Abstract
Butyltins (BTs) contaminate the environment and are found in human blood. BTs, tributyltin (TBT) and dibutyltin (DBT), diminish the cytotoxic function and levels of key proteins of human natural killer (NK) cells. NK cells are an initial immune defense against tumors, virally-infected cells and antibody-coated cells and thus critical to human health. The signaling pathways that regulate NK cell functions include mitogen-activated protein kinases (MAPKs). Studies have shown that exposure to BTs leads to the activation of specific MAPKs and MAPK kinases (MAP2Ks) in human NK cells. MAP2K kinases (MAP3Ks) are upstream activators of MAP2Ks, which then activate MAPKs. The current study examined if BT-induced activation of MAP3Ks was responsible for MAP2K and thus, MAPK activation. This study examines the effects of TBT and DBT on the total levels of two MAP3Ks, c-Raf and ASK1, as well as activating and inhibitory phosphorylation sites on these MAP3Ks. In addition, the immediate upstream activator of c-Raf, Ras, was examined for BT-induced alterations. Our results show significant activation of the MAP3K, c-Raf, in human NK cells within 10 minutes of TBT exposure and the MAP3K, ASK1, after one hour exposures to TBT. In addition, our results suggest that both TBT and DBT are impacting the regulation of c-Raf.
Keywords: MAPK/ERK Pathway, c-Raf (Raf1), ASK1, Natural Killer cells, butyltin, Ras
Introduction
Butyltins (BTs) are toxic organotin compounds which contaminate the environment (Hoch, 2001). The BTs tributyltin (TBT) and dibutyltin (DBT) have various commercial applications (Roper, 1992; Takahashi et al., 1999; Gipperth, 2009). TBT was formerly used as a marine biocide in antifouling paints to prevent the growth of aquatic organisms on the hulls and decks of boats and ships (Antizar-Ladislao, 2008; Clark et al., 1988; Fent, 2006; Hoch, 2001). TBT’s use in marine antifouling paints has been banned since 2008 but it will continue to contaminate the environment for many years due to its chemical stability, non-marine uses, applications to ships prior to the ban, and marine uses in spite of the ban (Gipperth, 2009) It has also been used as a wood preservative (Antizar-Ladislao, 2008; Clark et al., 1988; Hoch, 2001). Due to its use as an aquatic pesticide, there are concerns about its effects on the marine ecosystem (Kannan et al., 1995; Clark et al., 1988). Studies have shown TBT to be harmful to mammals, including humans, primarily due to neurotoxicity (Antizar-Ladislao, 2008; Boyer, 1989; Nakanishi et al., 2006; 2007; Saitoh et al, 2001). Higher order organisms have become accidently contaminated as a result of leaching of the chemical from the paints into the water and/or the consumption of contaminated animals (Fent, 2006; Mora et al., 1989; Veltman et al. 2006). Humans can then be inadvertently exposed to the chemical by ingesting contaminated food (Antizar-Ladislao, 2008; Belfroid et al., 2000; Kannan et al., 1995; Sadiki et al., 1996; Takahashi et al., 1999). Significant levels of TBT (as high as 261 nM, 85 ng/mL) have been found in human blood (Whalen et al., 1999; Kannan et al., 1999). Studies have shown that TBT causes thymic atrophy and thymus dependent immunosuppression in rodents (Snoeij et al., 1987, 1989; Vos et al. 1990).
DBT is used as a stabilizer of plastics and as a deworming agent in poultry (Epstein et al., 1991; Kannan et al., 1995; Takahashi et al., 1999). It is commonly found in Polyvinyl chloride plastics (Sadiki et al., 1996). Human exposure results from the usage of plastic cooking products containing DBT (Kannan et al., 1995; Takahashi et al., 1999) and/or the leaching of the chemical into drinking water from plastic water bottles and PVC pipes; as well as, ingestion of contaminated food (Azenha et al., 2002; Forsyth et al., 1992; 1997; Kannan et al., 1995; Sadiki et al., 1996; Takahashi et al., 1999). Dibutyltin concentrations have been detected in the livers of humans and other mammals (Takahashi et al., 1999). Specifically, DBT has been found at concentrations between 0.8 and 28.3 ng/g in human livers (Nielsen et al., 2002) and has been found in human blood at levels as high as 300 nM (94ng/mL) (Whalen et al., 1999; Kannan et al., 1999).
TBT and DBT have been shown to have an immunotoxic effect on human natural killer cells by significantly decreasing both their cytotoxic function and levels of cytolytic and cell-surface proteins (Aluoch et al., 2006; Dudimah et al., 2007a,b; Catlin et al., 2005; Odman-Ghazi et al, 2003; Whalen et al., 2002). Natural killer (NK) cells are part of the innate immune system, the body’s first defense against foreign invaders. NK cells kill tumor cells, virally-infected cells, or antibody-coated cells through receptor-mediated activation leading to the release of the cytotoxic proteins granzyme and perforin (Lowin et al., 1994; Shresta et al., 1995). NK cells also contribute to adaptive immunity in various manners. NK cells have recently been shown to stimulate the maturation of dendritic cells (Walzer et al., 2005; Wehner et al., 2011). Therefore, interference with NK function could render humans susceptible to the development of primary tumors, blood-borne metastasis, and severe infections.
