Abstract
Some species of Talaromyces secrete large amounts of red pigments. Literature has linked this character to species such as Talaromyces purpurogenus, T. albobiverticillius, T. marneffei, and T. minioluteus often under earlier Penicillium names. Isolates identified as T. purpurogenus have been reported to be interesting industrially and they can produce extracellular enzymes and red pigments, but they can also produce mycotoxins such as rubratoxin A and B and luteoskyrin. Production of mycotoxins limits the use of isolates of a particular species in biotechnology. Talaromyces atroroseus sp. nov., described in this study, produces the azaphilone biosynthetic families mitorubrins and Monascus pigments without any production of mycotoxins. Within the red pigment producing clade, T. atroroseus resolved in a distinct clade separate from all the other species in multigene phylogenies (ITS, β-tubulin and RPB1), which confirm its unique nature. Talaromyces atroroseus resembles T. purpurogenus and T. albobiverticillius in producing red diffusible pigments, but differs from the latter two species by the production of glauconic acid, purpuride and ZG–1494α and by the dull to dark green, thick walled ellipsoidal conidia produced. The type strain of Talaromyces atroroseus is CBS 133442
Introduction
Monascus species are known to produce six major azaphilone pigments being the yellow monascin and ankaflavin; the orange monascorubrin and rubropunctatin and the red monascorubramine and rubropunctamine, in addition to more than 20 related pigments [1,2]. Another azaphilone series of yellow pigments is even more widespread in Talaromyces, i.e. the mitorubrins [3–5]. The red pigment producer Monascus purpureus has been used primarily in Southern China, Japan and Southeast Asia for making red rice wine, red soybean cheese and Anka (red rice) [6]. A problem is that some samples of Monascus–fermented rice have been found to contain the mycotoxin citrinin [7], but also that Monascus isolates also often produce mevinolin, a drug that is also unwanted in foods [2]. The production of such mycotoxins and drugs limits the use of Monascus for industrial purposes, but since citrinin has not been found in any Talaromyces species, the latter may be a good alternative for red pigment production. Studies have shown that polyketide azaphilone Monascus red pigments and/or their amino acid derivatives are naturally produced by Talaromyces aculeatus, T. pinophilus, T. purpurogenus and T. funiculosus [8,9]. Talaromyces amestolkiae, T. ruber and T. stollii also produce azaphilone polyketides, as recently described by Yilmaz et al. [10], but in those three species the pigment are not diffusing into the growth medium. Talaromyces amestolkiae and T. stollii were isolated from immuno-compromised patients and are potential human pathogens, while T. purpurogenus produces mycotoxins such as rubratoxins A and B, rugulovasins, and luteoskyrin [10]. These factors limit the use of these species for biotechnological production of azaphilone pigments.
In the current study we describe a new Talaromyces species, T. atroroseus, which secretes large amounts of Monascus red pigments, without the production of any known mycotoxins.
Materials and Methods
Strains
Cultures were obtained from the CBS-KNAW Fungal Biodiversity Centre culture collection, Utrecht, the Netherlands. Fresh isolates deposited in the working collection of the Department of Applied and Industrial Mycology (DTO) housed at CBS, and strains from the IBT collection at DTU Systems Biology in Kongens Lyngby, Denmark were also included in this study. Strains are listed in Table 1. KAS strain numbers are from the fungal collection of Keith A. Seifert, Ottawa, Canada.
Table 1. Strains used in this study of Talaromyces atroroseus and related species.
| CBS No. | Other Collection No. | Species | Information and Origin |
|---|---|---|---|
| 206.89 | IFO 6580, IBT 3960; DTO 41F4 | T. albobiverticillius | Unknown, Japan |
| 238.95 | IBT 11181, CBS 123796 | T. atroroseus | Red sweet bell pepper, Kgs. Lyngby, Denmark |
| 234.60 | DTO 37A4 | T. atroroseus | Unknown, Germany |
| 257.37 | DTO 37A3 | T. atroroseus | Ex air in nitrite factory, Germany |
| 313.63 | DTO 41G2 | T. albobiverticillius | Vitis vinifera fruit, South Africa |
| 364.48 | ATCC 9777, IMI 040037, NRRL 1061, | T. atroroseus | Unknown, Darien, Manchuria, China |
| QM 6760, DTO 178A3, IBT 4458, IBT 11180 | |||
| 391.96 | DTO 41G8 | T. atroroseus | Unknown, Tanzania |
| 113139 | IBT 3967, NRRL 1147, DTO 177I2 | T. atroroseus | Unknown, USA |
| 113167 | DTO 39I2, DTO 39I3 | T. albobiverticillius | Unknown, unknown |
| 113168 | IBT 31347, DTO 39H9, DTO 177I9 | T. albobiverticillius | Sputum of patient, male, Copenhagen, |
| Denmark | |||
| 113153 | IBT 3458, NRRL 1136, DTO 37A7 | T. atroroseus | Ex mixed culture, Arlington Farm, Virginia |
| USA | |||
| 124294 | IBT 23082 | T. atroroseus | Tropical rainforest, Peru |
| 133440 | BCRC 34774, DTO 166E5, IBT 31667 | Type of T. albobiverticillius | Decaying leaves of a broad–leaved tree, Taiwan |
| 133441 | BCRC 34775, DTO 166E6, IBT 31668 | T. albobiverticillius | Decaying leaves of a broad–leaved tree. Taiwan |
| 133442 | KAS 3778, DTO 178A4, IBT 32470 | Type of T. atroroseus | House dust, South Africa |
| 133443 | IBT 29388, DTO 189D4 | T. atroroseus | Contamination in petri dish, Lyngby, Denmark |
| 133444 | IMI 163167, IBT 23702, DTO 189C2 | T. albobiverticillius | Punica granata, unknown |
| 133447 | DTO 81I2 | T. atroroseus | Swab sample from cheese warehouse, |
| the Netherlands | |||
| 133448 | DTO 157G5 | T. albobiverticillius | Pomegranate, Turkey |
| 133449 | IBT 29464, DTO 189D5 | T. atroroseus | Mouse dung, Høve Strand, Denmark |
| 133450 | FRR 75, IBT 4454, DTO 188I9 | T. atroroseus | Soil Murrumbidgee irrigation Area, |
| New South Wales, Australia | |||
| 133452 | NRRL 2120, IBT 3547, DTO 193H9 | T. albobiverticillius | Cotton duck, Panama |
| 113154 | R.B., IMI 090178, NRRL 1214, IBT 3645, | T. atroroseus | ”Parasite” in Aspergillus niger culture, |
| IBT 4428, CBS 127571 | Kansas City, Missouri, USA | ||
| TA85S-28-H2, AZ ,IAM 15392, JCM 23216, IBT 32650 | T. atroroseus | Soil, Thailand | |
| IBT 20955 | T. atroroseus | Air root in white mangrove, | |
| Can de Aruca, Paria Bay, Venezuela | |||
| IBT 4466 | T. albobiverticillius | Punica granata, imported to Denmark |
Morphological analysis
Macroscopic characters were studied on agar media Czapek-Dox yeast autolysate agar (CYA), CYA supplemented with 5 % NaCl (CYAS), yeast extract sucrose agar (YES), creatine sucrose agar (CREA), dichloran 18 % glycerol agar (DG18), oatmeal agar (OA) and malt extract agar (Oxoid) (MEA). The isolates were also tested on CYA at 37 °C and on Blakeslee malt extract agar (MEA2). All media were prepared as described by Samson et al. [11]. The strains were inoculated in three points onto media in 90-mm Petri dishes and incubated for 7 d at 25 °C in darkness. After incubation, the colony diameters on the various agar media were measured. Colonies were photographed with a Canon EOS 400D. Species were characterized microscopically by preparing slides from MEA. Lactic acid was used as mounting fluid. Specimens were examined using a Zeiss AxioSkop2 plus microscope.
DNA extraction, PCR amplification and sequencing
Strains were grown for 7 to 14 d on MEA prior to DNA extraction. DNA was extracted using the UltracleanTM Microbial DNA isolation Kit (MoBio, Solana Beach, U.S.A.). The extracted DNA was stored at -20 °C. The ITS regions, regions of the β-tubulin and RPB1 genes were amplified and sequenced according to methods previously described [12–15].
