 0.1 ± 0.02, P<0.01), indicating a mismatch between oxygen supply and demand.
Figure 2.
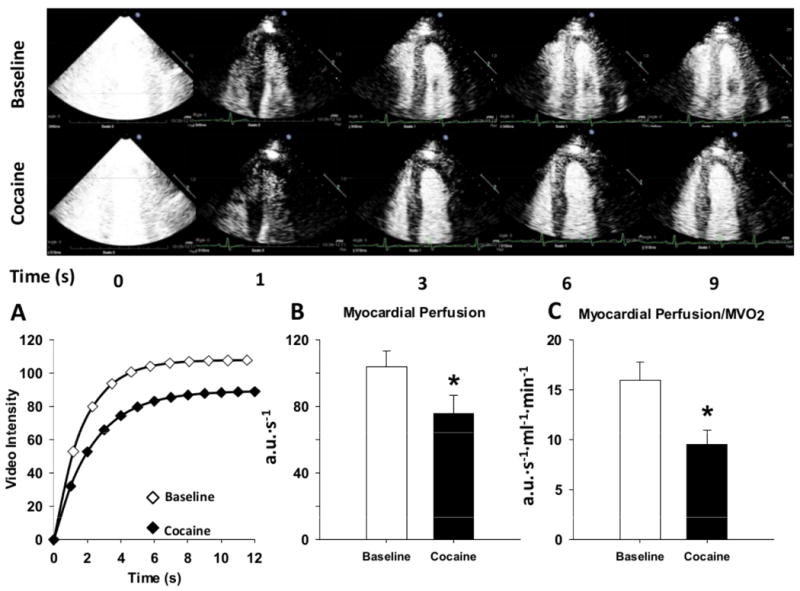
The myocardial contrast echocardiography response at baseline and following a non-intoxicating low-dose of intranasal cocaine (2 mg/kg). Top panel shows two-dimensional MCE images of the left ventricle of a representative cocaine-naïve healthy subject at various time intervals after the destructive pulse sequence (denoted as T0). Note that with cocaine, the rate of bubble replenishment (β) appears only somewhat reduced, while the maximum video intensity (capillary blood volume) never becomes as bright as the baseline condition. Bottom panel illustrates: A) a typical time-intensity plot obtained at baseline and during low-dose intranasal cocaine exposure, B) summary data showing myocardial perfusion at baseline and in response to cocaine, C) myocardial perfusion normalized to myocardial oxygen consumption (MVO2) at baseline and following cocaine. Summary data reported as mean ± SE. *P < 0.05.
Discussion
The mechanistic underpinning of cocaine-induced coronary vasoconstriction and the evidence base for treating acute cocaine-induced ACS are limited.2 Using myocardial contrast echocardiography (MCE) in cocaine-naïve healthy young adults, we show that a non-intoxicating low-dose intranasal cocaine challenge evokes a sizeable decrease in myocardial perfusion. Moreover, the predominant effect is to decrease myocardial capillary blood volume rather than microvascular flow velocity, suggesting a specific action of cocaine to constrict terminal feed arteries.
The prior clinical research on cocaine-induced coronary vasoconstriction required invasive cardiac catheterization and focused on large epicardial coronary arteries. In contrast, MCE provides a non-invasive approach to study cocaine’s effects on the human coronary microcirculation, which contains 80% of total myocardial blood volume and regulates nutrient exchange in cardiac myocytes.25 Using echogenic gas-filled microspheres with similar size and rheologic properties to red blood cells, the destruction-refill kinetic data permit repeated quantitative measures of microvascular flow velocity and capillary blood volume. While MCE is technically challenging and has not yet gained wide use in the clinical diagnosis of coronary artery disease, the technique has been well validated in multiple laboratories21, 26-32 and proven to be a powerful clinical research tool for elucidating mechanisms of vascular regulation in other conditions (e.g., coronary occlusion,27, 31, 32 insulin-mediated vasodilation29, 30, 33-37). However, MCE has not been used previously to study the vascular effects of cocaine.
