Abstract
Background
Semen quality appears to have declined over the past decades but reasons for this decline are unresolved. The concurrent increase in sedentary behavior may be a contributing factor. The objective of this study was to evaluate the relationship of physical activity and television (TV) watching with sperm parameters in a population of young, healthy men
Methods
Men aged 18-22 years (n=189) from the Rochester Young Men's Study (2009-2010) were used in this analysis. Physical activity (hours/week of moderate and vigorous exercise) and TV watching (hours/week of TV, video, or DVD watching) over the past 3 months was assessed via questionnaire. Semen quality was assessed by sperm concentration, motility, morphology, and total sperm count.
Results
Sperm concentration and total sperm count were directly related to physical activity after multivariable adjustment (p-trend=0.01 and 0.04); men in the highest quartile of moderate-to-vigorous activity (≥15 hours/week) had 73% (95% CI 15 to 160%) higher sperm concentration than men in the lowest quartile (< 5 hours/week). TV watching was inversely associated with sperm concentration and total sperm count in multivariable analyses (p-trend=0.05 and 0.06); men in the highest quartile of TV watching (>20 hours/week) had 44% (95% CI 15 to 63%) lower sperm concentration than men in the lowest quartile (0 hours/week). These measures of physical and leisure time activities were not significantly associated with sperm motility or morphology.
Conclusions
In this population of healthy men, higher moderate-to-vigorous activity and less TV watching were significantly associated with higher total sperm count and sperm concentration.
Keywords: physical activity, physical inactivity, sperm concentration, sperm morphology, sperm motility
Introduction
There has been much discussion about whether semen quality has been declining over the past decades.[1-8] Despite the inconsistent findings, the majority of data support a decline in sperm concentration in most Western countries [9] and the causes of the observed decline are still debated. One possible explanation could be the concurrent decrease in physical activity and increase in sedentary behavior over the same time frame.[10] Physical activity has been associated with many health benefits, including reduced risks of obesity, diabetes and cardiovascular disease.[11] Yet, strenuous exercise has also been proposed as a risk factor for male factor infertility,[12] based largely on data showing lower testosterone levels and semen quality among long-distance runners and cyclists.[13-21] Past studies, however, have largely evaluated the relation between exercise and semen quality in a limited range of activities and focused exclusively on high-intensity training. The relation of moderate levels of physical activity with semen quality has yet to be thoroughly evaluated.
Television (TV) watching, on the other hand, has been associated with many detrimental health consequences [22] but its effects on semen quality remain unexplored. Substantial and persistent increase in scrotal temperature can, in experimental settings, markedly disturb sperm production.[23] While sedentary work has been correlated to a moderate increase in scrotal temperatures, estimates of its impact on semen quality have been inconsistent.[24-29] Physical inactivity has also been linked to increased oxidative stress levels which could play an important role in the pathophysiology of male factor infertility.[30, 31]
The objective of this study was to evaluate the relationships between semen quality and both physical activity and TV watching among young, healthy men. We hypothesized that increased physical activity was associated with higher sperm count, concentration and motility, and a lower proportion of morphologically abnormal sperm. Furthermore, we hypothesized that increased TV watching time was associated with decreased semen quality parameters.
Methods
Study population
Participants were part of the Rochester Young Men's Study (RYMS), a cross-sectional study conducted during 2009–2010 at the University of Rochester (Rochester, NY, USA). Men were recruited into RYMS through flyers and newspapers at college campuses in the Rochester area. Subjects were eligible if they were born in the US after December 31st, 1987, able to read and speak English, and able to contact their mother and ask her to complete a questionnaire. A total of 389 potential participants contacted the study. Of these, 305 (78.4%) met all eligibility criteria and 222 men participated in the study. Our analysis only includes men with complete information on both physical activity and TV watching (n=189). The University of Rochester Research Subjects Review Board approved the study and written informed consent was obtained from all subjects before their participation.
Physical Activity and TV Watching
Men were asked to report the number of hours they spent in a normal week over the past 3 months engaged in vigorous, moderate, or mild exercise. Our main exposure of physical activity was hours per week of moderate to vigorous activity defined as the sum of those two categories. This type of activity corresponded to any exercise that makes you somewhat to very windy or sweaty. Alternate measures of physical activity were also calculated including total metabolic equivalents (METs) and moderate to vigorous METs. Mild (<3 METs), moderate (3-6 METS), and vigorous (>6 METs) activities were given an average MET level of 2, 4.5, and 6 respectively to calculate the total METs per person [32]. TV watching was assessed in the same questionnaire by asking men to select the category of TV watching time per workday or weekend day that corresponded to their average habits over the past 3 months. Categories for response were “none/almost none”, “1-3 hours/day”, “4-6 hours/day”, “7-9 hours/day”, and “over 10 hours/day”. The median value for each category was used to assign TV watching time and a weighted average of weekend and workdays was taken to give the average amount of TV watching time per week.
Semen collection and analysis
Semen samples were collected by masturbation at the clinic where upon arrival men were asked to report the time of their previous ejaculation. The men had been asked to abstain from ejaculation for at least 48 hr before sample collection; however they were not excluded if this was not the case (n=26). Abstinence times > 240 hr (n = 7) were truncated at 240 hr. Sample processing was initiated within 30 min of collection. Ejaculate volumes were estimated by specimen weight, assuming a semen density of 1.0 g/mL. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific Inc., Horsham, PA, USA). Two chambers of the hemocytometer were counted, and the average was used in this analysis. Motility was analyzed using World Health Organization 1999 criteria and was classified as both progressive (A + B) and total (A+B+C).[33] Smears for morphology were air-dried, fixed, and shipped to the University Department of Growth and Reproduction at the Rigshospitalet (Copenhagen, Denmark). The slides were Papanicolaou stained and assessed using strict criteria.[34] To increase consistency and comparability of methods, six sets of duplicate semen samples were sent over the course of the study from the University of Copenhagen's Department of Growth and Reproduction to the Andrology Laboratory (University of Rochester), which is Clinical Laboratory Improvement Amendments certified.