Data analysis
Sequence contigs were assembled using Seqman from DNAStar Inc. Newly generated ITS, β-tubulin and RPB1 sequences were included in a data set obtained from the Samson et al. [15] study. Data sets were aligned using Muscle software within MEGA5 [16]. Neighbour–joining analysis on the individual data sets was performed in MEGA5 and confidence in nodes determined using bootstrap analysis with 1000 replicates. Talaromyces galapagensis (CBS 751.74T) was selected as a suitable out-group in all the phylogenies. The newly generated sequences were deposited in GenBank (accession numbers, see Table 1 and Figures 1–3).
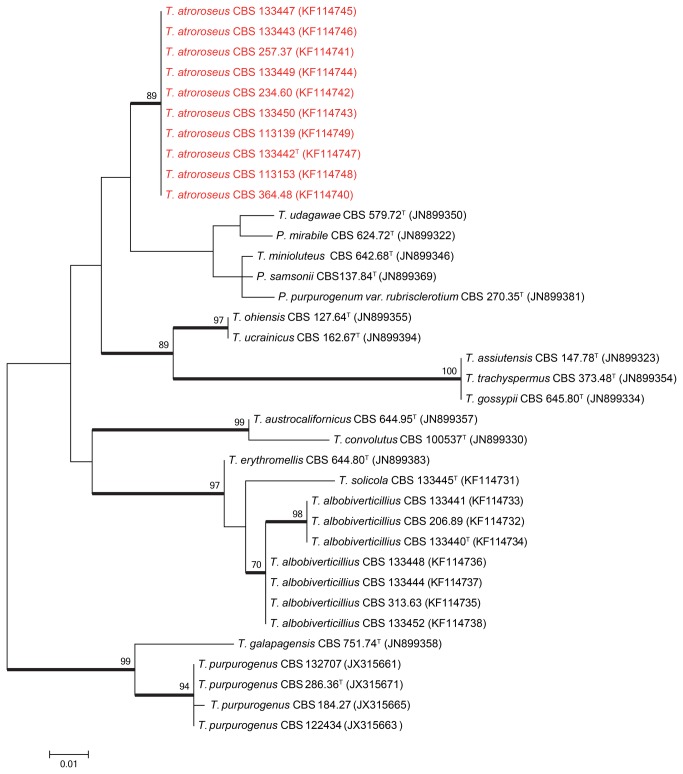
Figure 1. Maximum likelihood tree comparing the ITS gene region of Talaromyces species closely related to T. atroroseus.
Talaromyces galapagensis and T. purpurogenus were used as outgroup. Support in nodes is indicated above thick branches and is represented by bootstrap values higher than 70%. GenBank accession numbers are given between brackets, (T = ex-type). Red coloured names indicate T. atroroseus strains.
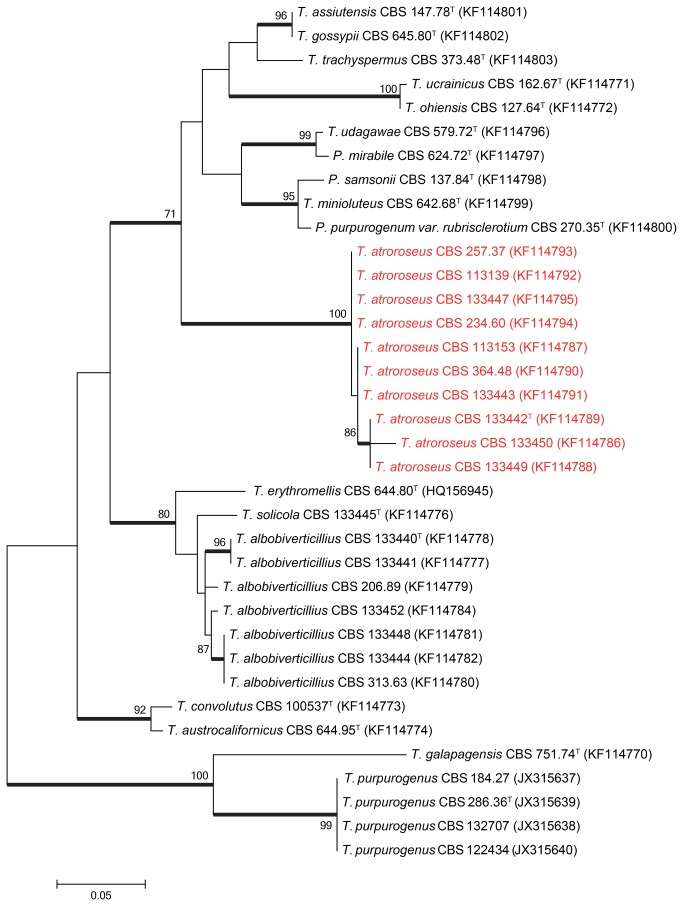
Figure 2. Maximum likelihood tree comparing the β–tubulin gene region of Talaromyces species closely related to T. atroroseus.
Talaromyces galapagensis and T. purpurogenus were used as outgroup. Support in nodes is indicated above thick branches and is represented by bootstrap values higher than 70%. GenBank accession numbers are given between brackets, (T = ex-type). Red coloured names indicate T. atroroseus strains.
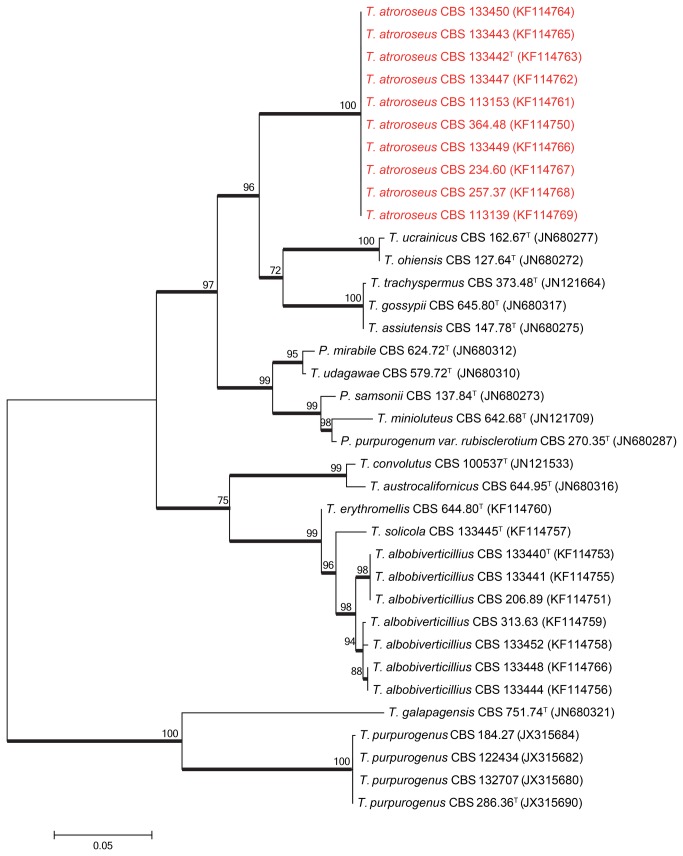
Figure 3. Maximum likelihood tree comparing the RPB1 gene region of Talaromyces species closely related to T. atroroseus.
Talaromyces galapagensis and T. purpurogenus were used as outgroup. Support in nodes is indicated above thick branches and is represented by bootstrap values higher than 70%. GenBank accession numbers are given between brackets, (T = ex-type). Red coloured names indicate T. atroroseus strains.
Extrolites
Cultures grown on CYA and YES for 7 d at 25 °C were used for extrolite extractions. Extracts were analysed by HPLC using alkylphenone retention indices and diode array UV–VIS detection as described by 17–19, using three 6 mm agar plugs. Standards of extrolites from the collection at DTU Systems Biology (Denmark) were used to compare the extrolites from the species under study [18].