We therefore validated the technique for this specific purpose in our own laboratory by showing: 1) a high degree of within-subject test-retest reproducibility; and 2) the expected increase in myocardial perfusion with low-dose dobutamine –a positive inotrope used as an internal coronary vasodilator control. That low-dose dobutamine caused a large increase in microvascular flow velocity with unchanged capillary blood volume is a novel finding that replicates in conscious human subjects the same pattern of microvascular response to dobutamine seen previously with MCE in anesthetized dogs.20, 38 From a mechanistic standpoint, increased cardiac work produced by low-dose dobutamine causes metabolic dilation of intramuscular feed arterioles but does not cause capillary recruitment; the latter only occurs at higher levels of catecholamine stimulation when increases in blood velocity alone are insufficient to meet increased myocardial oxygen demands.20
The seminal finding of our study is that cocaine—in marked contrast to dobutamine— decreases myocardial perfusion despite increasing myocardial oxygen demand. Moreover, the decrease in capillary blood volume with unchanged microvascular flow velocity strongly suggests that the major site of cocaine’s action is at the most distal coronary microvessels— terminal arterioles that control inflow to individual capillary networks. This interpretation is supported by previous studies showing that interventions that selectively affect the tone of terminal arterioles (such as insulin30, 35-37 or mild exercise39) selectively affect capillary blood volume without changing microvascular flow velocity. In marked contrast, acute stenosis or spasm of the large epicardial coronary arteries produces a qualitatively different MCE response characterized by an isolated decrease in microvascular flow velocity with little or no change in capillary blood volume.27
Thus, the new MCE data extend previous clinical research on cocaine-induced coronary constriction. Our data: a) substantiate the interpretation of the coronary sinus flow data in the seminal catheterization laboratory studies by Hillis, Lange, and coworkers using the same low non-intoxicating dose of topical intranasal cocaine (2mg/kg);9, 14 but b) differ from those of Majid and coworkers40 who found that intravenous infusion of an intoxicating dose of cocaine affected neither myocardial perfusion by MCE performed with intracoronary infusion of microbubbles nor coronary artery diameter by quantitative coronary angiography and actually increased coronary sinus flow by thermodilution. However, the negative findings in the latter study may be due to either: 1) acute cardiovascular tolerance as the cocaine challenge was administered to cocaine-addicted patients admitted 24-48h earlier with documented prolonged cocaine-related ACS, and/or 2) MCE technique, which used bolus intracoronary injections, preventing measurement of capillary blood volume which requires continuous steady state infusion. Indeed, the major new finding of our study is that, in the human coronary microcirculation, cocaine appears to exert its greatest effect at the level of the capillaries. These new MCE data in humans also extend previous animal studies in several important ways. Our data: a) are consistent with earlier studies in anesthetized dogs41-43 and pigs44-46 showing decreased coronary blood flow, and decreased myocardial perfusion by Thallium scintography47 as well as conventional (i.e., radiolabelled) microspheres41, 47, 48; but b) differ at first glance from more recent studies in conscious dogs and non-human primates in which proximal coronary artery flow increased with cocaine (suggesting that the decreased flow in earlier studies was an artifact of anesthesia).49 In the later study, however, coronary sinus pH fell despite increased large artery flow, suggesting impaired microvascular perfusion— which we have now shown directly in conscious humans.
Our study has several limitations. We excluded almost half the subjects screened due to suboptimal echocardiographic windows, which limits application of MCE to a broader population. This initial proof-of-concept study did not have a placebo control; however, the highly reproducible baseline data serve as a time control and cocaine’s preferential effect on capillary blood volume over microvascular flow velocity shows specificity. We did not perform invasive coronary angiography to exclude cocaine-induced epicardial coronary vasoconstriction. However, two pieces of information point to a microvascular site of action. First, multiple prior angiographic studies show only a 8-12% reduction in epicardial artery diameter using the same dose of topical intranasal cocaine used in our study.9-14 This small reduction in epicardial coronary artery diameter is well below the threshold 85% reduction required to restrict flow,15 as shown here (i.e, no change in microvascular flow velocity). Second, reduced resting myocardial blood flow from epicardial artery stenosis is manifest on MCE by a larger reduction in microvascular flow velocity (beta) than capillary blood volume (A),27 but we have seen the opposite, which further points to a distal arteriolar mechanism. While we cannot exclude the presence of coronary artery disease in our subjects, by design all had extremely low Framingham risk scores.