Covariate assessment
A physical examination of each participant was performed on the same day as semen sampling. Assessments included weight, height, testis size by palpation using Prader's orchidometer (Andrology Australia, Clayton, Victoria, Australia), and the presence of varicocele or other genital abnormalities. Men also completed questionnaires concerning demographics, medical and reproductive history, psychological stress, medication use (antibiotics, antidepressants, and hormones), and smoking habits. Substantial psychosocial stress was defined as indicating a positive response to 2 or more questions out of 6 questions on stressful life events. Diet was assessed using a validated questionnaire.[35] Diet quality was summarized by two previously described dietary patterns [36]: a Prudent pattern (characterized by high intakes of fish, chicken, fruit, cruciferous vegetables, tomatoes, leafy green vegetables, legumes, and whole grains) and Western pattern (characterized by high intakes of red and processed meat, butter, high fat dairy, refined grains, pizza, snacks, high energy drinks, mayonnaise, and sweets). All covariates were 100% complete.
Statistical Analysis
Men were classified into quartiles according to their average moderate to vigorous physical activity and TV watching per week. Descriptive statistics were calculated for demographic characteristics across quartiles of activity. Multivariable linear regression was used to evaluate the associations between quartile of activity and sperm parameters. Sperm concentration and sperm count were log-transformed to normalize distributions. The association between activity and sperm parameters was also evaluated as continuous linear and quadratic variable. Tests for non-linearity used the likelihood ratio test, comparing the model without any activity term to the model with the linear and quadratic term. Tests for trend were conducted across quartiles using a variable with the median physical activity and TV watching level in each quartile as a continuous variable in the linear regression models. All results are presented as adjusted means for the median level of each covariate. For sperm concentration and count (which were log-transformed for linear regression), adjusted means were obtained by exponentiating (“back-transforming”) the estimated beta coefficients for the median level of each covariate.
Confounding was evaluated using a hybrid approach combining prior knowledge using directed acyclic graphs (DAGs) and a statistical approach based on change in point estimates.[37] A set of variables was determined by a review of the prior literature and a detailed DAG was created identifying variables that should be included in the models. An exploratory confounding evaluation was also used with covariates being included in the model if they changed the exposure coefficient by more than 15% and were significant at the P= 0.10 level. Variables retained in the final multivariable models were abstinence time (hr), race (white/other), smoking status (current/former or never), BMI (kg/m2), recruitment period (2009/2010), total energy intake (kcals), TV watching (for physical activity analyses) and moderate to vigorous exercise (for TV watching analyses). Motility analyses were additionally adjusted for time from semen collection to start of semen analysis.[38]
Effect modification by BMI (< 25 kg/m2 and ≥ 25 kg/m2), smoking status (current and never/former smokers), physical activity (<8.25 hrs/week (median) and ≥8.25 hrs/week moderate-to-vigorous activity), and TV watching (<14 hrs/week (median) and ≥14 hrs/week) were tested using cross-product terms in the final multivariate model. SAS version 9.2 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Men had a median age of 19.6 years (range 18 to 22 yr), 81.5% were Caucasian, 58.4% had normal BMI (< 25 kg/m2), 77.4% were non-smoking, and had a low prevalence of relevant reproductive morbidity (Table 1). The median (interquartile range; IQR), hours per week of moderate to vigorous physical activity was 8.25 (5 to 14 hrs/week) and median hours per week of TV was 14 (4 to 20 hrs/week). Median sperm concentration was 53 × 106/ml (21 to 96 × 106/ml), median percent progressively motile sperm was 60% (50 to 70%), and median percent morphologically normal sperm was 8.5% (5.0 to 12.0%). Basic demographic characteristics did not differ significantly by levels of physical activity or TV watching (Table 1). Men who were more physically active had stronger adherence to a Prudent dietary pattern and men who watched more TV had stronger adherence to a Western dietary pattern (P <0.001 for both). Physical activity and TV watching were not highly correlated (Spearman correlation coefficient = 0.19).
Table 1.
Demographics of participants in the Rochester Young Men's Study according to quartile of activity.