An intracellular signaling pathway is activated when a NK cell binds to a target cell (Wei et al., 1998). This pathway ultimately leads to the activation of mitogen-activated protein kinases (MAPKs). Their activation results in the lysis of the bound target cell via the final release of the NK cell’s cytotoxic proteins (Wei et al., 1998). We hypothesize that premature activation of this pathway by BTs may interfere with the NK cell’s natural ability to lyse its target cell by rendering it incapable of triggering this pathway during a subsequent encounter with a tumor cell or infected cell. Our previous studies have shown a significant increase in the activation of the MAPKs, p38, p44/42, JNK, and the MAPK Kinases (MAP2Ks), MKK3/6 and MEK1/2, by TBT within ten minutes of exposure in human NK cells (Aluoch et al., 2005; 2006; 2007). DBT also induces the activation of p38, p44/42, MKK3/6, and MEK1/2 after brief exposure in human NK cells (Odman-Ghazi et al., 2010). The purpose of this study is to investigate whether exposure of human NK cells to TBT and DBT also result in the activation/phosphorylation of the MAP3Ks, c-Raf and/or ASK-1, in a Ras-dependent or independent manner. Ras is a GTPase normally involved in the activation of the MAP3K, c-Raf. In its inactive state, Ras is normally found bound to GDP; but upon activation, Ras binds GTP. Ras specifically binds to the Ras Binding Domain (RBD) of c-Raf.
Materials and Methods
Isolation of NK Cells from Human Blood
Highly purified natural killer cells were isolated from buffy coats collected from healthy adult male and female donors (American Red Cross, Portland, OR; Life blood, Memphis, TN) using the RosetteSep Human NK Cell Enrichment Antibody Cocktail protocol (Stem Cell Technologies, Vancouver, BC, Canada) (600 μL of antibody cocktail was added per 40 mL of blood). The mixture was incubated at room temperature 20 minutes. Following the incubation period, 7–8 mL were layered onto 4 mL of Ficoll-Hypaque (1.077 g/mL). The mixture was then centrifuged at 1200g for 50 minutes. NK cells were collected and washed twice with PBS solution and stored in complete media (RPMI-1640 supplemented with 10% heat-inactivated bovine calf serum (BCS), 2 mM L-glutamine and 50 U penicillin G with 50 μg streptomycin/ml) at 1 million cells/mL at 37 °C and air/CO2, 19:1 (Whalen et al., 2002). Donors are given numbers based on the source of the buffy coat Red Cross = RC# and Life blood = LB#.
Chemical Preparation
TBT (Sigma-Aldrich, St. Louis, MO) was dissolved in deionized water to yield a 1mM stock solution. This solution was then diluted in media to achieve the desired final concentration of 300, 200, 100, 50 or 25 nM (Whalen et al., 2002). DBT (Sigma-Aldrich) was dissolved in dimethylsulfoxide (DMSO) to make a stock solution which is diluted to 10, 5, 2.5, 1 and 0.5 μM concentrations in cell culture medium (Aluoch et al., 2005; Odman-Ghazi et al., 2010).
Cell Treatments
Purified NK cells (1.5 million cells/mL) were exposed to (1) no TBT (control), (2) 300 nM TBT, (3) 200 nM TBT, (4) 100 nM TBT, (5) 50 nM TBT or (6) 25 nM TBT followed by an incubation period of 10 minutes, 1 hour, or 6 hours at 37°C and 19:1 air/CO2. Purified NK cells (1.5 million cells/mL) were also exposed to (1) no DBT (control), (2) 10 μM DBT, (3) 5 μM DBT, (4) 2.5 μM DBT, (5) 1 μM DBT or (6) 0.5 μM DBT followed by an incubation period of 10 minutes, 1 hour or 6 hours at 37°C and 19:1 air/CO2.
Cell Lysates
Cell lysates were prepared from NK cells exposed (as described above) using 500 μL of lysis buffer (Active motif, Carlsbad, CA) per 10 million cells. The cell lysates were stored frozen at −80°C up to the point when they were run on SDS-PAGE. All controls and TBT- or DBT-exposed cells for a given experimental set-up were from an individual donor. Each of the experimental set-ups was repeated a minimum of four times using cells from different donors.
Western blots
The samples were run on a 10% SDS-PAGE and then transferred to a PVDF membrane. The PVDF membrane was then immunoblotted with anti-c-Raf1 (total), anti-phospho-c-Raf1 (S338), anti-phospho-c-Raf1 (S259), anti-phospho-c-Raf 1 (S289/296/301) and anti-ASK-1 (total), anti-ASK-1 (S967) and anti-ASK-1 (T845) antibodies (Cell Signaling Technology, Inc.; Danvers, MA). In addition, anti-β-actin was used to verify that equal amounts of protein were loaded. Antibodies were visualized using an ECL chemiluminescent detection system (GE Healthcare; Piscataway, NJ) and a Kodak Image Station 4000R (Rochester, NY). The density of each protein band was determined using the Kodak Image Station analysis software. The samples from each experimental condition were run on a separate gel and blot. Each set of experimental conditions had its own internal control (untreated cells). Therefore, any observable changes or differences in protein expression were determined based on the internal control. This determination provides relative quantitation by evaluating whether a given treatment changed in expression relative to the untreated cells. The density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes. A minimum of 4 separate experiments were carried out for analysis (Aluoch et al., 2005; Odman-Ghazi et al., 2010).
ELISA Assays
Activated GTP-Ras was determined by using a Ras Activation Elisa Assay Kit (EMD Millipore Co., Billerica, MA). NK cell lysates exposed to either C, 300, 200, 100, 50 or 25 nM TBT or C, 10, 5, 2.5, 1 or 0.5 μM DBT concentrations at an exposure time of ten minutes were assayed for Ras in the GTP bound- activated- form that binds to the Ras Binding Domain (RBD) of Raf. Activated Ras was detected and measured in the cell lysate samples, prepared as stated above. The captured activated Ras that binds to the RBD of Raf-1 was detected using the HRP conjugated secondary antibody provided. After adding the chemiluminescent substrate, the intensity of the signal was measured using a luminometer (Fluoroskan Ascent FL, Thermo Fisher, Pittsburgh, PA). Each experimental condition had its own internal control (untreated cells).
