To the editor
Proteus syndrome (PS) is characterized by progressive, mosaic, segmental overgrowth and occurs sporadically (Biesecker 2001; 2006). The mosaic nature and sporadic occurrence with lack of familial transmission led to the hypothesis that PS is caused by a post-zygotic somatic mutation, which was confirmed with the discovery of a mosaic activating c.49G>A, p.Glu17Lys AKT1 mutation (Lindhurst et al. 2011). To date, all patients who meet the clinical diagnostic criteria for PS and have been tested have this mutation (Lindhurst and Biesecker, unpublished results).
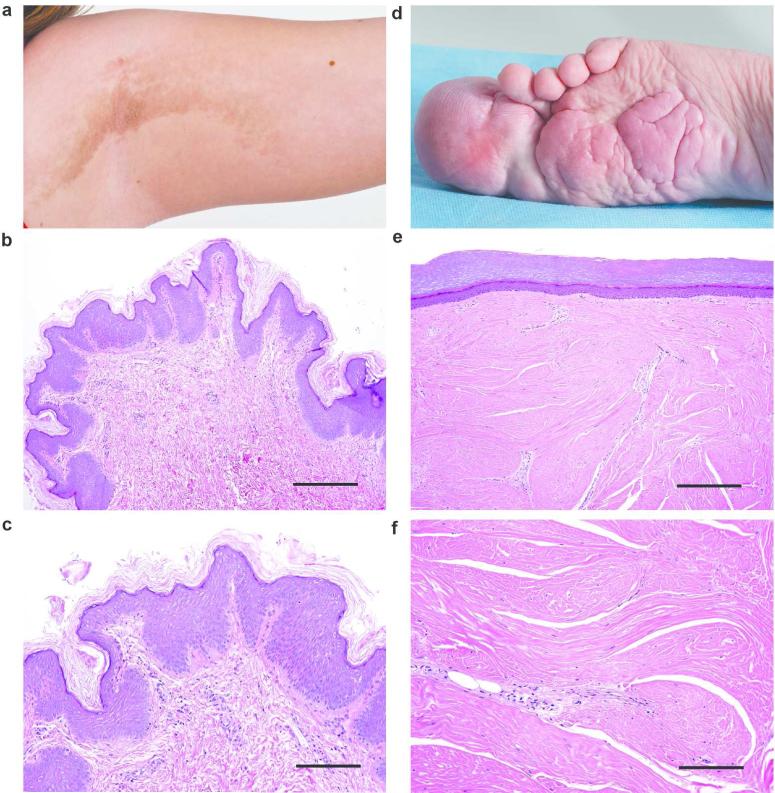
While any organ or tissue can be affected, skeletal overgrowth and dermatologic lesions are the most common manifestations of PS (Turner et al. 2004; Beachkofsky et al. 2010). Cerebriform connective tissue nevi (CCTN) are a highly specific and common lesion in patients with PS (Biesecker et al. 2001; Nguyen et al. 2004). These lesions are very firm and contain deep sulci that resemble the brain, for which the lesion is named. Histology sections of CCTN show massively expanded dermis filled with thick collagen bundles (Figure 1d-f) (McCuaig et al. 2012). Epidermal nevi (EN) can be non-syndromic or occur as part of several syndromes including PS (Happle 2010). The keratinocytic EN found in PS have a rough surface, are dark in color, usually follow the lines of Blaschko, and exhibit epidermal hyperkeratosis, papillomatosis, and acanthosis (Figure 1a-c) (Nguyen et al. 2004). EN are generally noticed in the first year of life and are stable in extent whereas CCTN grow progressively after first appearing later in the first or second year (Twede et al. 2005).
Figure 1.
Representative examples of an EN and CCTN. Panel a shows the rough surface of the linear EN on the infraaxillary vault in patient 101. Hematoxylin and eosin stain (b, scale bar = 100 μm; c, scale bar = 50 μm) of a skin biopsy from the EN in panel a. The lesion is characterized by papillomatosis, mild hyperkeratosis, and acanthosis. Panel d shows the CCTN encompassing the hallux of patient 75. Hematoxylin and eosin stain of skin obtained after amputation of the hallux (e, scale bar = 100 μm; f, scale bar = 50 μm). Note the extensive dense collagen matrix, compression of the papillary dermis and the smooth surface of the lesion.
It is unknown which cells determine the formation of these lesions. Based on the histology, we hypothesized that CCTN were generated by AKT1 p.Glu17Lys in the dermis and that EN were generated by this mutation in the epidermis. To test this hypothesis, we isolated fibroblasts and keratinocytes from lesional (CCTN or EN) and non-lesional (“normal”) skin samples and measured the level of the mutant allele in each cell type.