| Total Cohort | Moderate to Vigorous Activity | TV Watching | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Quartile (Range, hrs/week) | Q1 (<5) | Q4 (>14) | Q1 (0) | Q4 (≥20) | |||
| N (number of men) | 189 | 44 | 42 | p-value* | 34 | 49 | p-value* |
| Age, yrs † | 19.6 (18.9, 20.4) | 19.5 (18.9, 20.3) | 19.6 (19.0, 20.7) | 0.99 | 19.3 (18.7, 20.5) | 19.4 (18.7, 20.4) | 0.89 |
| Caucasian, n (%) | 154 (81.5) | 35 (79.6) | 33 (78.6) | 0.48 | 29 (85.3) | 38 (77.6) | 0.81 |
| BMI, kg/m2 | 24.6 (22.7, 26.7) | 24.6 (22.7, 26.2) | 24.9 (23.9, 27.5) | 0.31 | 24.5 (22.0, 25.8) | 24.6 (23.0, 27.9) | 0.18 |
| Current Smoker, n (%) | 43 (22.8) | 14 (31.8) | 5 (11.9) | 0.11 | 7 (20.6) | 12 (24.5) | 0.82 |
| Psychosocial Stress, n (%)‡ | 80 (42.3) | 20 (45.5) | 21 (50.0) | 0.50 | 16 (47.1) | 26 (53.1) | 0.22 |
| Abstinence Time, hrs | 70.6 (53.8, 99.3) | 71.0 (60.5, 89.7) | 63.5 (49.1, 91.0) | 0.14 | 76.1 (63.3, 91.0) | 76.1 (49.7,110.8) | 0.81 |
| History of Cryptorchism, n (%) | 5 (2.7) | 1 (2.3) | 1 (2.3) | 0.93 | 1 (2.3) | 1(2.3) | 0.93 |
| Varicocele, n (%) | 5 (2.7) | 1 (2.3) | 1 (2.4) | 1.0 | 1 (2.9) | 0 (0.0) | 0.28 |
| Hydrocele, n (%) | 3 (1.6) | 0 (0.0) | 1 (2.4) | 0.39 | 1 (2.9) | 0 (0.0) | 0.88 |
| Inguinal Hernia Repair, n (%) | 10 (5.3) | 4 (9.3) | 2 (4.8) | 0.68 | 3 (8.8) | 3 (6.3) | 0.41 |
| History of Genital Disease, n (%)§ | 6 (3.2) | 2 (4.6) | 1 (2.4) | 0.95 | 0 (0.0) | 3 (6.1) | 0.48 |
| Testis Size, mL | 26.3 (25.5, 32.5) | 26.1 (23.5, 30.8) | 28.8 (26.0, 33.8) | 0.09 | 26.9 (23.5, 34.0) | 26.3 (26.0, 32.3) | 0.68 |
| Calories, kcal/day | 2938.5 (2263.4, 3677.6) | 2795.8 (2211.4, 3433.1) | 3273.3 (2750.1, 4235.2) | 0.02 | 2867.4 (2133.1, 3257.3) | 3239.8 (2780.8, 3943.7) | 0.02 |
| Prudent Dietary Pattern, score ¶ | -0.2 (-0.6, 0.3) | -0.5 (-0.9, -0.2) | 0.0 (-0.5, 0.8) | <0.001 | 0.1 (-0.6, 0.5) | -0.4 (-0.6, 0.3) | 0.16 |
| Western Dietary Pattern, score ¶ | -0.2 (-0.7, 0.6) | 0.0 (-0.5, 0.5) | 0.2 (-0.5, 0.9) | 0.12 | -0.4 (-0.9, 0.1) | 0.3 (-0.4, 1.3) | <0.001 |
| Vigorous Activity, hrs/week | 4.0 (2.0, 8.0) | 1.0 (0.8, 2.0) | 12.0 (10.0, 15.0) | <0.001 | 3.0 (2.0, 7.0) | 4.0 (2.0, 10.0) | 0.30 |
| Moderate Activity, hrs/week | 4.0 (2.0, 6.0) | 2.0 (1.0, 2.0) | 10.0 (6.0, 11.0) | <0.001 | 2.3 (1.0, 5.0) | 5.0 (3.0, 10.0) | 0.09 |
| Mild Activity, hrs/week | 4.0 (2.0, 10.0) | 3.0 (1.0, 5.5) | 8.0 (3.0, 14.0) | 0.001 | 2.3 (1.0, 6.0) | 5.0 (3.0, 10.0) | 0.25 |
| TV watching, hrs/week | 14.0 (4.0, 20.0) | 7.0 (0.0, 14.0) | 14.0 (4.0, 20.0) | 0.19 | 0.0 (0.0) | 20.0 (20.0, 35.0) | <0.001 |
For continuous variables, Kruskal–Wallis analyses of variance were used to test for associations across quartiles of activity. For categorical variables, chi-squared tests and fisher exact tests (when one or more cell counts were ≤ 5) were used to test the associations between quartiles of activity.
Values presented are median (interquartile range) unless otherwise indicated.
Psychosocial stress is defined as marking a positive response to 2 or more questions (out of 6) on stressful life events.
History of genital disease is defined as having history of an infection of the testes, gonorrhea, genital warts or herpes, or chlamydia.
Dietary patterns were constructed using factor analysis. The two patterns are independent with a mean of 0 and standard deviation of 1. A higher score indicates higher adherence to Prudent or Western dietary pattern.
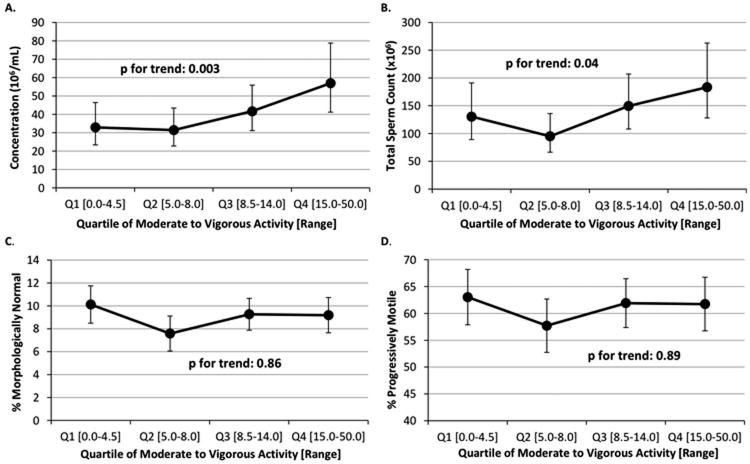
Moderate to vigorous physical activity was positively related to sperm concentration (Figure 1). In the fully adjusted model, men in the second, third, and fourth quartiles of physical activity had -5% (95% CI -34 to 39%), 27% (95% CI -14 to 86%), and 73% (95% CI 15 to 160%) higher sperm concentration than men in the lowest quartile (P for trend=0.003). The relation with total sperm count (sperm concentration × volume) were -27% (95% CI -52 to 10%), 14.6 (95% CI -25 to 75%), and 41% (95% CI -11 to 121%) higher total sperm count than men in the first quartile (P, trend=0.04). Sperm motility (total and progressive), sperm morphology, and sample volume were unrelated to physical activity. When intensity of physical activity was investigated, both moderate and vigorous activities had positive associations with sperm concentration; however, light physical activity was not related to sperm concentration (Table 2). Total METs and moderate to vigorous METs also showed consistent positive associations with sperm concentration.