Additional limitations should be considered. Increased LV pressure from diastolic dysfunction theoretically could contribute to the present results by causing extravascular compression of coronary microvessels. While we did not measure LV end-diastolic pressure, previous invasive hemodynamic studies show that this low-dose of intranasal cocaine has no effect on end-diastolic pressure.50 For ethical reasons, our studies are limited to low-dose cocaine which does not expose cocaine-naïve subjects to the potentially addicting effect of cocaine intoxication. Because none of the subjects experienced chest pain or had any ischemic EKG changes with this cocaine dose, the present MCE data do not prove that constriction of coronary microvessels is the major mechanism causing either chest pain or ACS in the clinical setting of cocaine intoxication. Further studies will be needed to determine if the microvascular response differs when an acute cocaine challenge is superimposed on chronic cocaine addiction or common cardiovascular risk factors.
Despite these limitations, the present MCE data document acute cocaine-induced vasoconstriction in the human coronary microcirculation and set the stage for future studies to elucidate the underlying mechanism and to evaluate potential countermeasures in a controlled clinical research setting.
Supplementary Material
Clinical Impact.
Cocaine is the second most widely abused drug in the world (second only to marijuana) and constitutes a major cause of cardiovascular disease, especially acute coronary syndrome (ACS). The incidence of cocaine-induced ACS has increased steadily over the last two decades, as cocaine use has increased worldwide. Treatment of cocaine-induced ACS however remains largely empirical because the underlying pathogenesis is incompletely understood, and an efficient method to evaluate putative countermeasures is lacking. Using myocardial contrast echocardiography in cocaine-naïve healthy young adults, we show that a non-intoxicating low-dose intranasal cocaine challenge evokes a sizeable decrease in myocardial perfusion. Moreover, the predominant effect is to decrease myocardial capillary blood volume rather than microvascular flow velocity, suggesting a specific action of cocaine to constrict terminal feed arteries. These findings establish myocardial contrast echocardiography as an efficient non-invasive method for future studies to elucidate the underlying mechanism of cocaine-induced coronary vasoconstriction, and to evaluate potential countermeasures in a controlled clinical research setting.
Acknowledgments
Funding Sources: This study was funded by: grants to Dr. Victor from the National Institutes of Health (R01DA10064) and the Lincy Foundation; and the NIH/National Center for Advancing Translational Science UCLA CTSI (UL1TR000124). Dr. Nelson is the recipient of a research fellowship grant from the Heart and Stroke Foundation of Canada.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Carrillo X, Curtis A, Muga R, Serra J, Sanvisens A, Bayes-Genis A. Acute coronary syndrome and cocaine use: 8-year prevalence and inhospital outcomes. Eur Heart J. 2011;32:1244–50. doi: 10.1093/eurheartj/ehq504. [DOI] [PubMed] [Google Scholar]
- 2.McCord J, Jneid H, Hollander JE, de Lemos JA, Cercek B, Hsue P, Gibler WB, Ohman EM, Philippides G, Newby LK. Management of Cocaine-Associated Chest Pain and Myocardial Infarction: A Scientific Statement From the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897–907. doi: 10.1161/CIRCULATIONAHA.107.188950. [DOI] [PubMed] [Google Scholar]
- 3.United Nations Office on Drugs and Crime (UNODC) World Drug Report 2012. United Nations Publication. 2012 [Google Scholar]
