Abstract
Radical cyclizations of cyclic ene sulfonamides provide stable bicyclic and tricyclic aldimines and ketimines in good yields. Depending on the structure of the precursor, the cyclizations occur to provide fused and spirocyclic imines with five-, six-, and seven-membered rings. The initial radical cyclization produces an α-sulfonamidoyl radical that undergoes elimination to form the imine and a phenylsulfonyl radical. In a related method, 3,4-dihydroquinolines can also be produced by radical translocation reactions of N-(2-iodophenylsulfonyl)tetrahydroisoquinolines. In either case, very stable sulfonamides are cleaved to form imines (rather than amines) under mild reductive conditions.
Introduction
The quintessential reaction of β-sulfonyl radicals is fragmentation to form a sulfonyl radical and a multiple bond.1 Such β-elimination reactions have broad synthetic utility. The radical prior to fragmentation can be centered on either carbon or nitrogen (Figure 1). For example, the elimination reaction of β-sulfonyl alkyl radicals in Figure 1a is commonly applied to make alkenes from β-functionalized sulfones, allyl sulfones and alkenyl sulfones.2 The example reaction from Zard is a typical addition/fragmentation reaction of an allyl sulfone.3
Figure 1.
Three classes of radicals that can undergo β-elimination of sulfonyl radicals to make C=C and C=N bonds
Carbon-nitrogen double bonds can also be made by two different kinds of sulfonyl radical eliminations. The elimination of β-sulfonyl aminyl and related radicals has been developed by Kim and others into a useful method to make assorted imines, hydrazones and, as exemplified in Figure 1b, oximes.4
The reverse positioning of the sulfonyl group and the radical—β-elimination of a sulfonyl group from an α-sulfonamidoyl radical (Figure 1c)—is less common and has a checkered history. Often, this history involves reactions of N-alkenylsulfonamides, hereafter called ene sulfonamides. In 1958, McKusick and coworkers reported that the isomerization of ene sulfonamide 1 to β-sulfonyl enamine 2 was induced by X-rays (Figure 2a).5 This isomerization can also be triggered by photolysis or thermolysis with AIBN, and studies supported a sulfonyl radical addition/elimination mechanism.5c
Figure 2.
Possible examples of sulfonyl radical eliminations to make imine intermediates under non-reducing conditions.
More recently, Zard6 and Renaud7 have introduced transformations based on carbon-radical additions to functionalized ene-sulfonamides to make functionalized ketones or heterocycles, depending on the associated functional groups. In an example from Renaud7 (Figure 2b), treatment of in situ generated catechol borane 3 (the radical precursor) with ene sulfonamide 4 and di-t-butyl hydroperoxide (DTHP) gave α-ketoester 5 in 20% overall yield from 1-octene (the precursor of 3).
Murphy has also observed that N-sulfonyl groups are lost in several transformations.8 For example, treatment of diazonium ion 9 with tetrakisdimethylaminoethylene (TDAE) provided indole 10 in 33% yield (Figure 2c) as one of several products.8b
All of the transformations in Figure 2 are multi-step processes which may or may not include sulfonyl radical elimination steps. Take Renaud’s reaction (b), for example. Addition of the radical derived from 3 to alkene 4 provides α-benzenesulfonamidoyl radical 6. One sensible route to the product 5 is sulfonyl radical elimination to an imine 7 followed by hydrolysis. However, the conditions are oxidizing, so direct oxidation of 6 to a sulfonyl iminium ion 89 then hydrolysis could provide a path to the product 5 that does not involve sulfonyl radical elimination.
The evidence for such sulfonyl radical eliminations under reducing conditions is even less clear cut, as shown by the two examples in Figure 3.10 In an approach to (–)-kainic acid, Cossy reported in 1999 that cyclization of enantiopure 11 with tributyltin hydride unexpectedly provided imine 13 (Figure 3a).11 She had expected a cyclative hydrostannation to occur starting with tin radical addition to the alkyne. The actual product 13 was indeed cyclized, but it contained neither tin nor the sulfonyl group. This, and Somfai’s result coming up in Figure 3b, led Cossy to suggest that enamidyl radical 12 was the key intermediate in this reaction.
Figure 3.
Tin hydride cyclizations of similar ene sulfonamides give contrasting results
Somfai in turn had reported in 1994 that syringe pump addition of tin hydride to ene sulfonamide 14 provided the reduced spirocyclic sulfonamide 16 in 57% isolated yield.12 This reaction presumably involves α-sulfonamidoyl radical 15, which apparently abstracts hydrogen from tin hydride even under syringe pump conditions. Likewise, a homolog of 14 with a six-membered ring ene sulfonamide underwent reductive cyclization in similar yield.
The collective evidence in Figures 2 and 3 for the β-sulfonyl radical elimination reaction to form imines is circumstantial because the direct products of such reactions are not isolated. Further, the low yields and formation of competing products in some reactions combine to suggest that even if this elimination does occur at times, it is not very efficient.