The extrolite extractions from T. atroroseus CBS 133450, CBS 113154 and CBS 123796 were also analysed by ultra high performance liquid chromatography high-resolution mass spectrometry (UHPLC-HRMS). Liquid chromatography was performed on an Agilent 1290 Infinity LC system with a DAD-detector coupled to an Agilent 6550 iFunnel Q-TOF with an electrospray ionization source. The separation was performed on a 2.1 x 250 mm, 2.7 μm Poroshell 120 Phenyl-Hexyl column (Agilent) at 60 °C with a water-acetonitrile gradient (both with 20 mM formic acid) going from 10 % (vol/vol) to 100 % acetonitrile in 15 min followed by 2.5 min with 100 % acetonitrile and then returning to the start conditions for 2.5 min for equilibration before next sample. All time the flow rate was kept at 0.35 mL/min. HRMS was performed in ESI+ and extrolites were identified with targeted search on accurate mass of [M+H]+ and [M+Na]+ using Agilent MassHunter Qualitative Analysis B.06.00 software and a database of potential extrolites in T. atroroseus with support from UV-VIS spectra. The list of compounds searched for including the extrolite standards can be found in Table S1.
Nomenclature
1. The electronic version of this article in Portable Document Format (PDF) in a work with an ISSN or ISBN will represent a published work according to the International Code of Nomenclature of algae, fungi, and plants, and hence the new names contained in the electronic publication of a PLOS ONE article are effectively published under that Code from the electronic edition alone, so there is no longer any need to provide printed copies. In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Name Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix http://www.mycobank.org/MB. The online version of this work is archived and available from the following digital repositories. PubMed Central, LOCKSS.
2. Repository of Talaromyces atroroseus Yilmaz, Frisvad, Houbraken & Samson 2013 sp. nov. [urn:lsid:mycobank.org: 804901]
Results and Discussion
The relationship between the Talaromyces atroroseus sp. nov. and its close relatives were studied using multigene phylogenies, bason on ITS, RPB1 and β-tubulin sequences. The aligned datasets were 482, 888 and 374 bp long, respectively. The new species resolved in a clade together with other red pigment producing species such as T. albobiverticillius, and T. minioluteus. Talaromyces purpurogenus resolved in a distantly related clade (Figures 1–3). Within the red pigment producing clade, T. atroroseus resolved in a distinct clade separate from all the other species in all three phylogenies, confirming its unique nature.
Historically red pigment production caused a lot of confusion and resulted in numerous misidentifications in literature. This is especially true for Talaromyces purpurogenus, T. ruber, Penicillium sanguineum and P. crateriforme. Penicillium purpurogenum and P. rubrum were described by Stoll [20]. In their monograph Raper and Thom [21] also described P. purpurogenum and P. rubrum. No type material was available for P. rubrum therefore Raper and Thom [21] used two strains to describe P. rubrum, NRRL 1062 (= CBS 370.48) and NRRL 2120 (= CBS 133452). Pitt [22] synonymized P. rubrum, P. crateriforme and P. sanguineum with P. purpurogenum. The issues in the T. purpurogenus complex were clarified by Yilmaz et al. [10] who synonymized Penicillium crateriforme and P. sanguineum with T. purpurogenus and they described T. ruber as a distinct species. NRRL 1062 remained as T. ruber but NRRL 2120 (= CBS 133452) is a different species than T. ruber. Our results showed that NRRL 2120 is T. albobiverticillius. Raper and Thom [21] based the Penicillium purpurogenum description on NRRL 1061 (= CBS 364.48). However our results show that NRRL 1061 is a typical T. atroroseus strain.
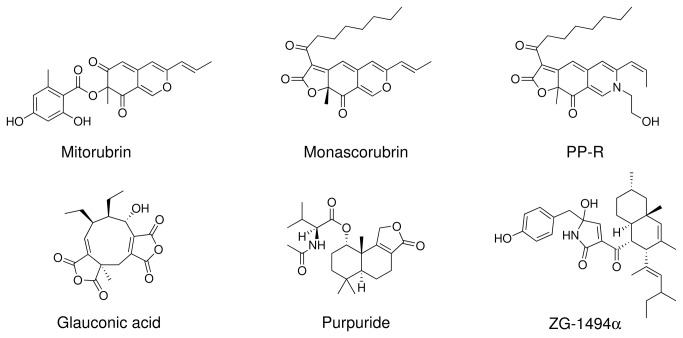
Both Talaromyces purpurogenus and T. atroroseus are common in soil, indoor environments, and fruits. Talaromyces atroroseus resembles T. purpurogenus and T. albobiverticillius in producing red diffusible pigments, but differs from the latter two species by the production of glauconic acid, purpuride and ZG–1494α (Table 2 and Figure 4) and by the dull to dark green thick walled ellipsoidal conidia produced. Barton et al. [26,27] and Barton and Sutherland [28] reported glauconic acid from P. purpurogenum IMI 090178, which in the present study has been re-identified as T. atroroseus, while ZG–1494α was reported from P. rubrum CBS 238.95 [36], which is also a typical T. atroroseus. Talaromyces atroroseus, T. purpurogenus and T. albobiverticillius differ from T. ruber, T. amestolkiae and T. stollii by their production of red diffusible pigment. In Table 3 many red pigment producers identified as Penicillium species are listed, that may either be T. purpurogenus, T. ruber, T. albobiverticillius or T. atroroseus. The strains listed in Table 3 were not available for us, so their exact identity cannot be verified.
Table 2. Reported extrolite production by strains verified as Talaromyces atroroseus during this study.
| Extrolite | Reported producer | Culture collection numbers | Reference |
|---|---|---|---|
| Glaucanic acid, Glauconic acid | Penicillium “R. B.”, P. purpurogenum | R.B. = IMI 090178 = NRRL 1214 = CBS 113154 = IBT 3645 = IBT 4428 | [23–30] |
| N-glutarylmonascorubramine, N-glutarylrubropunctamine | P. purpurogenum | IBT 11181 = CBS 238.95 = CBS 123796 | [9] |
| N-glutarylmonascorubramine | P. purpurogenum | R.B. = IMI 090178 = NRRL 1214 = CBS 113154 = IBT 3645 = IBT 4428 | [9] |
| Monascorubramine, PP-R | P. purpurogenum | IBT 11180 = CBS 364.48 = ATCC 9777 = IMI 040037 = NRRL 1061 = QM 6760 = IBT 4458 | [9] |
| PP-V, PP-R, PP-O, PP-Y | P. sp. | TA85S-28-H2 = AZ = IAM 15392 = JCM 23216 = IBT 32650 | [43–47] |
| Purpuride | P. purpurogenum | CBS 257.37 | [31] |
| Purpurogenone, Deoxypurpurogenone | P. purpurogenum | CBS 257.37 | [32–35] |
| ZG-1494α | P. rubrum | IBT 11181 = CBS 238.95 = CBS 123796 | [36] |
Strain numbers in bold are the strain numbers used in the references.
Figure 4. Structures of some of the most characteristic compounds produced by Talaromyces atroroseus.
All six compounds were detected in this study.
Table 3. Reported extrolite production from strains potentially belonging to Talaromyces atroroseus, but not examined during this study.
| Extrolite | Reported producer | Strain identifier / Culture collection number | Reference |
|---|---|---|---|
| 2,6,7-trihydroxy-3-methyl-naphthalene-1,4-dione | Penicillium purpurogenum | JS03-21* | [37] |
| BE-25327 | P. purpurogenum | F25327 = FERM P-12345 | [38] |
| Dhilirolide A, B, C, D | P. purpurogenum | IMI 357108 | [39] |
| Glauconic acid | P. glaucum | -* | [40] |
| Gluconic acid | P. purpurogenum var. rubrisclerotium (= T. pinophilus) | No. 2670 = NRRL 1064 = CBS 270.35 = ATCC 4713 = ATCC 52224 = NRRL 1142 = IBT 4302 | [41] |
| (-)-Mitorubrin, | P. purpurogenum | JS03-21* | [37] |
| Monascus red pigment | P. sp. | HKUCC 8070 | [42] |
| Orsellinic acid | P. purpurogenum | JS03-21* | [37] |
| Purpactin A, B, C | P. purpurogenum | FO-608 = FERM P-10776 | [48,49] |
| Purpurester A, B | P. purpurogenum | JS03-21* | [37] |
| Purpurquinones A, B, C | P. purpurogenum | JS03-21* | [37] |
| Red W59 (C30H34O9N3) | P. purpurogenum | -* | [50] |
| Red pigment | P. purpurogenum | GH2* | [51–53] |
| Red pigment | P. purpurogenum | SX01* | [54] |
| Red pigments | P. purpurogenum | DPUA 1275 | [56,57] |
| Red pigments | P. purpurogenum | -* | [58,59] |
| Red pigments | P. sp. | -* | [60] |
| SL 3238 (C27H41NO7) | P. purpurogenum | NRRL 3364 | [55] |
| TAN-931 | P. purpurogenum | JS03-21* | [37] |
Based on the reported morphology and extrolites the strains in the table are by the authors’ judgement belonging to Talaromyces atroroseus or a closely related species.