Statistical Analysis
Statistical analysis were determined using ANOVA and Student t-test; significance being a p-value<0.05. At least 4 separate experiments were carried out for analysis of each experimental condition.
Results
The effect of 10 minute exposures to 300, 200 100, 50 and 25 nM TBT on activation state of Ras in human NK cells
Activated Ras was determined in NK cells exposed for 10 min to Control (no TBT), 300, 200, 100, 50 and 25 nM TBT. Data are presented as percent of the control (Figure 1). A significant 10 to 15 percent decrease from the control was observed at the 300, 200, 100 and 50 nM exposures; specifically, a 10% decrease at the 300 nM concentration, 11% at 200 nM, 14% at 100 nM and 15% at the 50 nM concentration (Figure 1).
Figure 1. Effect of exposures to TBT on the activation state of Ras.
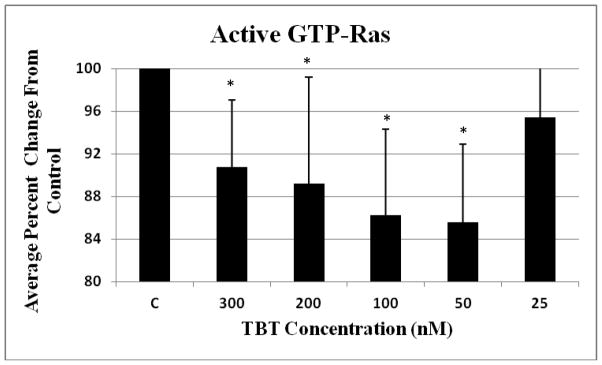
NK cells were exposed to 300-25 nM TBT for 10 minutes (Error bars represent average ± standard deviation from mean, n=4, NK cells prepared from 4 different donors, * represent p < 0.05).
The effect of 10 minute exposures to 10, 5, 2.5, 1 and 0.5 μM DBT on the activation state of Ras in human NK cells
When NK cells were exposed to DBT for 10 min, there was a significant 10 to 20% decrease in the amount of activated GTP-Ras compared to the control at the 10, 5 and 2.5 μM concentrations. The 10 μM concentration resulted in a 12% decrease, the 5 μM in an 18% decrease and the 2.5 μM in a 12% decrease from the control (Figure 2).
Figure 2. Effect of exposures to DBT on the activation state of Ras.
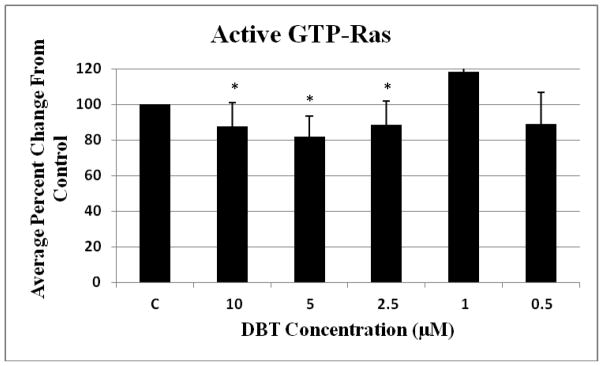
NK cells were exposed to 10-0.05 μM of DBT for 10 minutes (Error bars represent average ± standard deviation from mean, given n=4, * represent p < 0.05).
The effect of 10 minute exposures to 300, 200, 100, 50, and 25 nM TBT on the phosphorylation state of c-Raf and ASK1 in human NK cells
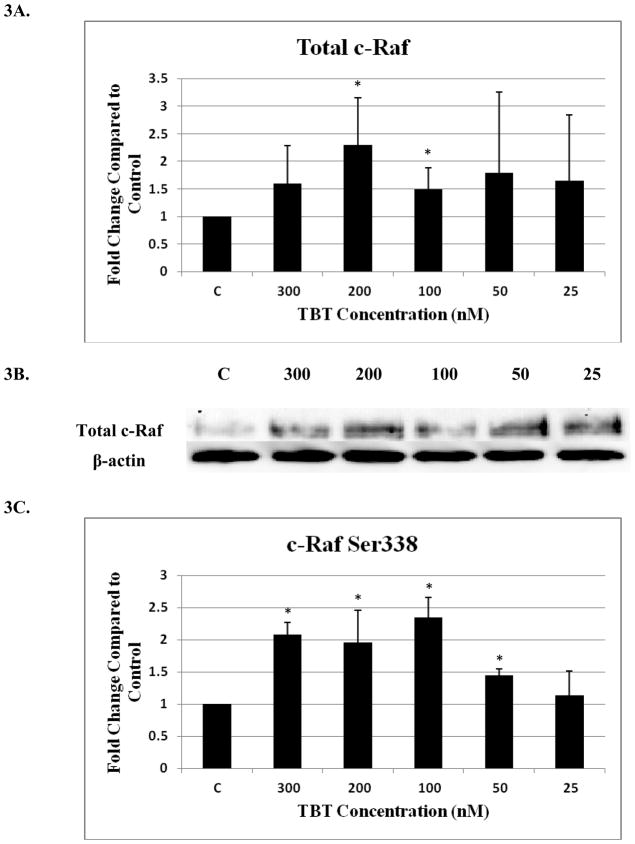
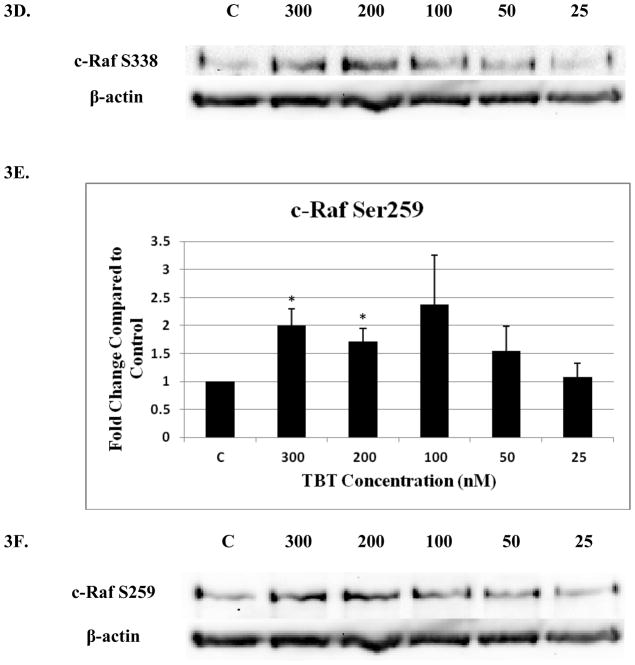
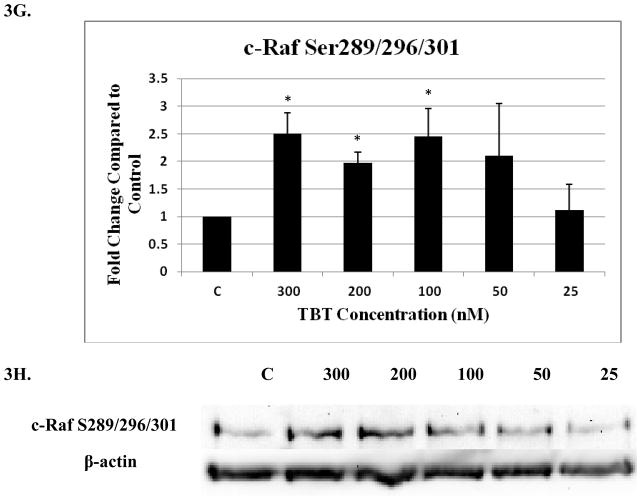
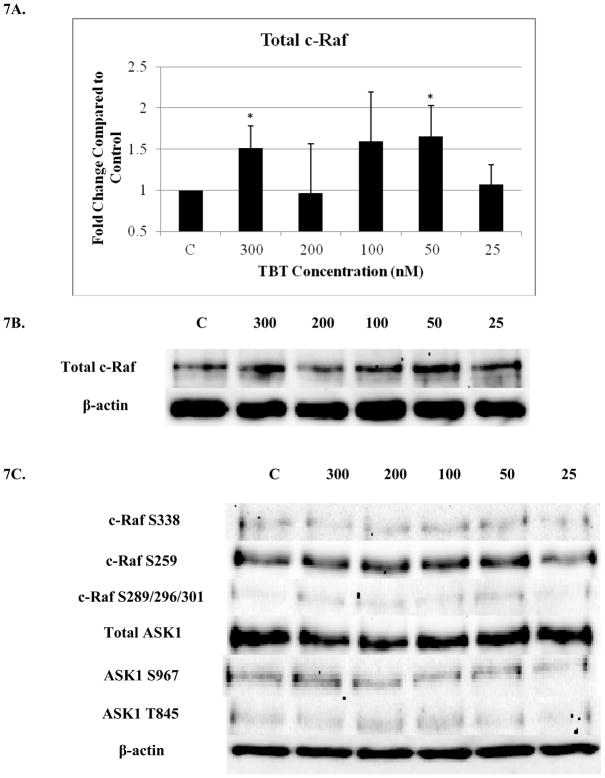