We now report that we have found strong evidence in the search for evidence of β-sulfonyl radical eliminations from α-sulfonamidoyl radicals. Specifically, we have isolated members of a family of stable imines—the primary products of such reactions—in good yields. The elimination has good synthetic potential because a strong N–SO2Ar bond is cleaved under mild conditions with concomitant ring formation. We also introduce a radical translocation variant that has value as a new deprotection reaction. Lastly, we revisit the contrasting results of Cossy and Somfai to better understand whether β-sulfonyl radical eliminations are involved.
Results and Discussion
Discovery and mechanism of the imine-forming reaction
We first encountered the imine-forming reaction during pilot studies directed towards the synthesis of the meloscine/epimeloscine (17a,b) class of natural products.13,14 The goal was to learn how to make the B-ring of ABD tricycles like 18 by radical cyclizations and to determine the favored configuration of such cyclizations when R ≠ H.
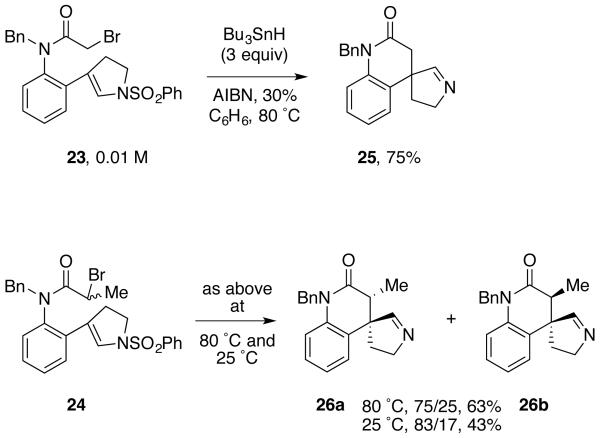
Scheme 1 shows the synthesis of two key precursors and the results of the first radical cyclizations. Initially we made precursors like 23 by a reliable Stille coupling route (detailed in the Supporting Information), but we later switched to the Suzuki coupling route in Scheme 1 because it was more flexible for varying substituents in target radical precursors. Coupling of 2-(pinacolatoboranyl)aniline 19 with readily available 4-iodo-1-(phenylsulfonyl)-2,3-dihydro-1H-pyrrole 2015 provided aniline 21 in 79% yield. Reductive N-benzylation to give 22 (69% yield) was followed by acylation with bromoacetyl bromide and 2-bromopropanoyl bromide. The α-bromoacetanilides 23 and 24 were obtained in 83% and 87% yields.
Scheme 1.
The syntheses of 23 and 24 typify the synthesis of the various radical precursors in this study
Bromoacetamide 23 has two axes that can provide rotational isomers. Rotation of the N–C(=O) bond (a) is a standard amide rotation that can provide E and Z isomers while rotation of the N–Ar bond (b) provides a pair of enantiomers. There is only one set of signals in the 1H NMR spectrum of 23 (>95%), which we assign to the depicted E-amide rotamer (C=O and N–Ar trans).16 All the geminal methylene protons of 23 are diastereotopic, confirming that it is a mixture of enantiomers about the N–Ar axis on the NMR time scale.
The corresponding α-bromopropanamide 24 adds a stereocenter to the rotatable amide and N–Ar bonds, so four diastereomers of 24 are possible.17 This amide is a 70/30 mixtures of diastereomers according to 1H NMR analysis. These must be the E-rotamers of the two possible diastereomers created by the stereocenter and the N–Ar axis. The diastereomers cannot be separated by chromatography, so the barrier to N–Ar bond rotation is less than about 22 kcal/mol. Related diastereomers with higher N–Ar rotation barriers have been separated and shown to give the same products on radical cyclization.17a So separation is pointless because the two diastereomers of 24 will give the same radical and hence the same result on radical cyclization.
The results of tin hydride reactions with 23 and 24 are summarized in Scheme 2. In a typical cyclization experiment, a benzene solution of 23 (1 equiv, 0.01 M), Bu3SnH (3 equiv) and AIBN (0.3 equiv) was heated at 80 °C for 30 min. The starting material was consumed and a single major new product was evident on TLC analysis. Evaporation of the solvent and flash chromatography provided the new product in 75% yield. This was not the expected product of reductive cyclization (see 18 in Figure 2), but instead the stable spirocyclic aldimine 25. The imine structure of 25 was clear from the various spectra. For example, the sulfonyl group was absent, and correlated resonances at 7.51 ppm (singlet) in the 1H NMR spectrum and 167.8 ppm in the 13C NMR are diagnostic of the imine CH atoms. Other NMR and HRMS data are fully consistent with structure 25.
Scheme 2.