* Strain not deposited in any accessible culture collection
Many Talaromyces species produce striking diffusing red pigments, especially T. purpurogenus, T. atroroseus, T. albobiverticillius, T. minioluteus, and T. marneffei. These red pigments are typically composed of the azaphilone pigments (Figure 5) monascorubrin, rubropunctatin, threonine derivative of rubropunctatin, monascorubramine, PP-R (= 7–(2–hydroxyethyl)-monascorubramine), rubropunctamine, N-glutarylrubropunctamine, and PP–V [8,9,43,44,61]. The same family of azaphilones are also known from red rice, where different species of Monascus have grown [1,2]. These red pigments are of interest for the industry as they are stable and non-toxic and can be used as food colorants [62]. The azaphilone pigments can react with amino acids, hence their name, and give intense dark red colours. In addition some of these species produce yellow azaphilone pigments, such as monascin, ankaflavin, monascusone A and B, xanthomonascin A, and another series of yellow mitrorubrin azaphilones: mitorubrin, mitorubrinol, mitorubrinol acetate, mitorubrinic acid, and many other related compounds [5]. Many of these pigments have been reported from or found in T. atroroseus in this study (Table 2 and Table 4). The potential for pigment production has in this study only been investigated in small scale on solid media; however, T. atroroseus also produce pigments in liquid cultures under the right conditions [8,46]. The potential for up scaling the production of red pigments needs to be investigated thoroughly.
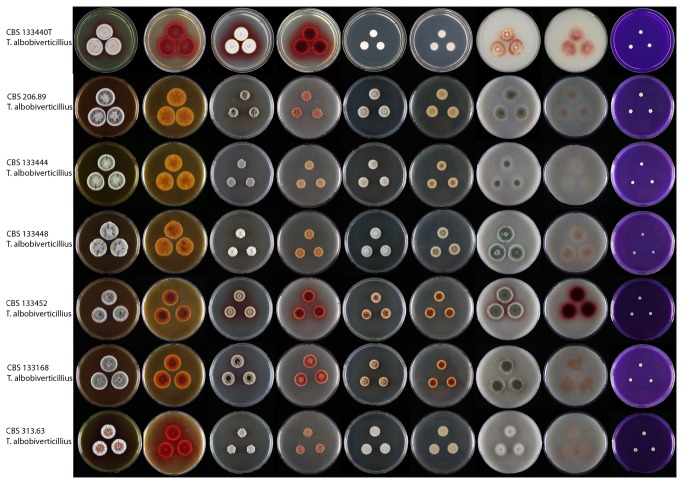
Figure 5. Strains of Talaromyces albobiverticillius on MEA, CYA, DG18, OA and CREA.
Colony obverse and reverse is shown for the first four media and obverse for CREA.
Table 4. Extrolites of Talaromyces atroroseus and T. albobiverticillius as examined by HPLC-DAD and/or UHPLC- HRMS and comparison to standards on the media CYA and YES.
| Species | Culture collection number | Extrolites * found |
|---|---|---|
| T. atroroseus | CBS 133450a | glauconic acidb, monascorubrinb, PP-Rb, purpurideb, purpuroquinone Ab, ZG-1494αb |
| CBS 113154a | glauconic acid, N-glutarylmonascorubramineb, monascorubrinb, PP-Ob, PP-Rb, purpurideb, purpuroquinone Ab, ZG-1494αb | |
| CBS 123796a | FK17-P2b2b, glauconic acid, N-glutarylmonascorubramineb, mitorubrin, mitorubrinol, monascorubrinb, PP-Ob, PP-Rb, purpurideb, purpuroquinone Ab, purpurogenone, ZG-1494αb | |
| CBS 257.37 | monascorubramine, purpuride, several Monascus-red pigments | |
| CBS 234.60 | glauconic acid, monascorubramine, purpuride, ZG-1494α | |
| CBS 391.96 | glauconic acid, monascorubramine, purpuride, ZG-1494α | |
| CBS 364.48 | glauconic acid, monascorubramine, PP-R, purpuride, rubropunctatin, ZG-1494α | |
| CBS 133447 | Glauconic acid, purpuride | |
| CBS 133442 | Glauconic acid, monascorubramin, purpuride, rubropunctatin | |
| CBS 113153 | glauconic acid, mitorubrin, monascorubramine, monascorubrin, purpuride | |
| CBS 113139 | monascin, monascorubramine | |
| IBT 3933 | glauconic acid, mitorubrin, monascorubramin, a purpactin | |
| IBT 20955 | glauconic acid, monascorubramine, monascorubrin, purpuride, ZG-1494α | |
| IBT 23082 | PP-R (only tested for Monascus pigments) | |
| CBS 133443 | glauconic acid, monascorubramine, purpuride | |
| CBS 133449 | glauconic acid, monascorubrin, purpuride | |
| JCM 23216 | Glauconic acid, monascorubramine, purpuride | |
| T. albobiverticillius | CBS 113168 | mitorubrin, mitorubrinic acid, monascorubramine, PP-R, rubropunctatin, vermicellin |
| CBS 313.63 | mitorubrin, monascorubramin, monascorubrin, rubropunctatin | |
| IBT 4466 | mitorubrinic acid, monascorubramine, a purpactin | |
| CBS 113167 | mitorubrin, mitorubrinic acid, monascorubrin, a purpactin | |
| CBS 133444 | mitorubrin, mitorubrinic acid, mitorubrinol | |
| CBS 133452 | mitorubrin, mitorubrinic acid, monascorubramine, rubropunctatin | |
| CBS 133441 | mitorubrin, mitorubrinic acid, monascin, monascorubramin, rubropunctatin, vermicellin |
a Strains examined by both HPLC-DAD and UHPLC- -HRMS
b Extrolites identified by UHPLC- -HRMS
* The extrolites only identified by HPLC-DAD might in some cases not be the actual metabolite but a derivative with the same chromophore and retention on the column
Even though sequence variations were observed for Talaromyces albobiverticillius strains, morphologically they were similar. Two strains used for the original description of T. albobiverticillius were received from Dr. Sung-Yuan Hsieh [63]. These included the type strain CBS 133440T and CBS 133441. These strains were isolated from soil in Taiwan and produce white conidial masses and intense soluble red pigment on various media (Figure 6). However, other freshly isolated T. albobiverticillius strains produce densely sporulating colonies and do not show any stability for red pigment production. Some of the Talaromyces albobiverticillius strains did not produce any soluble pigment such as CBS 133444 and CBS 133448. Strains that did produce red pigments include CBS 113168, and CBS 133452. On MEA only the degraded or mutated strains of T. albobiverticillius, such as CBS 133440T and CBS 313.63 produced red pigments. Micromorphologically all T. albobiverticillius strains produce long stipes (up to 380 µm) (Figure 5). Two strains of T. albobiverticillius (CBS 133440T and CBS 133441) have globose to subglobose, smooth conidia; however, the remaining strains produce ellipsoid to fusiform smooth conidia (Figure 5).