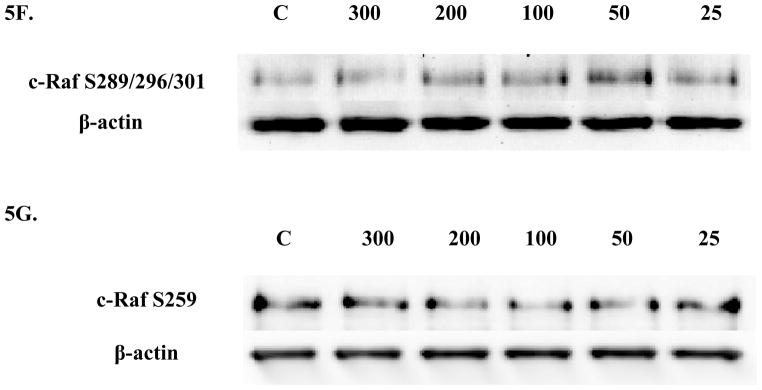
Four c-Raf antibodies were used: c-Raf (total protein levels), S338 (activation phosphorylation site), S259 (inhibitory phosphorylation site) and S289/296/301(feedback inhibition sites). There was a significant increase of total c-Raf (2.3 fold) at the 200 nM concentration and 1.5 at the 100 nM concentration of TBT (Figure 3A and B). Although not statistically significant, due to donor variability, four out of 5 donors showed an increase in phosphorylation at both the 300 and 50 nM concentrations; ranging from 1.2–2.8 fold at the 300 nM concentration and 1.2–4.3 fold at the 50 nM concentration. In addition, three out of five donors showed an increase varying from 1.4 to 3.7 fold for total c-Raf at the 25 nM concentration. Increased activation/phosphorylation of c-Raf at S338 was seen with exposures to 300, 200, 100, and 50 nM of TBT (2 fold at 300-100 nM and 1.45 fold at 50 nM) when compared to the control (Figure 3C and D). Elevated expression levels of S259 were also observed at the 300-200 nM (2 and 1.7 fold, respectively) (Figure 3E and F). While statistically insignificant, each of the four donors showed an increase in phosphorylation of S259 at the 100 nM exposure. A 1.8 fold increase was seen for donor RC684, 3.1 for donor RC694, 3.1 for donor RC708, and 1.5 for donor LB1 (Table 1). Three of four donors also showed an increase in S259 phosphorylation (1.4 to 2.1 fold) at the 50 nM exposure. Exposures to 300, 200, and 100 nM TBT significantly increased phosphorylation of Ser289/296/301, 2.5, 2.0, and 2.4 fold, respectively (Figure 3G and H). Increases for each of the four donors at the 50 nM concentration were also seen without collectively resulting in statistical significance. A 1.5 fold increase was seen for donor RC684, 3.4 fold increase for RC694, 2.3 fold increase for RC708 and 1.2 fold increase for LB1 (Table 1). Ten minute exposures of the human NK cells to these same concentrations of TBT (300-25 nM) showed no significant changes in the phosphorylation or total levels of ASK1 (p < 0.05) (Figure 4).