Figure 1.
Association between quartile of moderate to vigorous activity and semen quality parameters. Adjusted means are presented for the median abstinence time (70.6 hrs), race (white), smoking status (former or never smoker), BMI (24.6 kg/m2), recruitment period (2009), total calorie intake (2939 kcals/day), and TV watching (14 hrs/week). Adjusted means for motility were additionally adjusted for median time from semen collection to start of semen analysis (10.0 hours). Tests for trend were conducted across quartiles using a variable with the median activity level in each quartile as a continuous variable in the linear regression models.
Table 2.
Association between different intensities of physical activity and sperm concentration.
| Quartile of Physical Activity | |||||
|---|---|---|---|---|---|
|
|
|||||
| Adjusted Mean Sperm Concentration (95% CI)* | Q1 | Q2 | Q3 | Q4 | p-value for trend† |
| Intensity of Activity (hrs/week) | |||||
| Mild Activity Range [Min - Max] | [0.0-1.0] | [2.0-3.5] | [4.0-8.0] | [10.0-40.0] | |
| Mild Activity | 41.4 (29.6, 57.9) | 46.5 (33.5, 64.6) | 37.6 (27.2, 52.0) | 38.9 (28.5, 53.2) | 0.55 |
| Moderate Activity Range [Min - Max] | [0.0-1.5] | [2.0-3.0] | [4.0-6.0] | [7.0-30.0] | |
| Moderate Activity | 32.1 (21.6, 47.5) | 36.8 (26.9, 50.3) | 41.2 (30.7, 55.3) | 49.4 (35.8, 68.4) | 0.04 |
| Vigorous Activity Range [Min - Max] | [0.0-1.5] | [2.0-4.0] | [5.0-7.0] | [8.0-31.0] | |
| Vigorous Activity | 29.3 (20.5, 42.0) | 38.3 (28.5, 51.5) | 45.5 (32.8, 63.2) | 47.4 (34.7, 64.8) | 0.02 |
| Metabolic Equivalents (MET hrs/week) | |||||
| Total METs Range [Min - Max] | [4.0-32.5] | [33.0-56.5] | [57.0-82.0] | [87.5-315.0] | |
| Total METs | 32.6 (23.3, 45.6) | 33.0 (23.5, 46.3) | 45.8 (33.7, 62.2) | 48.6 (35.5 66.5) | 0.03 |
| Moderate to Vigorous METs Range [Min -Max] | [0.0-24.8] | [25.5-45.0] | [46.5-70.5] | [72.0-255.0] | |
| Moderate to Vigorous METs | 31.8 (22.5, 44.9) | 33.3 (24.1, 45.9) | 42.2 (30.8, 57.9) | 52.8 (38.9, 71.7) | 0.005 |
Adjusted means are presented for the median abstinence time (70.6 hrs), race (white), smoking status (former or never smoker), BMI (24.6 kg/m2), recruitment period (2009), total calorie intake (2939 kcals/day), and TV watching (14 hrs/week). Sperm concentration was log transformed for normality.
p-value for linear trend across quartiles. For mild activity, median for each quartile was 0, 2, 5, and 12 hrs/week; for moderate activity, median for each quartile was 0.25, 2, 5, 10 hrs/week; and for vigorous activity was 1, 3, 6, 10 hrs/week.
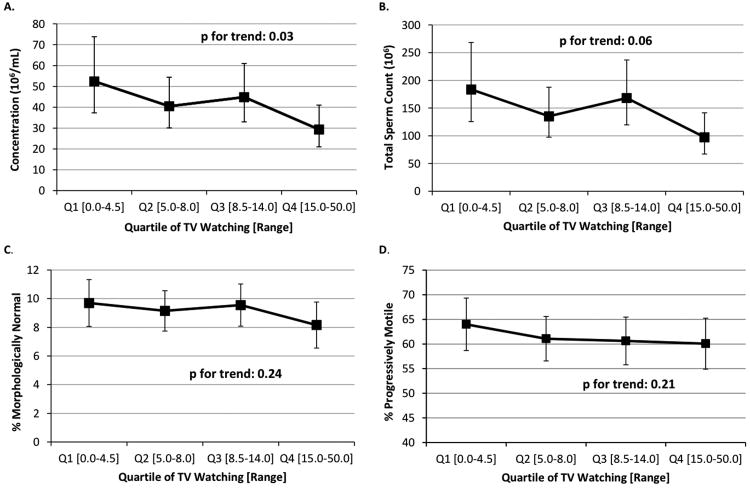
TV watching was inversely related to sperm concentration (Figure 2). In the multivariable model, men in the second, third, and fourth quartiles of TV watching had 23% (95% CI -16 to 49), 14% (95% CI -27 to 42%), and 44% (95% CI 15 to 63%) lower sperm concentration than men in the first quartile (P, trend=0.03). Findings for total sperm count closely mirrored those for concentration (P for trend=0.06). TV watching was unrelated to sperm motility (total or progressive), sperm morphology, or sample volume.