Skin samples were collected during surgical procedures or by punch biopsies from patients with PS under an IRB-approved protocol. The epidermis was separated from the dermis by treatment with dispase and keratinocyte and fibroblast cultures were established using standard protocols (Aasen and Belmonte 2010). A single keratinocyte culture was established from each biopsy. Fibroblasts were allowed to grow out of the dermal tissue until the dish was confluent. The dermal tissue was then transferred to a new dish to allow another fibroblast culture to be established from that same tissue. The number of fibroblast cultures established from a piece of dermis ranged from one to six. DNA was isolated from cultured cells harvested between passages one and four, and the mutation level was assayed using a PCR-based restriction fragment length polymorphism (RFLP) assay as described (Lindhurst et al. 2011). This assay has a lower limit of sensitivity of 0.5%. Each DNA preparation was tested at least twice and the values were averaged. In ten of the 20 samples, DNA was also isolated from the dermal tissue used to establish the fibroblast cultures after the final transfer.
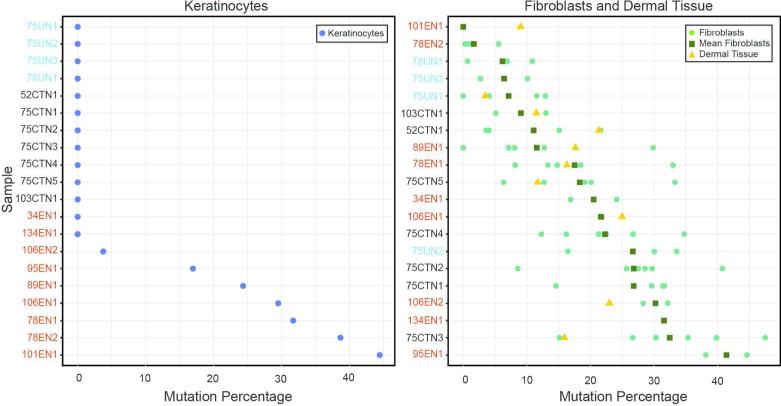
Seven CCTN biopsies were obtained from the feet of three patients (Table S1). The mutant allele was not detected in CCTN keratinocyte cultures whereas the mutation level from the CCTN fibroblast cultures was 9-32%. In the dermal tissue post-culturing, the mutation level was 12-21% (Figure 2).
Figure 2.
Percentage of AKT1 c.49G>A, p.Glu17Lys mutation in epidermal and dermal cells and tissue. One keratinocyte culture (left panel) was established from each epidermal sample. Mutation percentages are indicated by the blue circles. Multiple fibroblast cultures (right panel) were established from most pieces of dermal tissue. The mutation percentage for each fibroblast culture is indicated by a green circle. The mean mutation percentage for the fibroblast cultures from each sample is indicated by a green square and the mutation percentage in residual dermal tissue post culture is indicated by a yellow triangle. Samples are ordered from lowest to highest mutation value (keratinocytes) or mean mutation value (fibroblasts) and are color coded as follows: EN, red, CCTN, black, unaffected/unknown, light blue.
Nine EN samples were obtained from the hand, trunk, or neck from seven patients (Table S1). The mutation level in the keratinocyte cultures was 0-44% whereas the mutation level in the fibroblast cultures was 0-38%. The mutation level in the cultured dermal tissue was 9-25%. Interestingly, the two samples with the highest levels of mutation in the keratinocytes (101EN1 and 78EN2) had the lowest mutation percentages in dermal cells or tissue. Detection of the mutant allele in the EN keratinocytes is consistent with the identification of the AKT1 p.Glu17Lys mutation in skin scrapings of PS epidermal nevi (Wieland et al. 2013).
Four biopsies from two patients were obtained from tissue that appeared normal by gross examination but was from an area that was far removed from lesional tissue (designated as unaffected) or bordered a CCTN (designated as unknown) (Table S1). No evidence of the mutant allele was present in the keratinocyte cultures. The mean mutation level in the fibroblast cultures was 6-27%.