Figure 3. Effect of 10 min exposures to 300 to 25 nM TBT on c-Raf in human NK cells.
A) Levels of total c-Raf shown as fold change compared to control. Values are mean ± S.D. from 5 separate experiments using cells from different donors *p<0.05. B) Representative western blot for total c-Raf. C) Levels of c-Raf phosphorylated at S338 shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. D) Representative western blot for c-Raf phosphorylation at S338. E) Levels of c-Raf phosphorylated at S259 shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. F) Representative western blot for c-Raf phosphorylation at S259. G) Levels of c-Raf phosphorylated at S289/296/301 shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. H) Representative western blot for c-Raf phosphorylation at S289/296/301.
Table 1.
Increases in phosphorylation of c-Raf S259 and S289/296/301 following 10 minute exposures to TBT for individual donors.
| FOLD CHANGE FOR PO4-C-RAF S259 | ||||||
|---|---|---|---|---|---|---|
| Donor | RC684 | RC694 | LB1 | RC708 | AVG FI | SD |
| [TBT] | ||||||
| *300 | 1.7482 | 1.7848 | 2.0387 | 2.4026 | 1.9936 | 0.3017 |
| *200 | 1.8802 | 1.9489 | 1.4617 | 1.5915 | 1.7205 | 0.2319 |
|
| ||||||
| 50 | 1.3878 | 1.6438 | 1.0627 | 2.0947 | 1.5472 | 0.4356 |
| 25 | 1.0024 | 1.2927 | 0.7493 | 1.2573 | 1.0754 | 0.253 |
| FOLD CHANGE FOR PO4-C-RAF AT S289/296/301 | ||||||
| DONOR | RC684 | RC694 | LB1 | RC708 | AVG FI | SD |
| [TBT] | ||||||
| *300 | 1.9885 | 2.6902 | 2.4767 | 2.8513 | 2.5017 | 0.3749 |
| *200 | 2.2273 | 1.7691 | 2.0175 | 1.885 | 1.9747 | 0.1966 |
| *100 | 2.0845 | 2.6233 | 2.0171 | 3.0864 | 2.4528 | 0.502 |
|
| ||||||
| 25 | 1.1468 | 0.7339 | 0.826 | 1.7677 | 1.1186 | 0.4675 |
AVG FI = Average Fold Increase, SD = average ± standard deviation from mean, *p < 0.05. Outlined rows indicate concentrations of TBT where every donor showed an increase above the control, but where the combined data gave p>0.05.
Figure 4. Effect of 10 min exposures to 300 to 25 nM TBT on ASK1 in human NK cells.
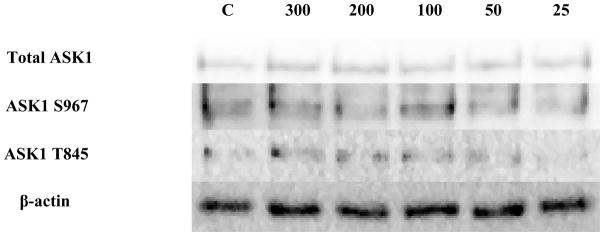
Representative western blot results showing no significant changes in total ASK1, ASK1 phosphorylation at S967, or ASK1 phosphorylation at T845 after exposure of NK cells to 25–300 nM TBT (n=4, p > 0.05).