- 4.Jacobsen TN, Grayburn PA, Snyder RW, Hansen J, Chavoshan B, Landau C, Lange RA, Hillis LD, Victor RG. Effects of intranasal cocaine on sympathetic nerve discharge in humans. J Clin Invest. 1997;99:628–34. doi: 10.1172/JCI119205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kontak AC, Victor RG, Vongpatanasin W. Dexmedetomidine as a Novel Countermeasure for Cocaine-Induced Central Sympathoexcitation in Cocaine-Addicted Humans. Hypertension. 2013;61:388–94. doi: 10.1161/HYPERTENSIONAHA.112.203554. [DOI] [PubMed] [Google Scholar]
- 6.Menon DV, Wang Z, Fadel PJ, Arbique D, Leonard D, Li JL, Victor RG, Vongpatanasin W. Central Sympatholysis as a Novel Countermeasure for Cocaine-Induced Sympathetic Activation and Vasoconstriction in Humans. J Am Coll Cardiol. 2007;50:626–33. doi: 10.1016/j.jacc.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 7.Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine Stimulates the Human Cardiovascular System via a Central Mechanism of Action. Circulation. 1999;100:497–502. doi: 10.1161/01.cir.100.5.497. [DOI] [PubMed] [Google Scholar]
- 8.Vongpatanasin W, Taylor JA, Victor RG. Effects of cocaine on heart rate variability in healthy subjects. Am J Cardiol. 2004;93:385–8. doi: 10.1016/j.amjcard.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Lange RA, Cigarroa RG, Yancy CW, Willard JE, Popma JJ, Sills MN, McBride W, Kim AS, Hillis LD. Cocaine-Induced Coronary-Artery Vasoconstriction. N Engl J Med. 1989;321:1557–62. doi: 10.1056/NEJM198912073212301. [DOI] [PubMed] [Google Scholar]
- 10.Pitts WR, Vongpatanasin W, Cigarroa JE, Hillis LD, Lange RA. Effects of the Intracoronary Infusion of Cocaine on Left Ventricular Systolic and Diastolic Function in Humans. Circulation. 1998;97:1270–3. doi: 10.1161/01.cir.97.13.1270. [DOI] [PubMed] [Google Scholar]
- 11.Daniel WC, Lange RA, Landau C, Willard JE, Hillis LD. Effects of the Intracoronary Infusion of Cocaine on Coronary Arterial Dimensions and Blood Flow in Humans. Am J Cardiol. 1996;78:288–91. doi: 10.1016/s0002-9149(96)00279-2. [DOI] [PubMed] [Google Scholar]
- 12.Pirwitz MJ, W JL. Influence of cocaine, ethanol, or their combination on epicardial coronary arterial dimensions in humans. Arch Intern Med. 1995;155:1186–91. [PubMed] [Google Scholar]
- 13.Brogan WC, III, Lange RA, Kim AS, Moliterno DJ, Hillis LD. Alleviation of cocaine-induced coronary vasoconstriction by nitroglycerin. J Am Coll Cardiol. 1991;18:581–6. doi: 10.1016/0735-1097(91)90617-i. [DOI] [PubMed] [Google Scholar]
- 14.Lange RA, Cigarroa RG, Flores ED, McBride W, Kim AS, Wells PJ, Bedotto JB, Danzinger RS, Hillis LD. Potentiation of Cocaine-Induced Coronary Vasoconstriction by Beta-Adrenergic Blockade. Ann Intern Med. 1990;112:897–903. doi: 10.7326/0003-4819-112-12-897. [DOI] [PubMed] [Google Scholar]
- 15.Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. The Am J Cardiol. 1974;34:48–55. doi: 10.1016/0002-9149(74)90092-7. [DOI] [PubMed] [Google Scholar]
- 16.Anwar Y, Tendler B, McCabe E, Mansoor G, White W. Evaluation of the Datascope Accutorr Plus according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit. 1997;2:105–10. [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St, John Sutton M, Stewart WJ. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rooke GA, Feigl EO. Work as a correlate of canine left ventricular oxygen consumption, and the problem of catecholamine oxygen wasting. Circ Res. 1982;50:273–86. doi: 10.1161/01.res.50.2.273. [DOI] [PubMed] [Google Scholar]
- 20.Le DE, Bin JP, Coggins MP, Wei K, Lindner JR, Kaul S. Relation between myocardial oxygen consumption and myocardial blood volume: A study using myocardial contrast echocardiography. J Am Soc Echocardiogr. 2002;15:857–63. doi: 10.1067/mje.2002.121275. [DOI] [PubMed] [Google Scholar]