Figure 2.
Association between quartile of TV watching and semen quality parameters. Adjusted means are presented for the median abstinence time (70.6 hrs), race (white), smoking status (former or never smoker), BMI (24.6 kg/m2), recruitment period (2009), total calorie intake (2939 kcals/day), and moderate to vigorous activity (8.25 hrs/week). Adjusted means for motility were additionally adjusted for median time from semen collection to start of semen analysis (10.0 hours). Tests for trend were conducted across quartiles using a variable with the median TV watching time in each quartile as a continuous variable in the linear regression models.
Similar results were also found when the semen quality parameters were dichotomized according to the WHO 2010 semen quality cut points. Men in the highest quartile of moderate-to-vigorous activity had an adjusted odds ratio of 0.25 (95% CI 0.05 to 1.20) for low sperm concentration (<15 × 106) compared to men in the lowest quartile (P for trend across quartiles=0.04). Men in the highest quartile of TV watching had an adjusted odds ratio of 5.45 (95% CI 1.22 to 24.40) of low sperm concentration compared to men in the lowest quartile (P for trend across quartiles=0.08). There was no significant association between moderate-to-vigorous activity and TV watching and odds of having low progressive motility (<32% progressive sperm) or low morphologically normal sperm (<4% normal) (data not shown).
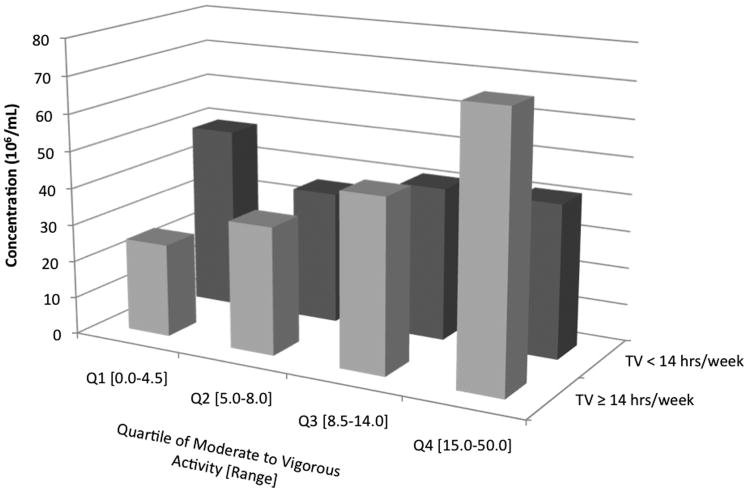
TV watching (dichotomized as above and below the median, 14 hrs) significantly modified the association between moderate to vigorous physical activity and sperm concentration (p-interaction=0.02) (Figure 3). Men who watched ≥ 14 hrs/week of TV had a significant positive association between physical activity and sperm concentration (P for trend= < 0.001) while men who watched <14 hrs/week of TV had no significant association between physical activity and sperm concentration (P for trend=0.69). Overall, men with the highest TV watching (≥ 14 hrs/week) and lowest physical activity (0 to 4.5 hrs/week) had the lowest adjusted mean sperm concentration, 24 × 106/mL (95% CI 14 to 40). There was no significant effect modification by BMI or smoking status.
Figure 3.
Effect modification of TV time on physical activity and sperm concentration (106/ml). Adjusted means are presented for the median abstinence time (70.6 hrs), race (white), smoking status (former or never smoker), BMI (24.6 kg/m2), recruitment period (2009), total calorie intake (2939 kcals/day), and TV watching (4 hrs/week and 14 hrs/week). Tests for trend were conducted across quartiles using a variable with the median activity level in each quartile as a continuous variable in the linear regression models. Tests for interaction were conducted were using a cross-product term (median activity level in each quartile as continuous variable × indicator variable for TV ≥ 14 hrs/week or < 14 hrs/week) in the final multivariate model. TV ≥ 14 hrs/week: p-trend < 0.001; TV < 14 hrs/week: p-trend 0.69; p-interaction 0.02.
Discussion
In this population of healthy young men, higher moderate to vigorous physical activity and lower TV watching were associated with higher sperm concentration and total sperm count but unrelated to sperm morphology, motility or sample volume. These associations with sperm counts suggest that lifestyle changes such as increases in physical activity may positively influence sperm count and concentration in reproductive-aged men.