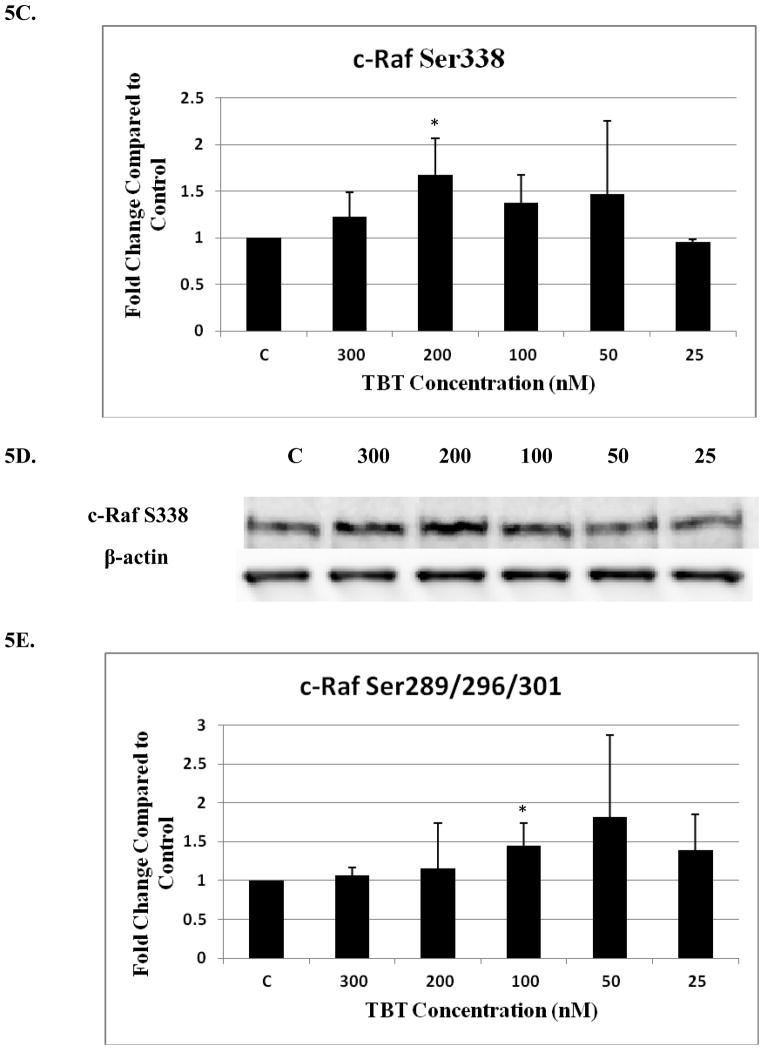
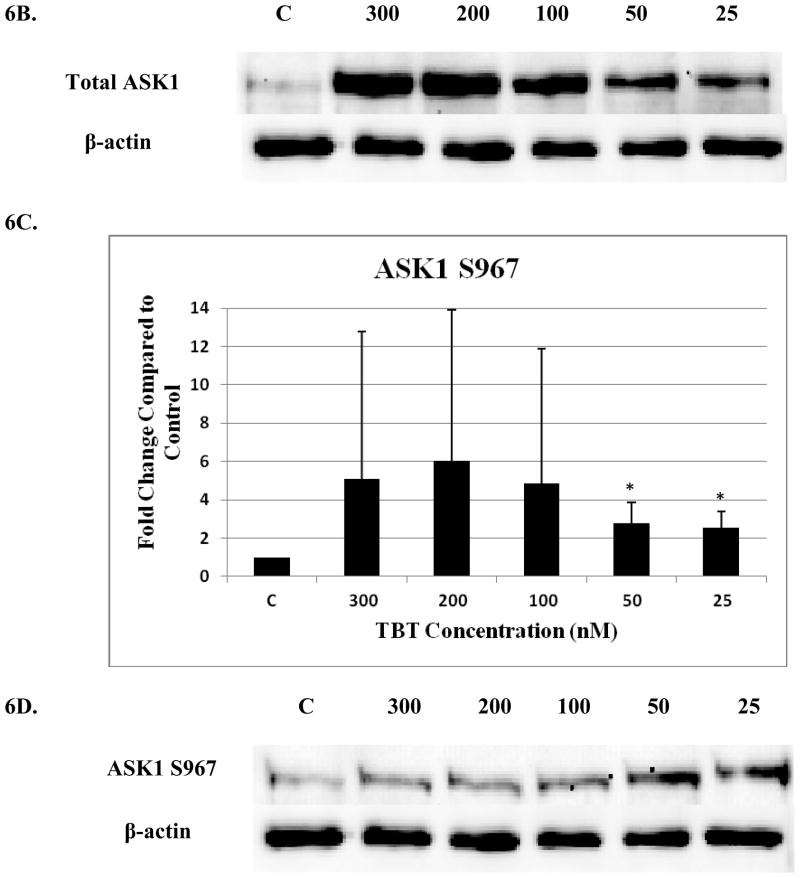
The effect of 1 hour exposures to 300, 200, 100, 50, and 25 nM TBT on the phosphorylation state of c-Raf and ASK1 in human NK cells
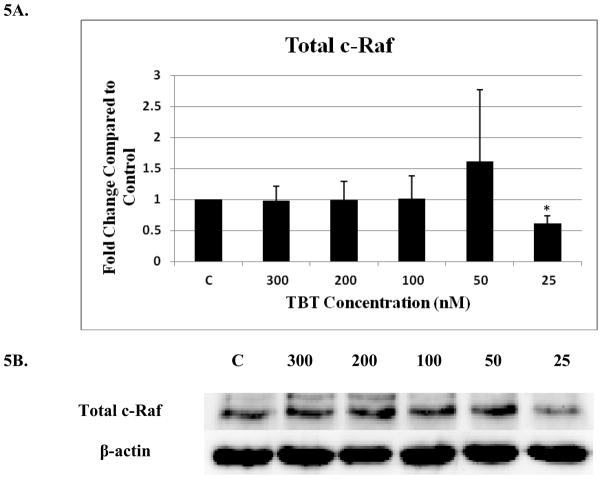
There was a significant decrease (40%) to 0.6 of the control (1 fold) for total c-Raf at the 25 nM concentration (p<0.05)(Figure 5A and B). A significant 1.7 fold increase in phosphorylation of S338 (activation) was seen with exposure to 200 nM TBT (Figure 5C and D) and a 1.45 fold increase for S289/296/301 at 100 nM TBT (p<0.05) (Figure 5E and F). NK cell exposure to 300 nM of TBT caused an increase in phosphorylation of S338 in 2 out of the 4 donors ranging from 1.4 fold to 1.5 fold (p>0.05). Three out of 4 donors also showed increases in the levels of phosphorylation of S338 at the 100 and 50 nM concentrations, ranging from 1.3 to 1.7 at the 100 and 1.2 to 2.2 at the 50 nM concentrations (p>0.05). Although, the data for c-Raf at S289/296/301 yielded statistically insignificant results for the 200, 50 and 25 nM TBT exposures, due to variability among donors, 3 out of 4 donors showed an increase at each exposure ranging from 1.2–1.6 at the 200 nM, 1.25–3.0 at the 50 nM and 1.3–1.9 at the 25 nM concentrations. No significant changes were seen for c-Raf at S259 (Figure 5G).