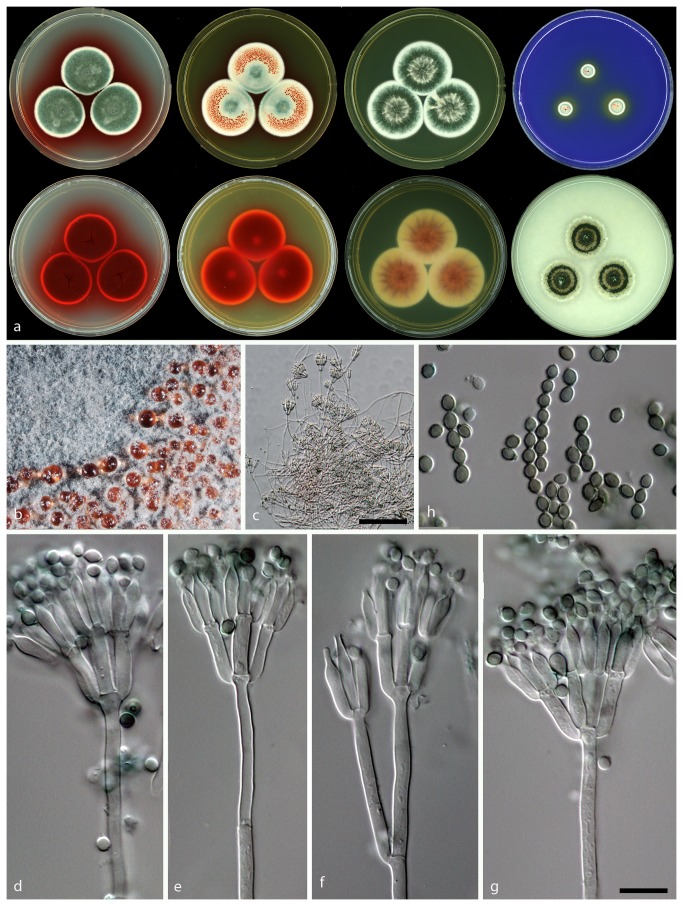
Figure 6. Morphological features of Talaromyces atroroseus sp. nov. CBS 133442.
a: Colonies incubated on CYA, CYA reverse, MEA, MEA reverse, YES, YES reverse, CREA and OA from left to right b: Colony texture on MEA, c–g: Conidiophores produced on MEA; h: Conidia. (– Scale Bar in c = 50 µm, in g = 10 µm and applies to d–h).
Even though two clades were observed in the phylogenies there are no concordance between observed clades and morphological characters as discussed above. As such, they are considered here as representing one species. Raper and Thom [21] mentioned a number of colour mutations they observed in strains of P. citrinum and P. chrysogenum. They stated that colour mutations are encountered as the most common and conspicuous types of mutations, especially considering mature conidia. Mutations can often be observed when a strain loses its green pigment in its conidia, resulting in a white or tanned colour. Colour mutants are regularly encountered among the strains which were exposed to artificial stimulations such as ultra-violet, X-ray radiations and neutron bombardment [21].
Talaromyces atroroseus is considered as the optimal producer of industrially important yellow and red soluble pigments. Another option as a suitable producer of red soluble azaphilone pigments is T. albobiverticillius. However T. albobiverticillius produces soluble red pigment only in some strains. We speculate that the mitorubrins produced by Talaromyces atroroseus are of the (-)-form, as they have been shown to be that for the closely related Talaromyces purpurogenus (at that time identified as Penicillium rubrum) [64,65]. However, Natsume et al. [66] and Suzuki et al. [67] found both (+) and (-)-forms in the genus Talaromyces, while mitorubrins in Hypoxylon and other related genera are of the (+)-form [68–70]. Although T. purpurogenus is another good producer of diffusible red azaphilone pigments, this species also produce a series of mycotoxins, such as rubratoxin A and B and luteoskyrin in addition to extrolites that may be toxic if injected intraperitoneally (spiculisporic acid) [71] or in the veins of cats (rugulovasine A and B) [72,73]. Talaromyces purpurogenus can thus not be recommended for industrial production for red pigments.
Talaromyces atroroseus Yilmaz, Frisvad, Houbraken & Samson sp. nov. Figure 6.
Mycobank MB804901 [urn:lsid:mycobank.org: 804901]
Holotype: CBS 133442 in Centraalbureau voor Schimmelcultures is designated as the holotype of Talaromyces atroroseus. It was isolated from indoor house dust, Stellenbosch, South Africa by C. Visagie in 2010.
Cultures ex type: CBS 133442 = IBT 32470 = DTO 178A4 = KAS 3778
Etymology: Named after the dark rosy diffusing azaphilone pigment mixture produced.
Diagnosis: Dark green ellipsoidal rough-walled conidia and a dark red diffusing pigment, strains of the species produce the unique combination of secondary metabolites: glauconic acid, ZG–1494α, purpuride, red Monascus pigments, mitorubrins, and purpactins in fresh isolates.
CYA 25 °C 7d: Colonies are 30–40 mm in diameter, low, plane; margins narrow (1–2 mm), entire, low; mycelia white; texture velvety; sporulation dense, conidia en masse dark to dull green; exudate absent; soluble pigment red; reverse coloration dark cherry red.
MEA 25 °C 7d: Colonies 35–40 mm in diameter, low, plane, having a pinkish colour because of exudates diffusing into mycelia; margins narrow (1–2 mm), entire, low; mycelia white; texture velvety overlaying floccose; sporulation moderately dense, conidia en masse bluish green; exudate red droplets especially close to margin; soluble pigment absent, after prolonged incubation red pigments produced; reverse coloration dark red.
YES 25 °C 7d: Colonies are 33–45 mm in diameter, raised at centre, sulcate; margins wide (2–3 mm), entire, low; mycelia white; texture velvety; sporulation dense, conidia en masse dark to dull green; exudates small red droplets; soluble pigment red in some isolates; reverse coloration brownish red.
CYAS 25 °C 7d: Commonly no growth, some strains up to 5 mm in colony diameter.
CREA 25 °C 7d: Colonies 9–13 mm in diameter, weak acid production close to colony periphery, some strains acid absent; reverse dark red.
OA 25 °C 7d: Colonies 30–35 mm in diameter, low, plane; margins wide (2–3 mm), entire, low; white mycelia; texture velvety; sporulation dense; conidia en masse dull to dark green, almost appears blackish green; exudates absent; soluble pigment absent; reverse coloration commonly greenish yellow to green, red in some isolates.
DG18 25 °C 7d: Colonies 27–30 mm in diameter, low, plane; margins wide (2 mm), entire, low; mycelia white; texture velvety, floccose mycelia present at centre; sporulation dense, conidia en masse greyish green, at margins bluish green; exudates absent; soluble pigment absent; reverse colour is beige.
Conidiophores mostly biverticillate, subterminal branches produced, have a greenish to brownish pigmentation; Stipes smooth walled, 90–150 × 2.5–3 µm; Branches 2–3 when present, 15–50 × 2–3 µm; Metulae in verticils of 3 to 5 per stipe, 8–15 × 3.0–4.0 µm; Phialides acerose, 3 to 6 per metula, 9.5–12.5 × 2.5–3 µm; Conidia rough walled, ellipsoidal, 2–3.5 × 1.5–2.5 µm.
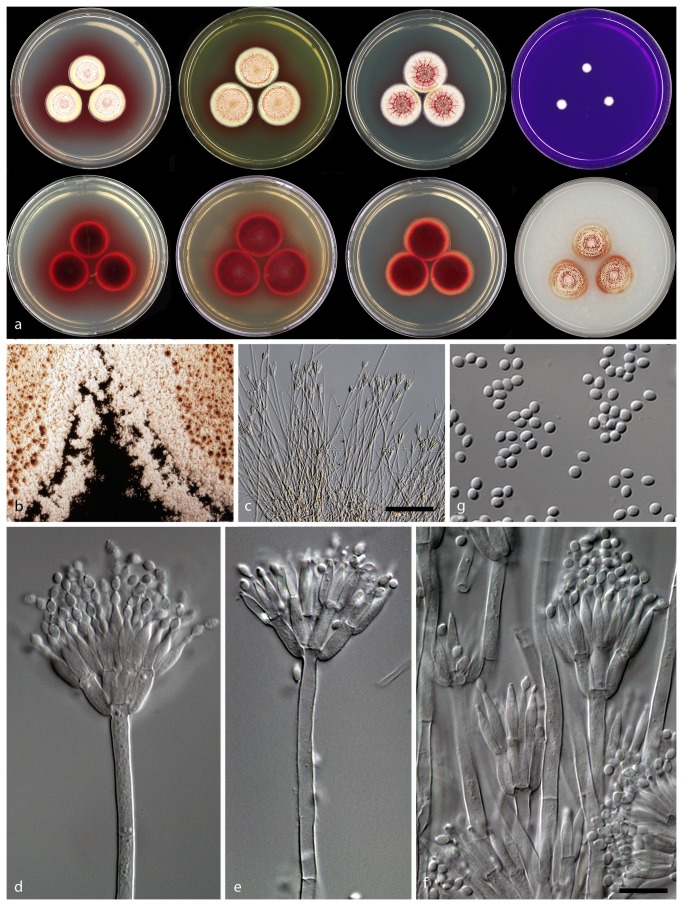
Talaromyces albobiverticillius (H.–M. Hsieh, Y.–M. Ju & S.–Y. Hsieh) Samson, Yilmaz, Frisvad & Seifert, Studies in Mycology 70: 174, 2011. MycoBank MB560683 (Figure 7)
Figure 7. Morphological features of Talaromyces albobiverticillius CBS 133440. : Colonies incubated on CYA, CYA reverse, MEA, MEA reverse, YES, YES reverse, CREA and OA from left to right b: Colony texture on MEA, c–f: Conidiophores produced on MEA; g: Conidia.