Results of previous research on physical activity and semen quality parameters have been inconsistent, with some studies finding a positive association,[17, 39] others finding no associations,[19, 21, 40, 41] and some finding an inverse association.[13, 15, 20, 42] This is likely due to differences in type, range, and intensity of physical activity across studies. Nevertheless, our findings are in agreement with a rodent model which showed that running slows testicular aging, possibly through decreased oxidative stress.[43] In the largest human study to date, Wise et al. found no association between overall self-reported physical activity and semen quality parameters in 2,261 men attending a fertility clinic.[19] However, in contrast to our study, the majority of their population reported no exercise (43%) and the median hours of exercise per week was much lower than ours (4.0 hrs/week) limiting their statistical power to detect an association. For instance, their top category of physical activity, ≥ 40 total MET hrs/week, is comparable to our second quartile of total MET hrs/week. In agreement with Wise, we found no significant differences in semen quality parameters when comparing our second quartile to the first. Of note, we found no detrimental effects of very high levels of physical activity on semen parameters which conflicts with several studies in which highly active men, particularly long-distance runners and cyclists, had reduced semen quality.[13-21] Although we may have had some elite athletes in our population, this raises the question of whether the detrimental effects of vigorous exercise seen previously might be specific to exercise modality or extreme activity levels. In support of this notion, Wise et al. found no association between total exercise and semen quality but found a detrimental effect of bicycling on sperm concentration.[19] Based on sport population figures from the National Collegiate Athletic Association it is most likely that the highly active men in our study were football, baseball, track, soccer, or basketball players which might explain why we did not see the detrimental effects.[44] In contrast to cycling and long distance running which are strongly associated with negative energy balance,[45, 46] these sports do not require as much energy expenditure.
TV watching and semen quality has not been studied previously. Of closest relevance, however, are studies on the relationship between sedentary behavior and semen quality parameters. In two different studies, Hjollund et al. showed that sedentary position at work was correlated with scrotal temperature in a dose-response manner,[25] but unrelated to semen quality.[26] In a large observational study of 1747 men, Støy et al. found a suggestive, but not statistically significant, decline in sperm concentration across quintiles of sedentary work.[24] More recently, Magnusdottir et al. showed that in men with normal semen quality, sedentary work was significantly more common among men with the lower sperm concentration (59%) compared to the men with the higher sperm concentration (22%).[28] However, in this study it was difficult to disentangle the effect of obesity from that of inactivity. The modifying effect of TV watching on the association between physical activity and sperm counts was unexpected as this has not been documented in previous literature. It is possible that this might be a chance finding, therefore further research is needed to confirm this result and explore the possible mechanisms of action.
Despite inconsistencies in the literature, an effect of physical activity and inactivity on sperm counts (concentration and total) has biological plausibility. Physical activity can impact reproductive function through its ability to regulate energy balance and affect BMI. At both extremes of the energy spectrum, disorders of chronic energy excess and energy deficiency are characterized by a wide range of reproductive disorders, including altered spermatogenesis in men.[47] As it seems we had few men with an excessive or deficient energy balance, this could possibly explain why we saw no detrimental effects of physical activity on semen quality. Physical activity not leading to exhaustion, has been shown to increase the expression of antioxidant enzymes throughout the body.[48] In contrast, physical inactivity has been associated with increased levels of oxidative stress.[30] Therefore regular exercise might work to prevent reactive oxygen species generation and protect male germ cells from oxidative damage.[49] Finally, the sedentary position and physical activity have been related to scrotal temperatures.[27] Given the correlation between scrotal temperatures and semen quality, this might also be a plausible mechanism. While important to note, given its extensive discussion in previous literature, this argument is controversial with some authors proposing that high scrotal temperatures are a consequence, rather than a cause, of impaired sperm production.[50]
While this study expands on previous research it does have several limitations. First, this was a cross-sectional and observational study, which limits our ability to determine causality of the observed relations. Additionally, it is not possible to conclude from these findings whether the observed differences in sperm counts translate into clinically relevant differences in fertility. Although some semen quality parameters, including sperm concentration, are known to predict spontaneous fertility, it is not possible to know whether the observed associations may translate into differences in reproductive success.[51] Like other observational studies, misclassification of physical activity and TV watching is possible. However, similar physical activity questionnaires have been validated.[52] We also did not collect information on the specific modality of physical activity which limited our ability to explore whether this had any impact on results. While we did see strong associations between physical activity and sperm counts, due to our small sample size, we cannot rule out that chance could play a role in this study. Additionally, we only had one semen sample from each man. Nevertheless, there are limited advantages to using more than one semen sample per man in epidemiologic studies.[53] Finally, the homogeneity of our study population may limit the generalizability of our findings to clinical groups and more diverse populations.
The RYMS study had a number of strengths. First, the study population was composed of healthy volunteers from a relatively homogenous setting (young, college men) with no knowledge of their fertility potential thereby decreasing the likelihood of reverse causation. We also had detailed information on a variety of lifestyle risk factors which improved our ability to adjust for confounding. Finally, our study's relatively large sample size and wide range of physical activity and TV watching compared to the majority of previous literature greatly improved our ability to discern an association.
In conclusion, higher physical activity and lower TV watching were associated with higher sperm count and concentration in young healthy men. These results are consistent with previous animal models of the role of physical activity on male reproductive aging. Our findings suggest that a more physically active lifestyle may improve semen quality. Further research is needed to confirm these findings and extend these results to other populations. Future studies should also evaluate the extent to which different exercise types affect semen quality as previous studies suggest there might be opposing effects of different types of activity on semen characteristics.
“What this paper adds” Box.
Section 1: What is already known on this subject
Physical activity has been associated with many health benefits, however in recent years, prolonged strenuous exercise has been proposed as a risk factor for male factor infertility.
Past studies have largely evaluated the relation between exercise and semen quality in only one or two types of activity (such as biking and running) and have focused exclusively on endurance or high-intensity athletic training.
Television watching has been associated with many detrimental health consequences, but its effects on semen quality have not been assessed.
Section 2: What this study adds:”?
Our study shows that higher physical activity and lower television watching were associated with higher sperm count and concentration in young healthy men.
Our findings suggest that a more physically active lifestyle may improve semen quality.