Figure 5. Effect of 1h exposures to 300 to 25 nM TBT on c-Raf in human NK cells.
A) Levels of total c-Raf shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. B) Representative western blot for total c-Raf. C) Levels of c-Raf phosphorylated at S338 shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. D) Representative western blot for c-Raf phosphorylation at S338. E) Levels of c-Raf phosphorylated at S289/296/301 shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. F) Representative western blot for c-Raf phosphorylation at S289/296/301. G) Representative western blot for c-Raf phosphorylation at S259.
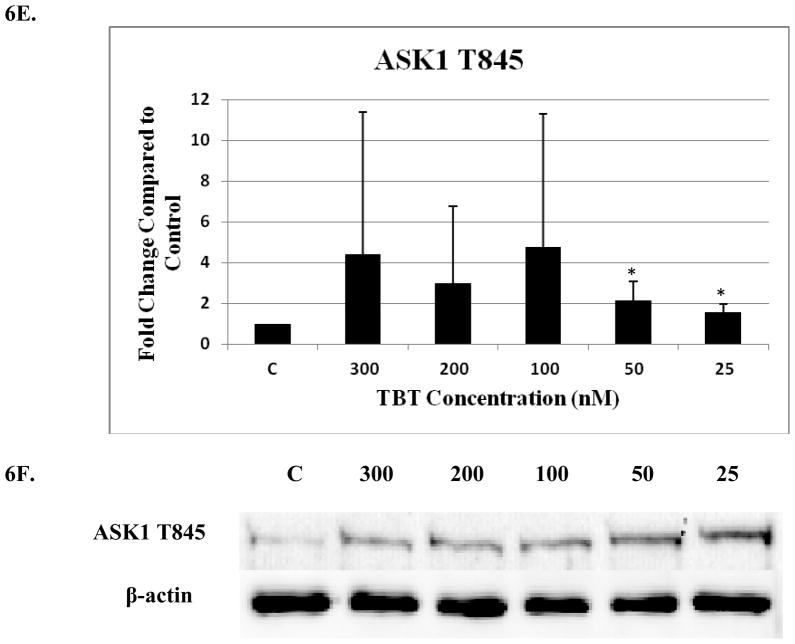
Exposure to 25 nM TBT for 1 h caused a 2.0 fold increase total ASK1 protein (p<0.05) (Figure 6a and b). The remaining four TBT concentrations did not show statistically significant increases in ASK. However, many of the donors showed increases in total ASK1 but there was considerable variation among donors (Table 2). Phosphorylation of ASK1 at S967 was significantly increased 2.8 fold at the 50 nM concentration and 2.5 fold at the 25 nM concentration, (p<0.05) (Figure 6C and D). Each of the donors showed an increased in ASK1 S967 phosphorylation at the 300 and 200 nM concentrations, however, the results were not statistically significant (Table 2). Three out of four donors showed an increase in S967 phosphorylation at the 100 nM exposure (p>0.05) (Table 2). Phosphorylation of ASK1 T845 was significantly increased at the 50 and 25 nM concentrations, 2.2 fold and 1.6 fold correspondingly (p<0.05) (Figure 6E and F). Table 2 shows that there were increases in phosphorylation of ASK1 T845 in 4 of 5 donors at the 300 nM and 25 nM exposures, and all 5 donors at the 100 nM exposure. However, there was a very wide range in the fold increase among the donors resulting in a p >0.05.
Figure 6. Effect of 1 h exposures to 300 to 25 nM TBT on ASK1 in human NK cells.
A) Levels of total ASK1 shown as fold change compared to control. Values are mean ± S.D. from 5 separate experiments using cells from different donors *p<0.05. B) Representative western blot for total ASK1. C) Levels of ASK1 phosphorylated at S967 shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. D) Representative western blot for ASK1 phosphorylation at S967. E) Levels of ASK1 phosphorylated at T845 shown as fold change compared to control. Values are mean ± S.D. from 5 separate experiments using cells from different donors *p<0.05. F) Representative western blot for ASK1 phosphorylation at T845.
Table 2.
Increases in total ASK1 and phosphorylation of ASK1 S967 and T845 following 1 hour exposures to TBT for individual donors.