( – Scale Bar in c = 50 µm, in f = 10 µm and applies to d–g).
Type. BCRC 34774
CYA 25 °C 7d: Colonies 15–20 mm in diameter, low, crateriform, in some isolates sulcate; margins narrow (1–2 mm), entire, low; mycelia white and yellow; texture floccose to velvety; sporulation sparse, in some isolates moderately dense; conidia en masse when sparse white, otherwise greyish green; exudates red small droplets; soluble pigmentation red; reverse coloration dark cherry red.
MEA 25 °C 7d: Colonies 24–28 mm in diameter, low, crateriform, in some isolates sulcate; margins wide (2–3 mm), entire, low; mycelia white and yellow; texture velvety with overlaying floccose in the centre; sporulation sparse, in some isolates moderately dense; conidia en masse when sparse white, otherwise greyish green; exudates clear and red droplets; soluble red pigment absent; reverse coloration dark red.
YES 25 °C 7d: Colonies 23–25 mm in diameter, raised at centre, sulcate; margins wide (2–3 mm), entire, low; mycelia white and yellow; texture velvety; sporulation sparse, in some isolates moderately dense; conidia en masse when sparse white, otherwise greyish green; exudates small orange to red droplets; soluble pigment red in some strains; reverse coloration red to pale brown.
CYAS 25 °C 7d: No growth.
CREA 25 °C 7d: Colonies 4–8 mm in diameter, no acid produced.
OA 25 °C 7d: Colonies 25–28 mm in diameter, low, plane; margins wide (3–4 mm), entire, low; mycelia white; texture velvety; sporulation sparse to moderately dense; conidia en masse when sparse white, otherwise greyish green; exudates absent; soluble pigment absent; reverse coloration red in the centre and the rest greenish yellow to green.
DG18 25 °C 7d: Colonies 15–35 mm in diameter, low, plane; margins narrow (1–2 mm), entire, low; mycelia white; texture velvety; sporulation sparse; sparse to moderately dense; conidia en masse when sparse white, otherwise greyish green; exudates clear to red droplets; soluble pigment red in some isolates absent; reverse coloration brownish red, in some isolates beige.
Conidiophores strictly biverticillate, subterminal branches absent; stipes smooth walled, 200–380 × 2.5–3.5 µm; metulae in verticals of 3–6, 8–12 × 1.5–4.5 μm; phialides acerose, 3–7 per metula, 8–13.5 × 2–3 μm; conidia smooth to finely roughened, spheroid to subglobose, in some isolates fusiform, 2–3.5 (4) × 1.5–2.5 μm.
Conclusion
Talaromyces atroroseus is a new species that produce large amounts of red pigments that can be potentially used for colouring foods, as it does not produce any known mycotoxins. Certain strains of T. albobiverticillius may also be used for these purposes.
Supporting Information
Table S1 contains the extrolites searched for by ultra high performance-liquid chromatography-diode array detection-high resolution mass spectrometric detection (UHPLC-DAD-HRMS) the fungal extracts analysed. The table also includes data on the available standards used in the study.
(DOCX)
Acknowledgments
We thank Agilent Technologies for the donation of LC-QTOF and LC-QQQ equipment to DTU Systems Biology via a Thought Leader Award. We also thank Cobus Visagie and Keith A. Seifert for a strain of Talaromyces atroroseus (KAS 3778) that served as type of the new species an was isolated from an indoor mold project funded by the Alfred P. Sloan Foundation. Ellen Kirstine Lyhne is thanked for technical assistance.
Funding Statement
Part of this work was supported by the Danish Research Agency for Technology and Production Grant 09-064967 and an equipment grant from Agilent Technologies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Feng Y, Shao Y, Chen F (2012) Monascus pigments. Appl Microbiol Biotechnol 96: 1421-1440. doi: 10.1007/s00253-012-4504-3. PubMed: 23104643. [DOI] [PubMed] [Google Scholar]
- 2. Patakova P (2013) Monascus secondary metabolites: production and biological activity. J Ind Microbiol Biotechnol 40: 169-181. doi: 10.1007/s10295-012-1216-8. PubMed: 23179468. [DOI] [PubMed] [Google Scholar]
- 3. Samson RA, Stolk AC, Frisvad JC (1989) Two new synnematous species of Penicillium . Stud Mycol 31: 133‑143. [Google Scholar]
- 4. van Reenen‑Hoekstra ES, Frisvad JC, Samson R.A, Stolk AC (1990). The Penicillium funiculosum complex ‑ well defined species and problematic taxa. In: RA , Pitt JI (eds.): Modern concepts in Penicillium and Aspergillus classification. New York: Plenum Press; . pp. 173‑191 [Google Scholar]
- 5. Frisvad JC, Filtenborg O, Samson RA, Stolk AC (1990) Chemotaxonomy of the genus Talaromyces . Antonie Van Leeuwenhoek 57: 179‑189. doi: 10.1007/BF00403953. PubMed: 2181929. [DOI] [PubMed] [Google Scholar]
- 6. Lin YL, Wang TH, Lee MH, Su NW (2008) Biologically active components and nutraceuticals in the Monascus–fermented rice: a review. Appl Microbiol Biotechnol 77: 965-973. doi: 10.1007/s00253-007-1256-6. PubMed: 18038131. [DOI] [PubMed] [Google Scholar]
- 7. Liu BH, Wu TS, Su MC, Chung CP, Yu FY (2005) Evaluation of citrinin occurrence and cytotoxicity in Monascus fermentation products. J Agric Food Chem 53: 170-175. doi: 10.1021/jf048878n. PubMed: 15631525. [DOI] [PubMed] [Google Scholar]
- 8. Mapari SAS, Hansen ME, Meyer AS, Thrane U (2008) Computerized screening for novel producers of Monascus–like pigments in Penicillium species. J Agric Food Chem 56: 9981–9989. doi: 10.1021/jf801817q. PubMed: 18841978. [DOI] [PubMed] [Google Scholar]
- 9. Mapari SAS, Meyer AS, Thrane U, Frisvad JC (2009) Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb Cell Fact 8: 24. doi: 10.1186/1475-2859-8-24. PubMed: 19397825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yilmaz N, Houbraken J, Hoekstra ES, Frisvad JC, Visagie CM et al. (2012) Delimitation and characterisation of Talaromyces purpurogenus and related species. Persoonia 29: 39-54. doi: 10.3767/003158512X659500. PubMed: 23606764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2010) Food and indoor fungi. CBS laboratory manual series 2. Utrecht: CBS KNAW Fungal Biodiversity Centre; p. 390. [Google Scholar]
- 12. Houbraken J, Due M, Varga J, Meijer M, Frisvad JC et al. (2007) Polyphasic taxonomy of Aspergillus section Usti . Stud Mycol 59: 107-128. doi: 10.3114/sim.2007.59.12. PubMed: 18490949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Houbraken J, López Quintero CA, Frisvad JC, Boekhout T et al. (2011) Five new Penicillium species, P. araracuarense, P. elleniae, P. penarojaense, P. vanderhammenii and P. wotroi, from Colombian leaf litter. Int J Syst Evol Microbiol 61: 1462-1475. [DOI] [PubMed] [Google Scholar]