Acknowledgments
We thank Lynda Kochman, Jodi Stevens, Kelly Brewer and Rita Herko for their assistance in data collection; Ken Edell and Lauren Parlett for data management, and the young men for their participation.
Funding: The authors are supported by NIH grant T32DK007703-16 and P30DK46200 and European Union DEER Grant 212844. All sources of funding had no role in the research.
Footnotes
Competing Interests: None.
Author Contributions: All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis
Study concept and design: Swan.
Acquisition of data: Mendiola, Chavarro, Swan, Jørgensen.
Analysis and interpretation of data: Gaskins, Chavarro, Swan.
Drafting of the manuscript: Gaskins, Afeiche, Chavarro.
Critical revision of the manuscript for important intellectual content: Afeiche, Mendiola, Jørgensen, Swan, Chavarro.
Statistical analysis: Gaskins.
Contributor Information
Audrey J Gaskins, Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts, USA; Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts, USA.
Jaime Mendiola, Division of Preventive Medicine and Public Health, University of Murcia School of Medicine, Murcia, Spain.
Myriam Afeiche, Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts, USA; Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts, USA.
Niels Jørgensen, University Department of Growth and Reproduction, University of Copenhagen, Rigshospitalet, Copenhagen, Denmark.
Shanna H Swan, Department of Obstetrics and Gynecology, School of Medicine, University of Rochester, Rochester, New York, USA; Department of Preventive Medicine, Mount Sinai School of Medicine, New York, New York, USA.
Jorge E Chavarro, Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts, USA; Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts, USA.
References
- 1.Carlsen E, Giwercman A, Keiding N, et al. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jorgensen N, Joensen UN, Jensen TK, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger J, Kunstmann JM, Czyglik F, et al. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 4.Irvine S, Cawood E, Richardson D, et al. Evidence of deteriorating semen quality in the United Kingdom: birth cohort study in 577 men in Scotland over 11 years. BMJ. 1996;312:467–71. doi: 10.1136/bmj.312.7029.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Z, Hu H, Song M, et al. Semen quality analysis of military personnel from six geographical areas of the People's Republic of China. Fertil Steril. 2011;95:2018–23. 23 e1–3. doi: 10.1016/j.fertnstert.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 6.Bujan L, Mansat A, Pontonnier F, et al. Time series analysis of sperm concentration in fertile men in Toulouse, France between 1977 and 1992. BMJ. 1996;312:471–2. doi: 10.1136/bmj.312.7029.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisch H, Goluboff ET, Olson JH, et al. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–14. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- 8.Paulsen CA, Berman NG, Wang C. Data from men in greater Seattle area reveals no downward trend in semen quality: further evidence that deterioration of semen quality is not geographically uniform. Fertil Steril. 1996;65:1015–20. [PubMed] [Google Scholar]
- 9.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health. 2005;26:421–43. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- 11.Blair SN, Morris JN. Healthy hearts--and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–6. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Arce JC, De Souza MJ. Exercise and male factor infertility. Sports Med. 1993;15:146–69. doi: 10.2165/00007256-199315030-00002. [DOI] [PubMed] [Google Scholar]
- 13.De Souza MJ, Arce JC, Pescatello LS, et al. Gonadal hormones and semen quality in male runners. A volume threshold effect of endurance training. Int J Sports Med. 1994;15:383–91. doi: 10.1055/s-2007-1021075. [DOI] [PubMed] [Google Scholar]
- 14.Miller BE, Hackney AC, De Souza MJ. The endurance training on hormone and semen profiles in marathon runners. Fertil Steril. 1997;67:585–6. doi: 10.1016/s0015-0282(97)80096-9. author reply 6-7. [DOI] [PubMed] [Google Scholar]
- 15.Arce JC, De Souza MJ, Pescatello LS, et al. Subclinical alterations in hormone and semen profile in athletes. Fertil Steril. 1993;59:398–404. [PubMed] [Google Scholar]
- 16.Vaamonde D, Da Silva ME, Poblador MS, et al. Reproductive profile of physically active men after exhaustive endurance exercise. Int J Sports Med. 2006;27:680–9. doi: 10.1055/s-2005-872906. [DOI] [PubMed] [Google Scholar]
- 17.Vaamonde D, Da Silva-Grigoletto ME, Garcia-Manso JM, et al. Response of semen parameters to three training modalities. Fertil Steril. 2009;92:1941–6. doi: 10.1016/j.fertnstert.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Viru AM, Hackney AC, Valja E, et al. Influence of prolonged continuous exercise on hormone responses to subsequent exercise in humans. Eur J Appl Physiol. 2001;85:578–85. doi: 10.1007/s004210100498. [DOI] [PubMed] [Google Scholar]
- 19.Wise LA, Cramer DW, Hornstein MD, et al. Physical activity and semen quality among men attending an infertility clinic. Fertil Steril. 2011;95:1025–30. doi: 10.1016/j.fertnstert.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safarinejad MR, Azma K, Kolahi AA. The effects of intensive, long-term treadmill running on reproductive hormones, hypothalamus-pituitary-testis axis, and semen quality: a randomized controlled study. J Endocrinol. 2009;200:259–71. doi: 10.1677/JOE-08-0477. [DOI] [PubMed] [Google Scholar]
- 21.Gebreegziabher Y, Marcos E, McKinon W, et al. Sperm characteristics of endurance trained cyclists. Int J Sports Med. 2004;25:247–51. doi: 10.1055/s-2004-819933. [DOI] [PubMed] [Google Scholar]