- 21.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of Myocardial Blood Flow With Ultrasound-Induced Destruction of Microbubbles Administered as a Constant Venous Infusion. Circulation. 1998;97:473–83. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 22.Johns M, Henderson R. Cocaine use by the otolaryngologist: a survey. Trans Sect Otolaryngol Am Acad Opthalmol Otolaryngol. 1977;84:969–73. [PubMed] [Google Scholar]
- 23.Crandall CG, Vongpatanasin W, Victor RG. Mechanism of Cocaine-Induced Hyperthermia in Humans. Ann Intern Med. 2002;136:785–91. doi: 10.7326/0003-4819-136-11-200206040-00006. [DOI] [PubMed] [Google Scholar]
- 24.Tuncel M, Wang Z, Arbique D, Fadel PJ, Victor RG, Vongpatanasin W. Mechanism of the Blood Pressure Raising Effect of Cocaine in Humans. Circulation. 2002;105:1054–9. doi: 10.1161/hc0902.104714. [DOI] [PubMed] [Google Scholar]
- 25.Kassab GS, Lin DH, Fung YC. Morphometry of pig coronary venous system. Am J Physiol Heart Circ Physiol. 1994;267:H2100–H2113. doi: 10.1152/ajpheart.1994.267.6.H2100. [DOI] [PubMed] [Google Scholar]
- 26.Thomas D, Xie F, Smith LM, O’Leary E, Smith K, Olson J, Nalty K, Hess R, Graham M, Therrien S, Porter TR. Prospective Randomized Comparison of Conventional Stress Echocardiography and Real-Time Perfusion Stress Echocardiography in Detecting Significant Coronary Artery Disease. J Am Soc Echocardiogr. 2012;25:1207–14. doi: 10.1016/j.echo.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Masugata H, Lafitte S, Peters B, Strachan GM, DeMaria AN. Comparison of real-time and intermittent triggered myocardial contrast echocardiography for quantification of coronary stenosis severity and transmural perfusion gradient. Circulation. 2001;104:1550–1556. doi: 10.1161/hc3801.095694. [DOI] [PubMed] [Google Scholar]
- 28.Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S. Noninvasive Quantification of Coronary Blood Flow Reserve in Humans Using Myocardial Contrast Echocardiography. Circulation. 2001;103:2560–5. doi: 10.1161/01.cir.103.21.2560. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab. 2007;293:E1250–E1255. doi: 10.1152/ajpendo.00451.2007. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free Fatty Acids Induce Insulin Resistance in Both Cardiac and Skeletal Muscle Microvasculature in Humans. J Clin Endocrinol Metab. 2011;96:438–46. doi: 10.1210/jc.2010-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peltier M, Vancraeynest D, Pasquet A, Taniyel RV, D’hondt AM, Melin JA, Vanoverschelde JL. Assessment of the physiologic significance of coronary disease with dipyridamole real-time myocardial contrast echocardiography: Comparison with technetium-99m sestamibi single-photon emission computed tomography and quantitative coronary angiography. J Am Coll Cardiol. 2004;43:257–64. doi: 10.1016/j.jacc.2003.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Jeetley P, Hickman M, Kamp O, Lang RM, Thomas JD, Vannan MA, Vanovershelde JL, van der Wouw PA, Senior R. Myocardial Contrast Echocardiography for the Detection of Coronary Artery Stenosis: A Prospective Multicenter Study in Comparison With Single-Photon Emission Computed Tomography. J Am Coll Cardiol. 2006;47:141–5. doi: 10.1016/j.jacc.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 33.Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiogensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293:E1804–E1809. doi: 10.1152/ajpendo.00498.2007. [DOI] [PubMed] [Google Scholar]