- 14. Houbraken J, Samson RA (2011) Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol 70: 1–51. doi: 10.3114/sim.2011.70.01. PubMed: 22308045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA et al. (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium . Stud Mycol 70: 159-183. doi: 10.3114/sim.2011.70.04. PubMed: 22308048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tamura K, Peterson D, Peterson N, Stecher G, Nei M et al. (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731-2739. doi: 10.1093/molbev/msr121. PubMed: 21546353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frisvad JC, Thrane U (1987) Standardized High‑Performance Liquid Chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone indices and UV‑VIS spectra (diode-array detection). J Chromatogr 404: 195‑214. doi: 10.1016/S0021-9673(01)86850-3. PubMed: 3680432. [DOI] [PubMed] [Google Scholar]
- 18. Nielsen KF, Månsson M, Rank C, Frisvad JC, Larsen TO (2011) Dereplication of microbial natural products by LC–DAD–TOFMS. J Nat Prod 74: 2338-2348. doi: 10.1021/np200254t. PubMed: 22026385. [DOI] [PubMed] [Google Scholar]
- 19. Houbraken J, Spierenburg H, Frisvad JC (2012) Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie Van Leeuwenhoek 101: 403-421. doi: 10.1007/s10482-011-9647-1. PubMed: 21965082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoll O (1903–1904) Beiträge zur Morphologischen und Biologischen Charakteristik von Penicillium–Arten. Dissertation Würzberg. 56 p. [Google Scholar]
- 21. Raper KB, Thom C (1949) Manual of Penicillia. Baltimore, MD, USA: Williams & Wilkins. 875 pp. [Google Scholar]
- 22. Pitt JI (1980) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces . New York: Academic Press; p. 634. [Google Scholar]
- 23. Yuill JL (1934) The acids produced from sugar by a Penicillium parasitic upon Aspergillus niger . Biochem J 28: 222-227. PubMed: 16745356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takashima M, Kitajama A, Otuka K (1955) Studies on the pigment of a white crystal “glauconic acid” and red pigments by Penicillium purpurogenum . J Agric Chem Soc JAPAN 29: 25-29. [Google Scholar]
- 25. Baldwin JE, Barton DHR, Bloomer JL, Jackman LM, Rodriguez-Hahn L et al. (1962) The constitutions of glauconic, glaucanic and byssochlamic acids. Experientia 18: 345-352. doi: 10.1007/BF02172243. PubMed: 13864341. [DOI] [PubMed] [Google Scholar]
- 26. Barton DHR, Jackman LM, Rodriguez-Hahn L, Sutherland JK (1965) The nonadrides. Part. II. The constitutions of glauconic and glaucanic acids. J Chem Soc 1965: 1772-1778 [Google Scholar]
- 27. Barton DHR, Godinho LDS, Sutherland JK (1965) The nonadrides. Part. III. The absolute configuration of glauconic and glaucanic acids. J Chem Soc 1965: 1779-1786 [Google Scholar]
- 28. Barton DHR, Sutherland JLK (1965) The nonadrides. Part I. Introduction and general survey. J Chem Soc 1965: 1769-1772 [Google Scholar]
- 29. Huff RK, Moppett CE, Sutherland JK (1968) A novel synthesis of a nine-membered ring. J Chem Soc Chem Commun 1968: 1192-1193 [Google Scholar]
- 30. Huff RK, Moppett CE, Sutherland JK (1972) Nonadrides 6. Dimerization of C-9 unit in vivo and in vitro . J Chem Soc 1972: 2584-2590 [Google Scholar]
- 31. King TJ, Roberts JC, Thompson DJ (1973) Studies in mycological chemistry. Part XXX and last. Isolation and structure of purpuride, a metabolite of Penicillium purpurogenum Stoll. J Chem Soc Perkin I 1973: 78-80. [DOI] [PubMed] [Google Scholar]
- 32. Roberts JC, Warren CWH (1955) Studies in mycological chemistry. Part IV. Purpurogenone, a metabolic product of Penicillium purpurogenum Stoll. J Chem Soc 1955: 2992-2998 [Google Scholar]
- 33. King TJ, Roberts JC, Thompson DJ (1970) The structure of purpurogenone, a metabolite of Penicillium purpurogenum Stoll, an X-ray study. J Chem Soc D: 1499. [Google Scholar]
- 34. Roberts JC, Thompson DJ (1971) Studies in mycological chemistry. Part XXVII. Reinvestigation of the structure of purpurogenone, a metabolite of Penicillium purpurogenum Stoll. J Chem Soc 1971: 3488-3492 [DOI] [PubMed] [Google Scholar]
- 35. Roberts JC, Thompson DJ (1971) Studies in mycological chemistry. Part XXVIII. Isolation and structure of deoxypurpurogenone, a minor pigment of Penicillium purpurogenum Stoll. J Chem Soc 1971: 3493-3495 [DOI] [PubMed] [Google Scholar]
- 36. West RR, van Ness J, Varming A-M, Rassing B, Biggs S et al. ZG (1494α) a novel platelet-activating acyltransferase inhibitor from Penicillium rubrum, isolation, structure elucidation and biological activity. J Antibiot 49: 967-973. [DOI] [PubMed] [Google Scholar]
- 37. Wang H, Wang Y, Wang W, Fu P, Liu P et al. (2011) Anti-influenza virus polyketides from the acid-tolerant fungus Penicillium purpurogenum JS03-21. J Nat Prod 74: 2014-2018. doi: 10.1021/np2004769. PubMed: 21879714. [DOI] [PubMed] [Google Scholar]
- 38. Kondo H, Kurama M, Nakajima S, Osada K, Okura A et al. (1993) New substance BE-25327 which is an estradiol agonist – useful for treating oestrogen-deficient gynaecological diseases, osteoporous, prostatic cancer and prostatomegaly. Jpn. Kokai Tokkyo Koho, JP 05032579 A2, 9 Feb [Google Scholar]
- 39. Silva ED, Williams DE, Jayanetti DR, Centko RM, Patrick BO et al. (2011) Dhilirolides A-D, meroterpenoids produced in culture by the fruit-infecting fungus Penicillium purpurogenum collected in Sri Lanka. Org Lett 13: 1174-1177. doi: 10.1021/ol200031t. PubMed: 21348535. [DOI] [PubMed] [Google Scholar]
- 40. Wijkman N (1931) On some new substances made through mould fungus. Justus. Liebigs Ann Chem 485: 61-73. doi: 10.1002/jlac.19314850106. [DOI] [Google Scholar]
- 41. Herrick HT, Mayo E (1928) The production of gluconic acid by the Penicillium luteum-purpurogenum group. II. Some optimal conditions for acid formation. J Biol Chem 77: 185-195. [Google Scholar]
- 42. Jiang Y, Li HB, Chen F, Hyde KD (2005) Production potential of water-soluble Monascus red pigment by a newly isolated Penicillium sp. J Agric Technol 1: 113-126. [Google Scholar]
- 43. Ogihara J, Kato J, Oishi K, Fujimoto Y, Eguchi T (2000) Production and structural analysis of PP-V, a homologue of monascorubramine, produced by a new isolate of Penicillium sp. J Biosci Bioeng 90: 549-554. doi: 10.1016/S1389-1723(01)80039-6. PubMed: 16232908. [DOI] [PubMed] [Google Scholar]
- 44. Ogihara J, Kato J, Oishi K, Fujimoto Y (2001) PP-R, 7-(2-hydroxyethyl)-monascorubramine, a red pigment produced in the mycelia of Penicillium sp. AZ. J Biosci Bioeng 91: 44-47. doi: 10.1263/jbb.91.44. PubMed: 16232944. [DOI] [PubMed] [Google Scholar]
- 45. Ogihara J, Kato J, Oishi K (2002) Effect of ammonium nitrate on the production of PP-V and monascorubrin homologues by Penicillium sp. AZ. J Biosci Bioeng 93: 54-59. doi: 10.1263/jbb.93.54. PubMed: 16233165. [DOI] [PubMed] [Google Scholar]
- 46. Arai T, Umemura S, Ota T, Ogihara J, Kato J et al. (2012) Effects of inorganic nitrogen sources on the production of PP-V [(10Z)-12-carboxyl-monascorubramine] and the expression of the nitrate assimilation gene cluster by Penicillium sp. AZ. Biosci Biotechnol Biochem 76: 120-124. doi: 10.1271/bbb.110589. PubMed: 22232256. [DOI] [PubMed] [Google Scholar]