Radical cyclizations to ene sulfonamides provide stable cyclic aldimines
Cyclization of 24 was conducted under similar conditions at both 80 °C (AIBN initiation) and room temperature (rt, Et3B initiation18). The 80 °C experiment gave imine epimers 26a and 26b as a 75/25 mixture in 63% isolated yield. This ratio increased to 83/17 in the rt experiment, and the isolated yield was 43%.
The configuration of the major isomer from cyclization of 24 was initially assigned as 26a based on NOESY experiments on the mixture (see Supporting Information). Later we succeeded in crystallizing the major diastereomer from the mixture, and its X-ray crystal structure confirmed both the constitution and the configuration. This structure, shown in Figure 5, provides clues as to why these imines are so stable. The lactam ring (B) of 26a adopts a distorted half-chair conformation in which the smaller imine CH-group on the spiro carbon atom is pseudo-equatorial and the larger CH2-group is pseudo-axial. The larger group adopts the pseudo-axial location because of A-strain19 and because there are no 1,3-diaxial interactions. In the pseudo-equatorial location, the imine carbon atom is shielded by the fused aromatic ring (especially the adjacent ortho-hydrogen) on one side and by the substituents on C3 of the lactam on the other side.
Figure 5.
X-ray crystal structure of 26a (left) along with standard representation focusing on the lactam ring conformation and substituents (right)
The mechanism for formation of these products is illustrated in Figure 6a with the simpler precursor 23. A tributyltin radical abstracts bromine from 23 to give α-amide radical 27, which then undergoes 6-exo cyclization by adding to the β-carbon atom of the ene sulfonamide. The resulting α-sulfonamidoyl radical 28 ejects the phenylsulfonyl radical (PhSO2·) in a β-fragmentation reaction to give imine 25. This imine is robust, and it survives both heating with excess tin hydride (a potential ionic hydride source) and silica gel chromatography. It cannot tautomerize to an enamine. Its isolation is strong evidence implicating the β-elimination of α -sulfonamidoyl radicals because it is the primary product this reaction.
Figure 6.
Evidence for β-fragmentation: (a) Suggested steps and intermediates for formation of the imine and (b) possible fates of the tin and sulfur reaction components
Focusing on the phenylsulfonyl radical product1a,2a of the β -fragmentation reaction, we can further speculate that this abstracts a hydrogen atom from tin hydride to generate tributyltin radical (Bu3Sn·) and benzenesulfinic acid (PhSO2H).20a,b This is a chain transfer step provided that the original bromine abstraction reaction by the tin radical (23 → 27) competes effectively with possible back hydrogen atom transfer.21
Benzenesulfinic acid is an unstable compound prone to disproportionation and other reactions.2a And with a pKa of about 2.7, it can also be expected to undergo an acid/base reaction with Bu3SnH as shown in Figure 6b. If this reaction is quantitative, then 2 equiv of Bu3SnH are needed for the overall reaction. Indeed, the use of 1 equiv of Bu3SnH in the pilot reductions in Scheme 1 did not provide high conversions of precursor 24.22a Likewise, tin hydide addition/elimination reactions of allyl sulfones require 2 equiv of tin hydride.23 This suggests that a significant amount of tin hydride is consumed either by the indicated acid/base reaction or by other reactions with the sulfur-derived product(s).20b
Scope of the imine-forming reaction
Next we surveyed the scope of the imine-forming reaction by varying substituents and ring sizes, and the results of these studies are summarized in Table 1. The precursors were all made by suitable variations of the route outlined in Scheme 1, and complete details (experimental procedures, characterization of intermediates) are in the Supporting Information. The radicals derived from the precursors in Table 1 may undergo the initial cyclization at different rates. To maximize the chances for cyclization rather than direct reductive debromination, we switched to a standard syringe pump procedure for these reactions. The crude products were purified by flash chromatography to provide the isolated yields in Table 1.
Table 1. Scope of the new imine forming reaction. Isolated yields after flash chromatography are recorded.

|
Cyclization of 2-bromo-2-methylpropanamide 29, a more substituted analog of 23 and 24, provided imine 30 with a quaternary center adjacent to the spirocenter in 81% yield (entry 1). Precursors 31 and 33 have a six-membered ene sulfonamide ring, one without (31) and one with (33) additional methyl groups on the carbon bearing the radical precursor. Isolated yields of six-membered cyclic imines 32 and 34 were 73% and 50% (entries 2 and 3). These precursors all form spirocyclic aldimines on tin hydride reaction. The precursor 35 bears an addition ethyl group on the α-carbon atom of the ene sulfonamide. This gives spirocyclic ketimine product 36 in 61% yield.
Finally, we prepared a 2-bromo-2-methylpropanamide precursor 37 that has the ene sulfonamide as part of a seven-membered ring. Cyclization of 37 provided imine 38 with spirofused six- and seven-membered rings in 40% isolated yield (entry 5). In this seven-membered ring series, the geminal dimethyl group adjacent to the spiro-carbon was important for product stability.22b In contrast, the five- and six-membered imines were stable regardless of amide substitution pattern.
