Abstract
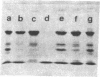
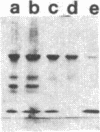
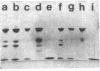
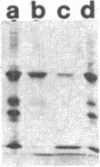
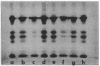
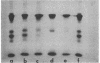
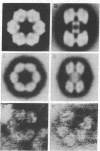
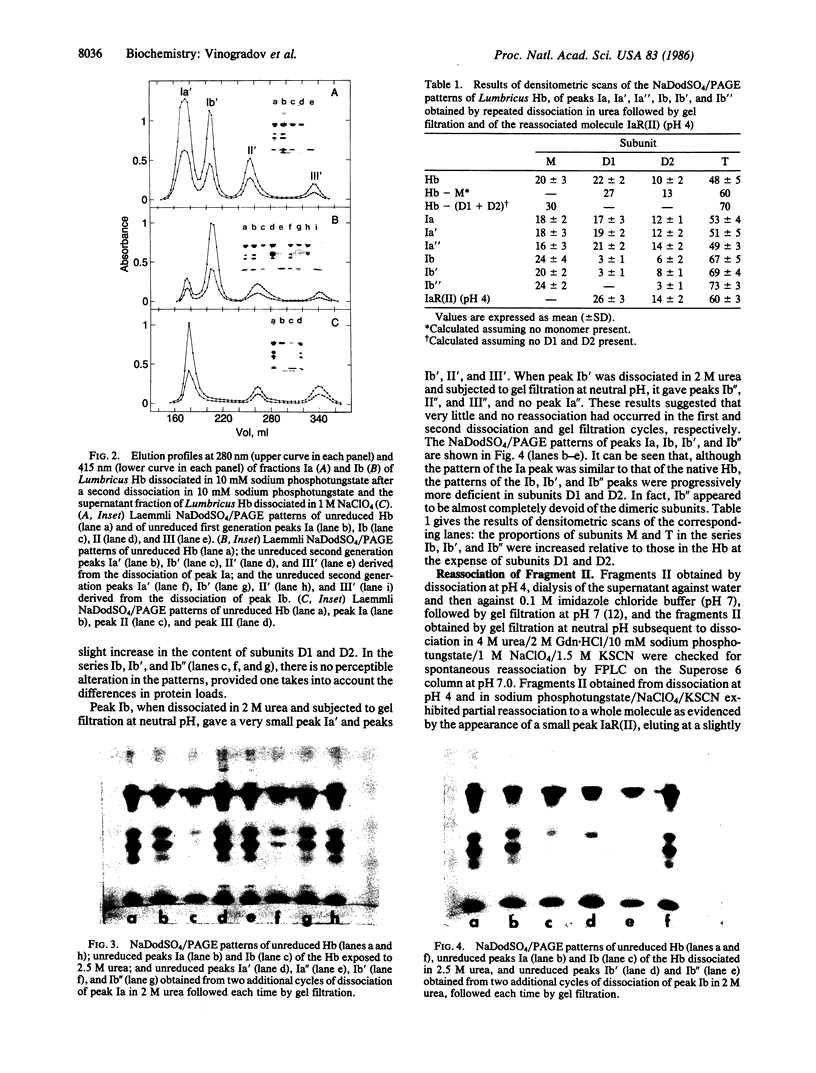
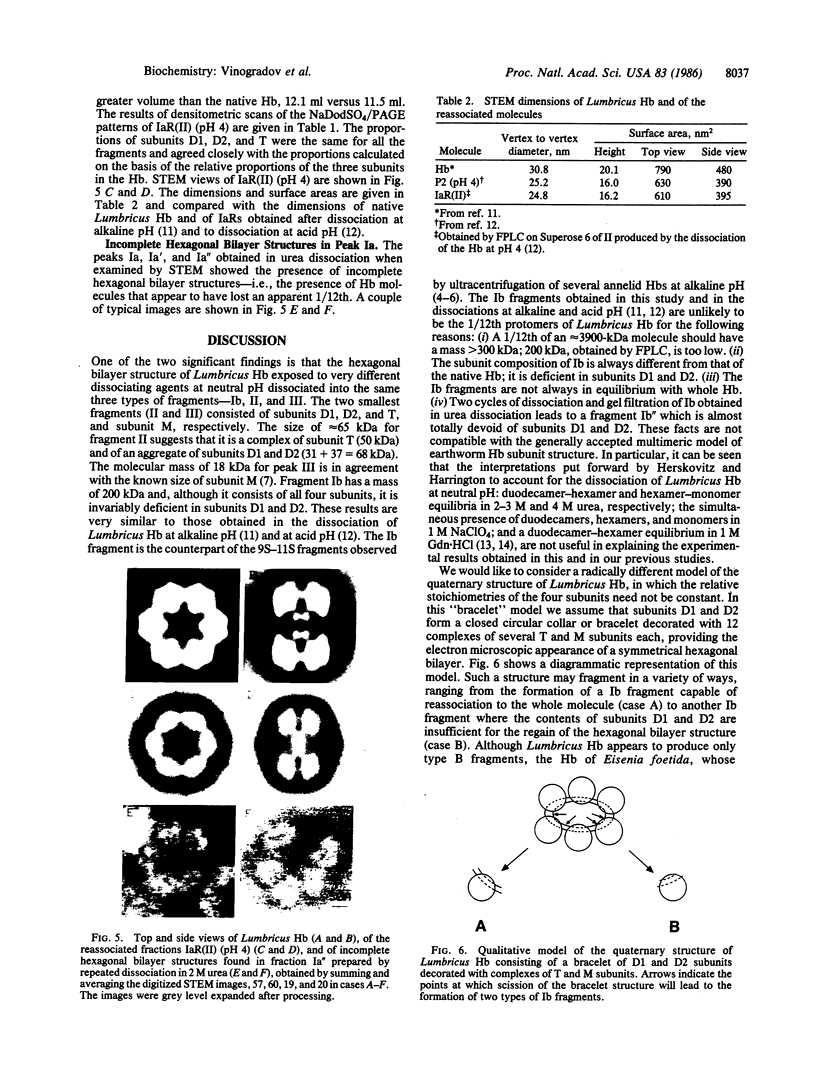
The complete dissociation of the hexagonal bilayer structure of Lumbricus terrestris hemoglobin (3900 kDa) at neutral pH, in the presence of urea, guanidine hydrochloride, sodium perchlorate, potassium thiocyanate, sodium phosphotungstate, and sodium phosphomolybdate, followed by gel filtration at neutral pH on Sephacryl S-200 or Superose 6, produced two fragments, II (65 kDa) and III (17 kDa); NaDodSO4/polyacrylamide gel electrophoresis showed that peak II consisted of subunits D1 (31 kDa, chain V), D2 (37 kDa, chain VI), and T (50 kDa, disulfide-bonded trimer of chains II, III, and IV) and that peak II consisted of subunit M (16 kDa, chain I). When dissociation was incomplete, two additional peaks were present, peak Ia eluting at the same volume as the whole hemoglobin and peak Ib (200 kDa). Scanning transmission electron micrographs of peak Ia showed it to consist of whole molecules and of incomplete hexagonal bilayer structures, missing an apparent 1/12th. Peak Ib contained all four subunits but was usually deficient in subunits D1 and D2, was not always in equilibrium with the whole molecule, and could be dissociated further into II and III. The patterns of dissociation observed at neutral pH were very similar to those observed previously at alkaline pH and at acid pH and appear to be incompatible with the generally accepted multimeric model of Lumbricus hemoglobin