- 47. Arai T, Koganei K, Umemura S, Kojima R, Kato J et al. (2013) Importance of the ammonia assimilation by Penicillium purpurogenum in amino derivative Monascus pigment, PP-V, production. AMB Express 3: 19. doi: 10.1186/2191-0855-3-19. PubMed: 23537394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tomoda H, Nishida H, Masuma R, Cao J, Okuda S et al. (1991) Purpactins, new inhibitors of acyl-CoA:cholesterol acyltransferase produced by Penicillium purpurogenum. I. Production, isolation and physico-chemical and biological properties. J Antibiot (Tokyo) 44: 136-143. doi: 10.7164/antibiotics.44.136. PubMed: 1750931. [DOI] [PubMed] [Google Scholar]
- 49. Nishida H, Tomoda H, Cao J, Okuda S, Omura S (1991) Purpactins, new inhibitors of acyl-CoA:cholesterol acyltransferase produced by Penicillium purpurogenum. II. Structure elucidation of purpactins A, B and C. J Antibiot (Tokyo) 44: 144-151. doi: 10.7164/antibiotics.44.144. PubMed: 2010354. [DOI] [PubMed] [Google Scholar]
- 50. Pharm Amano (1974) Novel pigment Red W59 extracted from Penicillium purpurogenum culture medium with organic solvents, Jap. Pat. JP 49093587 -A [Google Scholar]
- 51. Méndez-Zavala A, Contreras-Esquivel JC, Lara-Victoriano F, Rodriguez-Herrera R, Aguilar CN (2007) Fungal production of the red pigment using a xerophilic strain Penicillium purpurogenum GH-2. Rev Mex Ingen Quim 6: 267-273. [Google Scholar]
- 52. Méndez A, Pérez C, Montañéz JC, Martínez G Aguilar CN (2011) Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J Zhejiang Univ-Sci B (Biomed; & Biotechnol) 12: 961-968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Espinoza-Hernández TC, Rodríguez-Herrera R, Aguilar-González CN, Lara-Victoriano F, Reyes-Valdés MH et al. (2013) Characterization of three novel pigment-producing Penicillium strains isolated from the Mexican semi-desert. Afr J Biotechnol 12: 3405-3413. [Google Scholar]
- 54. Qui M, Xie R, Shi Y, Chen H, Wen Y et al. (2010) Isolation and identification of endophytic fungus SX01, a red pigment producer from Ginkgo biloba L. World J Microbiol Biotechnol 26: 993-998. doi: 10.1007/s11274-009-0261-6. [DOI] [Google Scholar]
- 55. Bollinger P, Haerri E, Sigg H (1972) Antibiotic derived from Penicillium species. US Pat. 3,655,880
- 56. Santos-Ebinuma VC, Teixeira MFS, Pessoa A Jr. (2013) Submerged culture conditions for the production of alternative natural colorants by a new isolated Penicillium purpurogenum DPUA 1275. J Microbiol Biotechnol 23: 802-810. doi: 10.4014/jmb.1211.11057. PubMed: 23676916. [DOI] [PubMed] [Google Scholar]
- 57. Santos-Ebinuma VC, Roberto IC, Teixeira MFS, Pessoa A Jr. (2013) Improving of red colorants production by a new Penicillium purpurogenum strain in submerged culture and the effect of different parameters in their stability. Biotechnol Prog 29: 778-785. doi: 10.1002/btpr.1720. PubMed: 23554384. [DOI] [PubMed] [Google Scholar]
- 58. Velmurugan P; Kamala, Kannan S, Balachandar V, Lakshmanaperumalsamy P, Chae J-C et al. (2010) Natural pigment extraction from five filamentous fungi for industrial applications and dyeing of leather. Carb Polym 79: 262-268. doi: 10.1016/j.carbpol.2009.07.058. Available online at: 10.1016/j.carbpol.2009.07.058 [DOI] [Google Scholar]
- 59. Velmurugan P, Lee YH, Venil CK, Lakshmanaperumalsamy P, Chae J-C et al. (2010) Effect of light on growth, intra cellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J Biosci Bioeng 109: 346-350. doi: 10.1016/j.jbiosc.2009.10.003. PubMed: 20226375. [DOI] [PubMed] [Google Scholar]
- 60. Gunasekaran S, Poorniammai R (2008) Optimization of fermentation conditions for red pigment production from Penicillium sp. under submerged cultivation. Afr J Biotechnol 7: 1894-1898. [Google Scholar]
- 61. Mapari SAS, Nielsen KF, Larsen TO, Frisvad JC, Meyer AS et al. (2005) Exploring fungal biodiversity for water–soluble pigments and potential natural food colorants. Current Opinion Biotechnol 16: 231-238. doi: 10.1016/j.copbio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 62. Mapari SAS, Thrane U, Meyer AS (2010) Fungal polyketide azaphilone pigments as future food colorants? Trends Biotechnol 28: 300-307. doi: 10.1016/j.tibtech.2010.03.004. PubMed: 20452692. [DOI] [PubMed] [Google Scholar]
- 63. Hsieh HM, Ju YM, Hsieh SY (2010) Penicillium albobiverticillium sp. nov., a new species producing white conidial masses from biverticillate penicillia. Fung Sci 25: 25-31. [Google Scholar]
- 64. Büchi G, White JD, Wogan GN (1965) The structures of mitorubrin and mitorubrinol. J American Chem Soc 87: 3484-3489. doi: 10.1021/ja01093a036. [DOI] [PubMed] [Google Scholar]
- 65. Marsini MA, Gowin KM, Pettus TRR (2006) Total synthesis of (+)-mitorubrinic acid. Org Lett 8: 3481-3483. doi: 10.1021/ol0610993. PubMed: 16869640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Natsume M, Takahashi Y, Marumo S (1985) (-)-Mitorubrinic acid, a morphogenic substance inducing chlamydospore-like cells, and its related new metabolite, (+)-mitorubrinic acid B, isolated from Penicillium funiculosum . Agric Biol Chem 49: 2517-2519. doi: 10.1271/bbb1961.49.2517. [DOI] [Google Scholar]
- 67. Suzuki S, Hosoe T, Nozawa K, Yaguchi T, Udagawa Si et al. (1999) Mitorubrin derivatives on ascomata of some Talaromyces species of Ascomycetous fungi. J Nat Prod 62: 1328-1329. doi: 10.1021/np990146f. PubMed: 10514327. [DOI] [PubMed] [Google Scholar]
- 68. Steglich W, Klaar M, Furtner W (1974) (+)-Mitorubrin derivatives from Hypoxylon fragiforme . Phytochem: 132874-132875. [Google Scholar]
- 69. Osmanova N, Schultze W, Ayoub N (2010) Azaphilones: a class of fungal metabolites with diverse biological activities. Phytochem Rev 9: 315-342. doi: 10.1007/s11101-010-9171-3. [DOI] [Google Scholar]
- 70. Gao J-M, Yang S-X, Qin J-C (2013) Azaphilones. Chemistry and Biology - Chem Rev 113: 4755-4811. [DOI] [PubMed] [Google Scholar]
- 71. Fujimoto H, Jisai Y, Horie Y, Yamazaki M (1988) On isolation of spiculisporic acid, a toxic metabolite from Talaromyces panasenkoi . Proc Jpn Assoc Mycotoxicol 27 pp. 15-19. [Google Scholar]
- 72. Nagaoka A, Kukuchi K, Nagawa Y (1972) Pharmacological studies of new indole alkaloids, rugulovasine A and B hydrochloride. I. Effects of both alkaloids on cardiovascular and central nervous system, and smooth muscles. Drug Res 22: 137-142. [PubMed] [Google Scholar]
- 73. Nagaoka A, Kikuchi K (1972) Pharmacological studies of new indole alkaloids, rugulovasine A and B hydrochloride. II. Hypotensive mechanism of both alkaloids in the anesthetized cats. Drug Res 22: 143-146. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 contains the extrolites searched for by ultra high performance-liquid chromatography-diode array detection-high resolution mass spectrometric detection (UHPLC-DAD-HRMS) the fungal extracts analysed. The table also includes data on the available standards used in the study.
(DOCX)