Imine products predominated in every case in Scheme 2 and Table 1, so this suggests that the β-fragmentation reaction of the intermediate α-sulfonamidoyl radicals is rather general. All the imines in Table 1 were again stable and easily isolable. Overall, this is an appealing method to make functionalized spirocyclic imines.
Imine-formation by radical translocation
If β-fragmentation is a core reaction of α-sulfonamidoyl radicals, then it should be possible to make such radicals by another route and again observe the formation of imines. We chose radical translocation24 to generate such radicals based in part on Murphy’s minor product in Figure 2c.25 However, we used halide rather than diazo radical precursors to retain the opportunity for tin hydride mediated reactions.
The results of four simple but informative experiments are summarized in Scheme 3. Reduction of N-(2-bromophenylsulfonyl)pyrrolidine 39 and the corresponding piperidine homolog 40 with Bu3SnH at 80 °C under the usual thermal conditions provided principally the directly debrominated products 41 and 42. We suspected that these products formed because the radical translocation (a 1,5-hydrogen atom transfer reaction) failed, not because the elimination reaction failed.
Scheme 3.
Radical translocation reactions lead to oxidative removal of N-(2-halosulfonyl) groups under mild reductive conditions provided that the precursors have suitably activated C–H bonds
To address this problem, we made the two N-(2-halophenylsulfonyl)tetrahydroisoquinolines 43a (X = I) and 43b (X = Br). These have a pair of C–H bonds that are additionally activated for radical translocation by the adjacent aryl ring. Reduction of 43a,b with Bu3SnH under the standard thermal conditions followed by flash chromatography provided the stable 3,4-dihydroisoquinoline 44 in 55% and 46% yield, repectively.
The dihydroisoquinoline product 44 arises from a sequence of iodine atom abstraction to give aryl radical 45 followed by radical translocation to give 46 then β-fragmentation to give the imine functional group embedded in the products 44. These results show that the imine-forming reaction is a characteristic of α-sulfonamidoyl radicals that is independent of their method of generation. Beyond that, such reactions could have value in synthesis. Sulfonyl groups are attractive N-protecting groups because sulfonamides are so stable.26 The knock on N-sulfonyl groups is that they are hard to remove by either hydrolysis or reduction.27 Here the 2-halosulfonyl group functions as “self-oxidizing protecting group”;28 it is removed under mild reductive conditions to give a valuable imine product that is oxidized on the carbon skeleton with respect to the precursor.
Understanding the contrasting prior results
To complete the study, we came full circle to the contrasting results of Cossy and Somfai (Figure 3). Recall that starting from similar ene sulfonamide precursors, Cossy obtained a fused imine 13 lacking the N-sulfonyl group11 but Somfai obtained a standard reduced spricycle 16 retaining the N-sulfonyl group.12
Figure 7 shows possible products in tin hydride reactions of Cossy’s substrate 11. At issue is which functional group in 11 is the radical precursor in the cyclization and which is the radical acceptor. Cossy had planned a hydrostannation reaction in which the alkyne is the radical precursor and the ene sulfonamide is the acceptor, path (a) (going downward from 11). Addition of tributyltin radical to 11 gives alkenyl radical 47. The expected final product of cyclization of 47 at that time was reduced alkenyl stannane 48, but this was not isolated.
Figure 7.
Products in the cyclization of 11 depend on which functional group is the precursor in the cyclization and which is the acceptor. Path (a), the alkyne is the precursor and the ene sulfonamide is the acceptor. Path (b), the ene sulfonamide is the precursor and the alkyne is the acceptor. The isolated product 13 is a secondary product of path (a) derived from 49, not a primary product from reductive desulfonylation path (b).
The absence of 48 led to the suggestion that the observed product 13 arose from a cyclization in which the ene sulfonamide was the radical precursor and the alkyne was the radical acceptor, path (b) (going to the right from 11). This is a reductive desulfonylation, not a hydrostannation. The problem with this suggestion is that the conversion of 11 to 12 is a homolytic substitution reaction at a sulfonyl group—a reaction that is widely thought to be unfavorable.29 Previous suggestions of such reactions have been refuted both experimentally30 and computationally.31 However, the focus of much of this prior work has been on substitution of sulfonyl groups by second-row radicals (especially carbon radicals) rather than tin radicals. In contrast, homolytic substitutions at sulfur atoms in lower oxidation states (for example, ArSR and ArS(O)R) with carbon, tin and other radicals are common.32
With the hindsight provided by the new results, we can see that Cossy’s original line of thinking (the alkyne is the precursor, path (a)) could well be correct. However, we now see that the expected product of the tin radical addition to the alkyne is imine 49 resulting from sulfonyl radical elimination rather than reduced sulfonamide 48 from hydrogen transfer. Could it be that imine 49 was formed in Cossy’s reaction, then protodestannylated to 13 by the in situ generated benzenesulfinic acid?33
To address this issue, we synthesized bromide 50 (Scheme 4) as described fully in the Supporting Information. The bromine atom in 50 that replaces the alkyne in 11 can only serve as a radical precursor, not a radical acceptor. Thus, the origin of any cyclized product formed in reduction of 50 is straightforward to determine.