subunit structure. A model is proposed in which it is postulated that the stoichiometries of some of the subunits need not be constant and that subunits D1 and D2 either form a "bracelet" decorated with complexes of T and M subunits or serve as "linkers" between the latter, to provide the appearance of a two-tiered hexagonal structure. Additional support for the proposed model comes from observations that the fragment II obtained subsequent to dissociation at pH 4, in sodium phosphotungstate, in sodium perchlorate, and in potassium thiocyanate was found to be in equilibrium with a hexagonal bilayer structure IaR(II), whose dimensions were approximately equal to 20% smaller than those of the native hemoglobin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Chiancone E. Assembly of multisubunit respiratory proteins. Annu Rev Biophys Bioeng. 1977;6:239–271. doi: 10.1146/annurev.bb.06.060177.001323. [DOI] [PubMed] [Google Scholar]
- Chung M. C., Ellerton H. D. The physico-chemical and functional properties of extracellular respiratory haemoglobins and chlorocruorins. Prog Biophys Mol Biol. 1979;35(2):53–102. doi: 10.1016/0079-6107(80)90003-6. [DOI] [PubMed] [Google Scholar]
- Crewe A. V. High-resolution scanning transmission electron microscopy. Science. 1983 Jul 22;221(4608):325–330. doi: 10.1126/science.6867711. [DOI] [PubMed] [Google Scholar]
- Frossard P. The erythrocruorin of Eisenia fetida. I. Properties and subunit structure. Biochim Biophys Acta. 1982 Jun 24;704(3):524–534. doi: 10.1016/0167-4838(82)90076-0. [DOI] [PubMed] [Google Scholar]
- Frossard P. The erythrocruorin of Eisenia fetida. II. Properties of the principal subunit. Biochim Biophys Acta. 1982 Jun 24;704(3):535–541. doi: 10.1016/0167-4838(82)90077-2. [DOI] [PubMed] [Google Scholar]