- 34.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic Hyperinsulinemia Enhances Human Skeletal Muscle Perfusion by Capillary Recruitment. Diabetes. 2001;50:2682–90. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 35.Vincent MA, Dawson D, Clark ADH, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal Muscle Microvascular Recruitment by Physiological Hyperinsulinemia Precedes Increases in Total Blood Flow. Diabetes. 2002;51:42–8. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- 36.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 37.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular Recruitment Is an Early Insulin Effect That Regulates Skeletal Muscle Glucose Uptake In Vivo. Diabetes. 2004;53:1418–23. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 38.Bin JP, Le DE, Jayaweera AR, Coggins MP, Wei K, Kaul S. Direct effects of dobutamine on the coronary microcirculation: comparison with adenosine using myocardial contrast echocardiography. J Am Soc Echocardiogr. 2003;16:871–9. doi: 10.1067/S0894-7317(03)00423-1. [DOI] [PubMed] [Google Scholar]
- 39.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290:E1191–E1197. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- 40.Majid P, Cheirif J, Rokey R, Sanders WE, Patel B, Zimmerman JL, Dellinger RP. Does cocaine cause coronary vasospasm in chronic cocaine abusers? A study of coronary and systemic hemodynamics. Clin Cardiol. 1992;15:253–8. doi: 10.1002/clc.4960150407. [DOI] [PubMed] [Google Scholar]
- 41.Hale SL, Alker KJ, Rezkalla S, Figures G, Kloner RA. Adverse effects of cocaine on cardiovascular dynamics, myocardial blood flow, and coronary artery diameter in an experimental model. Am Heart J. 1989;118:927–33. doi: 10.1016/0002-8703(89)90226-3. [DOI] [PubMed] [Google Scholar]
- 42.Abel F, Wilson S, Shao R, Fennell W. Cocaine depresses the canine myocardium. Circ Shock. 1989;81:309–12. [PubMed] [Google Scholar]
- 43.Fraker TD, Temesy-Armos PN, Brewster PS, Wilkerson RD. Mechanism of cocaine-induced myocardial depression in dogs. Circulation. 1990;81:1012–6. doi: 10.1161/01.cir.81.3.1012. [DOI] [PubMed] [Google Scholar]
- 44.Miao L, Nunez B, Susulic V, Wheeler S, Carrozza JP, Ross JN, Morgan JP. Cocaine-induced microvascular vasoconstriction but differential systemic haemodynamic responses in Yucatan versus Yorkshire varieties of swine. Br J Pharmacol. 1996;117:559–65. doi: 10.1111/j.1476-5381.1996.tb15227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunez B, Miao L, Carrozza J, Ross J, Douglas P, Gordon P, Katz SE, Kuntz R, Morgan JP. Cocaine-induced transmural myocardial infarction in a Yorkshire swine with normal coronary arteries: Evidence for microvascular and/or epicardial coronary artery spasm. Cardiovasc Pathol. 1994;3:93–7. doi: 10.1016/1054-8807(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 46.Zimring HJ, Fitzgerald RL, Engler RL, Ito BR. Intracoronary versus intravenous effects of cocaine on coronary flow and ventricular function. Circulation. 1994;89:1819–28. doi: 10.1161/01.cir.89.4.1819. [DOI] [PubMed] [Google Scholar]
- 47.Oster ZH, Som P, Wang GJ, Weber DA. Imaging of Cocaine-Induced Global and Regional Myocardial Ischemia. J Nucl Med. 1991;32:1569–72. [PubMed] [Google Scholar]
- 48.Kuhn F, Johnson M, Gillis R, Visner M, Schaer G. Effect of cocaine on the coronary circulation and systemic hemodynamics in dogs. J Am Coll Cardiol. 1990;16:1491. doi: 10.1016/0735-1097(90)90396-7. [DOI] [PubMed] [Google Scholar]
- 49.Shannon RP, Mathier MA, Shen YT. Coronary vascular responses to short-term cocaine administration in conscious baboons compared with dogs. J Am Coll Cardiol. 2000;35:1347–54. doi: 10.1016/s0735-1097(00)00547-7. [DOI] [PubMed] [Google Scholar]
- 50.Boehrer JD, Moliterno DJ, Willard JE, Snyder RW, 2nd, Horton RP, Glamann DB, Lange RA, Hillis LD. Hemodynamic effects of intranasal cocaine in humans. J Am Coll Cardiol. 1992;20:90–3. doi: 10.1016/0735-1097(92)90142-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