Scheme 4.

Tin hydride reduction of 50. The bromide, not the ene sulfonamide, functions as a radical precursor.
Reduction of 50 with Bu3SnH was conducted at room temperature under conditions similar to Cossy’s (Et3B initiation). The major product was the imine 51 (Scheme 4), which was isolated in 54% yield by flash chromatography and fully characterized. Clearly in substrate 50, the bromide functioned as the radical precursor and the ene sulfonamide functioned as the radical acceptor.
The results of this experiment suggest that the imine product 13 in Cossy’s reaction was formed by the path (b) in Figure 7 with the steps of 1) tin radical addition to the alkyne 11 to give 47, 2) 5-exo cyclization, and 3) β-fragmentation to eliminate the sulfonyl group and form the imine 49. Finally, 4) ionic protodestannylation of this primary product 49 produces the isolated product 13.
With new understanding of Cossy’s reaction, it is now Somfai’s result (no imine formation in Figure 3b) that with hindsight looks out of place. Thus, we resynthesized bromide 14 by Somfai’s published procedures12 to revisit its cyclization chemistry.
Syringe pump addition of tributyltin hydride to bromide 14 as described by Somfai gave somewhat variable results, as summarized in Figure 8 and described more fully in the PhD thesis of H. Zhang.34 Two products were consistently formed—an unstable product and stable product. The inconsistencies were with percent conversion and the ratio of the two products; however, the ratio trend was consistent: the unstable product was always major and the stable product was always minor.
Figure 8.
Syringe pump addition of tin hydride to 14 gives not one but two products: one stable to chromatography (16) and one unstable (52)
In a typical syringe pump experiment, the stable product was isolated in 15% yield by flash chromatography and proved to be Somfai’s reduced product 16 retaining the N-sulfonyl group. The 1H NMR spectra of several crude mixtures recorded before chromatography showed that the unstable product was spirocyclic imine 52.35 For example, the protons of the N-CH2 group resonated as a doublet of triplets, J = 2.1 Hz and 6.9 Hz at 3.86 ppm, in the 1H NMR spectrum. When an integration standard was added (1,3,5-trimethoxybenzene), the calculated yield of the imine 52 was typically 45–60%.
Syringe pump experiments like these are difficult to reproduce in the best of cases because there are many variables. And the cyclization of 14 is far from a best case scenario for two reasons. First, the benzenesulfinic acid byproduct is a good hydrogen atom donor that is nicely matched with the α-sulfonylamidoyl radical 15 for polarity reverse catalysis.36 This acid may be consumed less efficiently under syringe pump conditions because tin hydride is always deficient. Thus, the syringe pump procedure might provide more powerfully reducing conditions rather than less. Second, alkyl bromides are electrophiles and ene sulfonamides are nucleophiles, so non-radical reactions may compete, especially under the long heating involved in the syringe pump procedure.
To mitigate the first problem, we switched to the standard fixed concentration conditions so that tin hydride is never deficient during the reaction course.37 To eliminate the possibility of a competing intramolecular SN2 reaction, we replaced the bromide 14 by the analogous phenylselenide 53.
The results of two key experiments with 53 are summarized in Scheme 5. In both experiments, 53 (0.01 M) was reduced with 3 equiv of tin hydride at 80 °C (AIBN initiation). After 30 min, TLC analysis showed that the precursor was largely consumed. In the first reaction, the solvent was evaporated and the crude product was dissolved in CDCl3 with an integration standard. The major product was imine 52 in 60% yield. Resonances from the reduced product 16 were not evident in this spectrum.
Scheme 5.
Results of tin hydride reactions of phenylselenide 53 and alcohol 55
In the second reaction, a solution of sodium borohydride in ethanol was added after tin hydride treatment to reduce any imine present to an amine. This crude mixture was exposed to benzoyl chloride and pyridine, then the resulting product was purified by flash chromatography to provide benzamide 54 in 65% yield.
These results show that the formation of imine 51 by sulfonyl radical elimination is more rapid than hydrogen atom abstraction from tin hydride under fixed tin hydride conditions and syringe pump conditions. We understand why Somfai did not observe the imine 51 (because he purified his products by flash chromatography), though we are not sure why he isolated about 30% more reduced product 16 from his syringe pump experiment than we typically observed. From the vantage point of the β-fragmentation reaction, the key observation is that the cyclization results with 14 are generally consistent with Cossy’s prior results and with our new results.