We conclude that the AKT1 p.Glu17Lys activating mutation in keratinocytes is a key determinant of EN formation. Mutations in the FGFR3, PIK3CA, and RAS genes have been identified in epidermal cells of EN not associated with PS, supporting this hypothesis (Hafner et al. 2006; 2007; 2012). Histopathologically, these keratinocytic EN are strikingly similar to those found in PS and it should be noted that PIK3CA encodes the catalytic subunit of the PI3K complex which functions upstream of AKT in the same signaling pathway. In contrast, there was no correlation of the mutation levels in fibroblasts or cultured dermal tissue with the clinical lesion type. The inability to detect an AKT1 mutation in two of the EN keratinocyte cultures could be due to a sampling artifact if the mutant cells were not uniformly distributed throughout the lesion and the sample was from an area with low-level mosaicism. It seems unlikely that the lack of mutant keratinocytes in some EN indicates that these lesions form as a result of signaling from mutant cells in the dermis, since even organoid EN, such as nevus sebaceous have been shown to result from mutant cells in the epidermis. (Groesser et al. 2012) Mutant cells were found in dermal fibroblasts of both CCTN and normal-appearing skin, suggesting that the presence of mutant cells in the dermis is necessary but not sufficient to drive the formation of CCTN. The propensity of CCTNs to develop on soles and palms in PS may reflect a greater role of AKT1 in postnatal regulation of tissue architecture at these sites than elsewhere on the skin.
Supplementary Material
Acknowledgments
We thank the patients and their families for their participation in this study, Shawn McCandless for patient referral, Julie Sapp, Lauren Ivey, and Stephanie Dugan for their clinical support and advice, and Julia Fekecs for graphics support. This work was supported by the Intramural Research Program of the National Human Genome Research Institute.
Footnotes
Conflict of Interest
Leslie Biesecker is an uncompensated advisor to Illumina Corporation. Dr. Biesecker and Lindhurst receive royalties from Genentech for cell lines. The other authors state no conflict of interest.
References
- Aasen T, Belmonte JCI. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc. 2010;5:371–82. doi: 10.1038/nprot.2009.241. [DOI] [PubMed] [Google Scholar]
- Beachkofsky TM, Sapp JC, Biesecker LG, et al. Progressive overgrowth of the cerebriform connective tissue nevus in patients with Proteus syndrome. J Am Acad Dermatol. 2010;63:799–804. doi: 10.1016/j.jaad.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker L. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14:1151–7. doi: 10.1038/sj.ejhg.5201638. [DOI] [PubMed] [Google Scholar]
- Biesecker LG. The Multifaceted Challenges of Proteus Syndrome. JAMA. 2001;285:2240–3. doi: 10.1001/jama.285.17.2240. [DOI] [PubMed] [Google Scholar]
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783–7. doi: 10.1038/ng.2316. [DOI] [PubMed] [Google Scholar]
- Hafner C, Lopez-Knowles E, Luis NM, et al. Oncogenic PIK3CA mutations occur in epidermal nevi and seborrheic keratoses with a characteristic mutation pattern. Proc Natl Aca Sci USA. 2007;104:13450–4. doi: 10.1073/pnas.0705218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner C, Toll A, Gantner S, et al. Keratinocytic epidermal nevi are associated with mosaic RAS mutations. J Med Genet. 2012;49:249–53. doi: 10.1136/jmedgenet-2011-100637. [DOI] [PubMed] [Google Scholar]
- Hafner C, van Oers JMM, Vogt T, et al. Mosaicism of activating FGFR3 mutations in human skin causes epidermal nevi. J Clin Invest. 2006;116:2201–7. doi: 10.1172/JCI28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happle R. The group of epidermal nevus syndromes. J Am Acad Dermatol. 2010;63:1–22. doi: 10.1016/j.jaad.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Lindhurst MJ, Sapp JC, Teer JK, et al. A Mosaic Activating Mutation in AKT1 Associated with the Proteus Syndrome. N Engl J Med. 2011;365:611–9. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuaig CC, Vera C, Kokta V, et al. Connective tissue nevi in children: institutional experience and review. J Am Acad Dermatol. 2012;67:890–7. doi: 10.1016/j.jaad.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Nguyen D, Turner JT, Olsen C, et al. Cutaneous Manifestations of Proteus Syndrome. Correlations With General Clinical Severity. Arch Dermatol. 2004;140:947–53. doi: 10.1001/archderm.140.8.947. [DOI] [PubMed] [Google Scholar]
- Turner JT, Cohen MM, Jr., Biesecker LG. Reassessment of the Proteus syndrome literature: Application of diagnostic criteria to published cases. Am J Med Genet. 2004;130A:111–22. doi: 10.1002/ajmg.a.30327. [DOI] [PubMed] [Google Scholar]
- Twede JV, Turner JT, Biesecker LG, et al. Evolution of skin lesions in Proteus syndrome. J Am Acad Dermatot. 2005;52:834–8. doi: 10.1016/j.jaad.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Wieland I, Tinschert S, Zenker M. High-level somatic mosaicism of AKT1 c.49G>A mutation in skin scrapings from epidermal nevi enables non-invasive molecular diagnosis in patients with Proteus syndrome. Am J Med Genet. 2013;161:889–91. doi: 10.1002/ajmg.a.35764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.