- Harrington J. P., Herskovits T. T. The effects of salts on the subunit structure and dissociation of Lumbricus terrestris hemoglobin. Biochemistry. 1975 Nov 4;14(22):4972–4976. doi: 10.1021/bi00693a028. [DOI] [PubMed] [Google Scholar]
- Herskovits T. T., Harrington J. P. Solution studies on heme proteins: subunit structure, dissociation, and unfolding of Lumbricus terrestris hemoglobin by the ureas. Biochemistry. 1975 Nov 4;14(22):4964–4971. doi: 10.1021/bi00693a027. [DOI] [PubMed] [Google Scholar]
- Kapp O. H., Polidori G., Mainwaring M. G., Crewe A. V., Vinogradov S. N. The reassociation of Lumbricus terrestris hemoglobin dissociated at alkaline pH. J Biol Chem. 1984 Jan 10;259(1):628–639. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mainwaring M. G., Lugo S. D., Fingal R. A., Kapp O. H., Vinogradov S. N. The dissociation of the extracellular hemoglobin of Lumbricus terrestris at acid pH and its reassociation at neutral pH. A new model of its quaternary structure. J Biol Chem. 1986 Aug 15;261(23):10899–10908. [PubMed] [Google Scholar]
- Shlom J. M., Vinogradov S. N. A study of the subunit structure of the extracellular hemoglobin of Lumbricus terrestris. J Biol Chem. 1973 Nov 25;248(22):7904–7912. [PubMed] [Google Scholar]
- Vinogradov S. N., Shlom J. M., Hall B. C., Kapp O. H., Mizukami H. The dissociation of Lumbricus terrestris hemoglobin: a model of its subunit structure. Biochim Biophys Acta. 1977 May 27;492(1):136–155. doi: 10.1016/0005-2795(77)90221-5. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. N. The structure of invertebrate extracellular hemoglobins (erythrocruorins and chlorocruorins). Comp Biochem Physiol B. 1985;82(1):1–15. doi: 10.1016/0305-0491(85)90120-8. [DOI] [PubMed] [Google Scholar]