Finally, to test whether ene sulfonamides are competent radical precursors at all under these conditions, we treated alcohol 55 (Scheme 5) with tributyltin hydride under the standard thermal conditions (80 °C). No new products was detected, and most of the starting material was recovered. Thus, the sulfonyl groups of ene sulfonamides are not subject to facile homolytic substition reactions by tin radicals.29-31
Conclusions
Tin hydride mediated radical cyclizations of ene sulfonamides are general reactions that provide bicyclic and tricyclic aldimines and ketimines in good yields. Depending on the structure of the precursor, the cyclizations occur in 5-exo, 6-exo or 7-exo modes to provide fused or spirocyclic imines. With the exception of the small spirocyclic imine 52, all of the cyclic imines herein are robust compounds that are not reduced ionically by tin hydride under the reaction conditions and that are readily purified by flash chromatography. Reinvestigation of the contrasting prior results from Cossy11 and Somfai12 showed that imine products predominated in both cases.
Mechanistically, the initial radical cyclization produces an α-sulfonamidoyl radical that undergoes elimination to form the imine and a phenylsulfonyl radical. This β-elimination reaction has been postulated in the past based on the structures of downstream products that have been isolated in related reactions (Figures 2 and 3). The isolation of the many primary imine products in this work does not prove that imines are intermediates in all of these prior reactions. However, it cements the case for the β-elimination as a core reaction of α-sulfonamidoyl radicals.
In a related method, 3,4-dihydroquinolines can also be produced by radical translocation reactions of N-(2-halophenylsulfonyl)-tetrahydroisoquinolines. Accordingly, readily available N-(2-halophenylsulfonyl) groups can now serve as so-called self-oxidizing protecting groups,28 although C-H bonds that are activated towards 1,5-hydrogen transfer seem to be an important prerequisite. As with the cyclative transformation, very stable sulfonamides are cleaved to form imines (rather than amines) under mild reductive conditions.
Imines are synthetic intermediates in a vast collection of two-component and multi-component addition reactions.38 By far the most common way to make imines, whether stable species or transient reaction intermediates, is by the condensation of aldehydes or ketones with amines.39 The ability to made imines by a sulfonyl radical elimination, especially when coupled with a prior radical reaction (here, the cyclizations), provides a powerful alternative to the usual condensation route that differs both in the bonds that are formed and in the reaction conditions. These differences promise to offer expanded opportunities for imine chemistry in synthesis.
Supplementary Material
Figure 4.

Structures of meloscine natural products 17 and target tricyclic analogs 18
ACKNOWLEDGMENT
We thank the National Institutes of Health and the National Science Foundation for funding.
Footnotes
ASSOCIATED CONTENT
The Supporting Information contains details of all new experiments. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Chatgilialoglu C, Bertrand MP, Ferreri C. In: Sulfur-Centered Radicals. Alfassi ZB, editor. Wiley; West Sussex: 1999. pp. 311–354. [Google Scholar]; (b) Bertrand F, Le Guyader F, Liguori L, Ouvry G, Quiclet-Sire B, Seguin S, Zard SZ. C. R. Acad. Sci. Ser. II C. 2001;4:547–555. [Google Scholar]; (c) Rosenstein I. In: Radicals in Organic Synthesis. 1st ed. Renaud P, Sibi M, editors. Vol. 1. Wiley-VCH; Weinheim: 2001. pp. 50–71. [Google Scholar]
- 2.(a) Chatgilialoglu C. In: Chemistry of Sulphones and Sulphoxides. Patai S, Rappoport Z, editors. Wiley; Chichester: 1988. pp. 1089–1113. [Google Scholar]; (b) Bertrand MP. Org. Prep. Procedure Int. 1994;26:257–290. [Google Scholar]
- 3.Le Guyader F, Quiclet-Sire B, Seguin S, Zard SZ. J. Am. Chem. Soc. 1997;119:7410–7411. [Google Scholar]
- 4.Kim S, Lee IY, Yoon J-Y, Oh DH. J. Am. Chem. Soc. 1996;118:5138–5139. Kim S, Yoon JY. J. Am. Chem. Soc. 1997;119:5982–5983. Kim S, Kim N, Yoon JY, Oh DH. Synlett. 2000:1148–1150. Such eliminations can also be used to make C=O and N=bonds. For example, Kim SG, Jin RC. Synlett. 1992:629–630. Lee JY, Lim K-C, Meng X, Kim S. Synlett. 2010;2010:1647–1650.