- 22.Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–55. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl. 1995;18:169–84. doi: 10.1111/j.1365-2605.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 24.Stoy J, Hjollund NH, Mortensen JT, et al. Semen quality and sedentary work position. Int J Androl. 2004;27:5–11. doi: 10.1046/j.0105-6263.2003.00428.x. [DOI] [PubMed] [Google Scholar]
- 25.Hjollund NH, Storgaard L, Ernst E, et al. The relation between daily activities and scrotal temperature. Reprod Toxicol. 2002;16:209–14. doi: 10.1016/s0890-6238(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 26.Hjollund NH, Storgaard L, Ernst E, et al. Impact of diurnal scrotal temperature on semen quality. Reprod Toxicol. 2002;16:215–21. doi: 10.1016/s0890-6238(02)00025-4. [DOI] [PubMed] [Google Scholar]
- 27.Jung A, Leonhardt F, Schill WB, et al. Influence of the type of undertrousers and physical activity on scrotal temperature. Hum Reprod. 2005;20:1022–7. doi: 10.1093/humrep/deh697. [DOI] [PubMed] [Google Scholar]
- 28.Magnusdottir EV, Thorsteinsson T, Thorsteinsdottir S, et al. Persistent organochlorines, sedentary occupation, obesity, and human male subfertility. Hum Reprod. 2005;20:208–15. doi: 10.1093/humrep/deh569. [DOI] [PubMed] [Google Scholar]
- 29.Figa-Talamanca I, Cini C, Varricchio GC, et al. Effects of prolonged automobile driving on male reproduction function: a study among taxi drivers. Am J Ind Med. 1996;30:750–8. doi: 10.1002/(SICI)1097-0274(199612)30:6<750::AID-AJIM12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Laufs U, Wassmann S, Czech T, et al. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–14. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 31.Shiva M, Gautam AK, Verma Y, et al. Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin Biochem. 2011;44:319–24. doi: 10.1016/j.clinbiochem.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interactions. 4th. New York: Cambridge University Press; 1999. [Google Scholar]
- 34.Menkveld R, Stander FS, Kotze TJ, et al. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–92. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- 35.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27-36. [DOI] [PubMed] [Google Scholar]
- 36.Gaskins AJ, Colaci DS, Mendiola J, et al. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng HY, Hsueh YH, Messam LL, et al. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–90. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 38.Amann RP, Chapman PL. Total sperm per ejaculate of men: obtaining a meaningful value or a mean value with appropriate precision. J Androl. 2009;30:642–9. doi: 10.2164/jandrol.108.006825. [DOI] [PubMed] [Google Scholar]
- 39.Vaamonde D, Da Silva-Grigoletto ME, Garcia-Manso JM, et al. Physically active men show better semen parameters and hormone values than sedentary men. Eur J Appl Physiol. 2012 doi: 10.1007/s00421-011-2304-6. [DOI] [PubMed] [Google Scholar]
- 40.Hall HL, Flynn MG, Carroll KK, et al. Effects of intensified training and detraining on testicular function. Clin J Sport Med. 1999;9:203–8. doi: 10.1097/00042752-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Lucia A, Chicharro JL, Perez M, et al. Reproductive function in male endurance athletes: sperm analysis and hormonal profile. J Appl Physiol. 1996;81:2627–36. doi: 10.1152/jappl.1996.81.6.2627. [DOI] [PubMed] [Google Scholar]
- 42.Jensen CE, Wiswedel K, McLoughlin J, et al. Prospective study of hormonal and semen profiles in marathon runners. Fertil Steril. 1995;64:1189–96. [PubMed] [Google Scholar]
- 43.Chigurupati S, Son TG, Hyun DH, et al. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008;199:333–41. doi: 10.1677/JOE-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NCAA participation rates going up. National Collegiate Athletic Association; 2011. [cited 2012 April 25]; Available from: http://www.ncaa.com/news/ncaa/article/2011-11-02/ncaa-participation-rates-going. [Google Scholar]
- 45.Fudge BW, Westerterp KR, Kiplamai FK, et al. Evidence of negative energy balance using doubly labelled water in elite Kenyan endurance runners prior to competition. Br J Nutr. 2006;95:59–66. doi: 10.1079/bjn20051608. [DOI] [PubMed] [Google Scholar]
- 46.Black KE, Skidmore PM, Brown RC. Energy intakes of ultraendurance cyclists during competition, an observational study. Int J Sport Nutr Exerc Metab. 2012;22:19–23. doi: 10.1123/ijsnem.22.1.19. [DOI] [PubMed] [Google Scholar]
- 47.Redman LM. Physical activity and its effects on reproduction. Reprod Biomed Online. 2006;12:579–86. doi: 10.1016/s1472-6483(10)61183-2. [DOI] [PubMed] [Google Scholar]
- 48.Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–31. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Tremellen K. Oxidative stress and male infertility--a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 50.Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39:203–15. doi: 10.1111/j.1439-0272.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 51.Sripada S, Townend J, Campbell D, et al. Relationship between semen parameters and spontaneous pregnancy. Fertil Steril. 2010;94:624–30. doi: 10.1016/j.fertnstert.2009.02.085. [DOI] [PubMed] [Google Scholar]
- 52.Iwai N, Hisamichi S, Hayakawa N, et al. Validity and reliability of single-item questions about physical activity. J Epidemiol. 2001;11:211–8. doi: 10.2188/jea.11.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stokes-Riner A, Thurston SW, Brazil C, et al. One semen sample or 2? Insights from a study of fertile men. J Androl. 2007;28:638–43. doi: 10.2164/jandrol.107.002741. [DOI] [PubMed] [Google Scholar]