- 5.(a) Stacey FW, Sauer JC, McKusick BC. J. Am. Chem. Soc. 1959;81:987–992. [Google Scholar]; (b) Graftieaux A, Gardent J. Tetrahedron Lett. 1972;13:3321–3324. [Google Scholar]; (c) Hertler WR. J. Org. Chem. 1974;39:3219–3223. [Google Scholar]
- 6.(a) Quiclet-Sire B, Wendeborn F, Zard SZ. Chem. Commun. 2002;0:2214–2215. doi: 10.1039/b207061h. [DOI] [PubMed] [Google Scholar]; (b) Quiclet-Sire B, Tran NDM, Zard SZ. Org. Lett. 2012;14:5514–5517. doi: 10.1021/ol3026044. [DOI] [PubMed] [Google Scholar]
- 7.Darmency V, Renaud P. Chimia. 2005;59:109–110. [Google Scholar]
- 8.(a) Bommezijn S, Martin CG, Kennedy AR, Lizos D, Murphy JA. Org. Lett. 2001;3:3405–3407. doi: 10.1021/ol0166449. [DOI] [PubMed] [Google Scholar]; (b) Mahesh M, Murphy JA, LeStrat F, Wessel HP. Beilstein J. Org. Chem. 2009;5:1. doi: 10.3762/bjoc.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curran DP, Guthrie DB, Geib SJ. J. Am. Chem. Soc. 2008;130:8437–8445. doi: 10.1021/ja8012962. [DOI] [PubMed] [Google Scholar]
- 10.Murphy also observed a minor desulfonylation product that might arise from sulfonyl radical elimination in an unusual reductive indole-forming reaction, see reference 7a.
- 11.Cossy J, Cases M, Pardo DG. Tetrahedron. 1999;55:6153–6166. [Google Scholar]
- 12.Ahman J, Somfai P. J. Chem. Soc., Perkin Trans. 1. 1994:1079–1082. [Google Scholar]
- 13.Zhang H, Curran DP. J. Am. Chem. Soc. 2011;133:10376–10378. doi: 10.1021/ja2042854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Overman LE, Robertson GM, Robichaud AJ. J. Am. Chem. Soc. 1991;113:2598–2610. [Google Scholar]; (b) Selig P, Herdtweck E, Bach T. Chem. Eur. J. 2009;15:3509–3525. doi: 10.1002/chem.200802383. [DOI] [PubMed] [Google Scholar]; (c) Hayashi Y, Inagaki F, Mukai C. Org. Lett. 2011;13:1778–1780. doi: 10.1021/ol200311y. [DOI] [PubMed] [Google Scholar]; (d) Feldman KS, Antoline JF. Org. Lett. 2012 doi: 10.1021/ol203463n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiewel K, Luo Z, Sulikowski GA. Org. Lett. 2005;7:5163–5165. doi: 10.1021/ol051993e. [DOI] [PubMed] [Google Scholar]
- 16.(a) Itai A, Toriumi Y, Saito S, Kagechika H, Shudo K. J. Am. Chem. Soc. 1992;114:10649–10650. [Google Scholar]; (b) Curran DP, Hale GR, Geib SJ, Balog A, Cass QB, Degani ALG, Hernandes MZ, Freitas LCG. Tetrahedron: Asymmetry. 1997;8:3955–3975. [Google Scholar]
- 17.(a) Guthrie DB, Geib SJ, Curran DP. J. Am. Chem. Soc. 2011;133:115–122. doi: 10.1021/ja108795x. [DOI] [PubMed] [Google Scholar]; (b) Mandel J, Pan X, Hay EB, Geib SJ, Wilcox CS, Curran DP. J. Org. Chem. 2013;78:4083–4089. doi: 10.1021/jo400385t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura K, Ichinose Y, Nozaki K, Fugami K, Oshima K, Utimoto K. Bull. Chem. Soc. Jpn. 1989;62:143–147. [Google Scholar]
- 19.Hoffmann RW. Angew. Chem., Int. Ed. Eng. 2000;39:2055–2070. [Google Scholar]
- 20.Chatgilialoglu C, Alberti A, Ballestri M, Macciantelli D, Curran DP. Tetrahedron Lett. 1996;37:6391–6394. (b) Another possible outcome is that the sulfonyl radicals eventually dimerize. The products of such reactions would probably also react with tin hydrides. See, Chatgilialoglu C, Lunazzi L, Ingold KU. J. Org. Chem. 1983;48:3588–3589.
- 21.(a) Chatgilialoglu C, Newcomb M. In: Advances In Organometallic Chemistry. West R, Hill AF, editors. Vol. 44. Academic Press; San Diego: 1999. pp. 67–112. [Google Scholar]; (b) Vleeschouwer FD, Speybroeck VV, Waroquier M, Geerlings P, Proft FD. J. Org. Chem. 2008;73:9109–9120. doi: 10.1021/jo802018b. [DOI] [PubMed] [Google Scholar]
- 22.(a) With 2 equiv of tin hydride, small amounts of starting material sometimes remained in pilot experiments, so we settled on 3 equiv for the preparative experiments. (b) Cyclization of the related 2-bromoacetamide analog provided a crude spirocyclic imine product that was not stable to handling or isolation.
- 23.Ueno Y, Ohta M, Okawara M. J. Organomet. Chem. 1980;197:C1–C4. [Google Scholar]
- 24.(a) Curran DP, Kim D, Liu HT, Shen W. J. Am. Chem. Soc. 1988;110:5900–5902. [Google Scholar]; (b) Snieckus V, Cuevas JC, Sloan CP, Liu H, Curran DP. J. Am. Chem. Soc. 1990;112:896–898. [Google Scholar]; (c) Robertson J, Pillai J, Lush RK. Chem. Soc. Rev. 2001;30:94–103. [Google Scholar]; (d) Denes F, Beaufils F, Renaud P. Synlett. 2008:2389–2399. [Google Scholar]
- 25.Murphy JA, Roome SJ. J. Chem. Soc., Perkin Trans. 1. 1995:1349–1358. [Google Scholar]
- 26.(a) Kocienski PJ. Protecting Groups. Georg Thieme Verlag; Stuttgart: 1994. [Google Scholar]; (b) Greene TW, Wuts PGM. Protective Groups in Organic Synthesis. 3rd ed. Wiley-Interscience; New York: 1999. [Google Scholar]
- 27.(a) Knowles HS, Parsons AF, Pettifer RM, Rickling S. Tetrahedron. 2000;56:979–988. [Google Scholar]; (b) Viaud P, Coeffard V, Thobie-Gautier C, Beaudet I, Galland N, Quintard J-P, Le Grognec E. Org. Lett. 2012;14:942–945. doi: 10.1021/ol300003f. [DOI] [PubMed] [Google Scholar]
- 28.Curran DP, Yu HS. Synthesis. 1992:123–127. [Google Scholar]
- 29.Kampmeier JA, Evans TR. J. Am. Chem. Soc. 1966;88:4096–4097. [Google Scholar]
- 30.Crich D, Hutton TK, Ranganathan K. J. Org. Chem. 2005;70:7672–7678. doi: 10.1021/jo050990c. [DOI] [PubMed] [Google Scholar]
- 31.Aitken HM, Hancock AN, Schiesser CH. Chem. Commun. 2012;48:8326–8328. doi: 10.1039/c2cc33856d. [DOI] [PubMed] [Google Scholar]
- 32.(a) Crich D. Helv. Chim. Acta. 2006;89:2167–2182. [Google Scholar]; (b) Schiesser CH. Chem. Commun. 2006:4055–4065. doi: 10.1039/b608150a. [DOI] [PubMed] [Google Scholar]; (c) Li C. In: Encyclopedia of Radicals in Chemistry, Biology and Materials. Chatgilialoglu C, Studer A, editors. Vol. 1. Wiley; New York, NY: 2012. pp. 943–964. [Google Scholar]; (d) Jasch H, Hienrich MR. In: Encyclopedia of Radicals in Chemistry, Biology and Materials. Chatgilialoglu C, Studer A, editors. Vol. 2. Wiley; New York, NY: 2012. pp. 529–560. [Google Scholar]
- 33.Büchi G, Wüest H. Tetrahedron Lett. 1977;18:4305–4306. [Google Scholar]
- 34.PhD Thesis of Hanmo Zhang. University of Pittsburgh; 2013. [Google Scholar]
- 35.This compound has been previously observed and also described to be unstable. See: Burk RM, Overman LE. Heterocycles. 1993;35:204–210.
- 36.(a) Roberts BP. Chem. Soc. Rev. 1999;28:25–35. [Google Scholar]; (b) Crich D, Grant D, Krishnamurthy V, Patel M. Acc. Chem. Res. 2007;40:453–463. doi: 10.1021/ar600020v. [DOI] [PubMed] [Google Scholar]
- 37.Syringe pump experiments with phenyl selenide 53 gave similar results to bromide 14. Imine 52 was typically the major crude product but reduced product 15 was a significant minor product.
- 38.(a) Yus M, Gonzalez-Gomez JC, Foubelo F. Chem. Rev. 2011;111:7774–7854. doi: 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]; (b) Choudhury LH, Parvin T. Tetrahedron. 2011;67:8213–8228. doi: 10.1016/j.tet.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kobayashi S, Mori Y, Fossey JS, Salter MM. Chem. Rev. 2011;111:2626–2704. doi: 10.1021/cr100204f. [DOI] [PubMed] [Google Scholar]
- 39.(a) Pawlenko S. In: Organic Nitrogen Compounds with a C,N-Double Bond. 4th ed. Klamann D, Hagemann H, editors. E14b. Georg Thieme Verlag; Stuttgart: 1990. pp. 222–281. [Google Scholar]; (b) Abbaspour Tehrani K, De Kimpe N. In: Compounds with Two Carbon-Heteroatom Bonds: Heteroatom Analogues of Aldehydes and Ketones. Pawda A, Bellus D, editors. Vol. 27. Georg Thieme Verlag; Stuttgart-New York: 2004. pp. 245–312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.