| FOLD CHANGE FOR TOTAL ASK1 | |||||||
|---|---|---|---|---|---|---|---|
| DONOR | RC481 | RC482 | RC154 | RC688 | LB3 | AVG FI | SD |
| [TBT] | |||||||
| 300 | 1.254 | 1.6629 | 0.7419 | 11.6247 | 0.9284 | 3.2424 | 4.6989 |
| 200 | 0.6694 | 2.3915 | 1.1174 | 14.3919 | 0.7128 | 3.8566 | 5.9305 |
| 100 | 1.543 | 1.8302 | 1.0011 | 8.0857 | 1.5911 | 2.8102 | 2.9646 |
| 50 | 1.7274 | 2.2033 | 1.4526 | 4.0355 | 0.774 | 2.0386 | 1.2303 |
| *25 | 1.7803 | 2.2498 | 1.4238 | 3.2144 | 1.5352 | 2.0407 | 0.729 |
| FOLD CHANGE FOR PO4-ASK1 AT S967 | |||||||
| DONOR | RC481 | RC482 | RC154 | RC688 | AVG FI | SD | |
| [TBT] | |||||||
|
| |||||||
|
| |||||||
| 100 | 1.0175 | 1.6353 | 1.4345 | 15.415 | 4.8756 | 7.032 | |
| *50 | 1.764 | 2.4633 | 2.6214 | 4.304 | 2.7882 | 1.077 | |
| *25 | 1.5647 | 2.9122 | 2.1147 | 3.549 | 2.5352 | 0.8734 | |
| FOLD CHANGE FOR PO4-ASK1 AT T845 | |||||||
| DONOR | RC481 | RC482 | RC154 | RC688 | LB3 | AVG FI | SD |
| [TBT] | |||||||
| 300 | 1.3001 | 1.5428 | 0.8864 | 16.9168 | 1.3838 | 4.406 | 6.9979 |
| 200 | 0.684 | 2.2235 | 1.4371 | 9.6611 | 0.8697 | 2.9751 | 3.7853 |
|
| |||||||
| *50 | 2.3539 | 2.076 | 2.3245 | 3.2927 | 0.7955 | 2.1685 | 0.8967 |
| *25 | 1.8734 | 2.0552 | 1.5965 | 1.2168 | 1.0231 | 1.553 | 0.4332 |
AVG FI = Average Fold Increase, SD = average ± standard deviation from mean, *p < 0.05). Outlined rows indicate concentrations of TBT where every donor showed an increase above the control, but where the combined data gave p>0.05.
The effect of 6 hour exposures to 300, 200, 100, 50, and 25 nM TBT on the phosphorylation state of c-Raf and ASK1 in human NK cells
A 1.5 fold increase was observed at the 300 nM concentration for total c-Raf and 1.7 at the 50 nM concentration (p<0.05) (Figure 7A and B). A 1.3 to 2.4 fold increase for total c-Raf was seen for three out of the four donors at the 100 nM concentration. However, the wide range in fold increases due to donor variability yielded statistically insignificant results. Figure 7C depicts representative western blot results showing no significant changes in phosphorylation for c-Raf at the three amino acid sites or ASK1 in human NK cells after they were exposed to 300, 200, 100, 50 and 25 nM TBT for 6 hours as compared to control (no TBT).
Figure 7. Effect of 6 hour exposures to 300 to 25 nM TBT on c-Raf and ASK1 in human NK cells.
A) Levels of total c-Raf shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. B) Representative western blot for total c-Raf. C) Representative western blots for c-Raf phosphorylation at S338, S259, S289/296/301 and for total ASK1 and ASK1 phosphorylation at S967, T845. Four separate experiments were analyzed using cells from different donors, p>0.05.
The effect of 10 minute and 1 hour exposures to 10, 5, 2.5, 1 and 0.5 μM DBT on the phosphorylation state of c-Raf and ASK1 in human NK cells
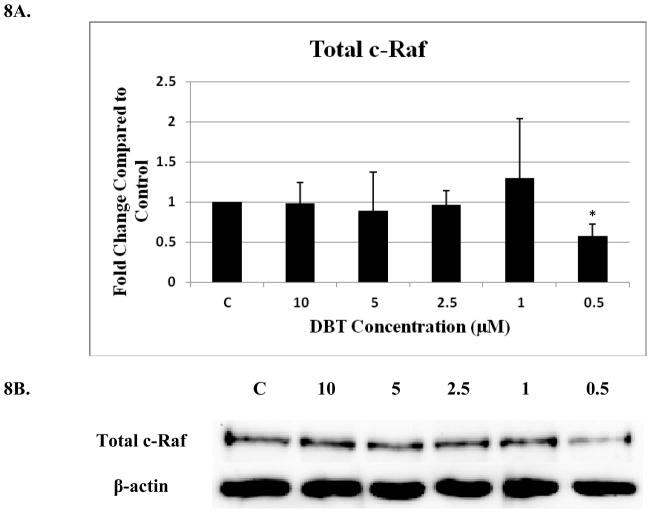
Examination of total c-Raf in NK cells exposed to DBT for 10 min showed a statistically significant decrease (43%) only at the lowest (0.5 μM) DBT exposure (Figure 8A and B). No significant changes in phosphorylation were seen at any of the c-Raf or ASK1 sites with a 10 min (Figure 8C) or 1 hour (Figure 9) of DBT.
Figure 8. Effect of 10 minute exposures to 10 to 0.5 μM DBT on c-Raf and ASK1 in human NK cells.
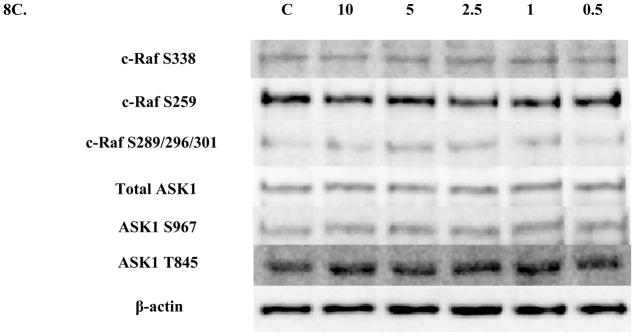
A) Levels of total c-Raf shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. B) Representative western blot for total c-Raf. C) Representative western blots for c-Raf phosphorylation at S338, S259, S289/296/301 and for total ASK1 and ASK1 phosphorylation at S967, T845. Five separate experiments were analyzed using cells from different donors, p>0.05.
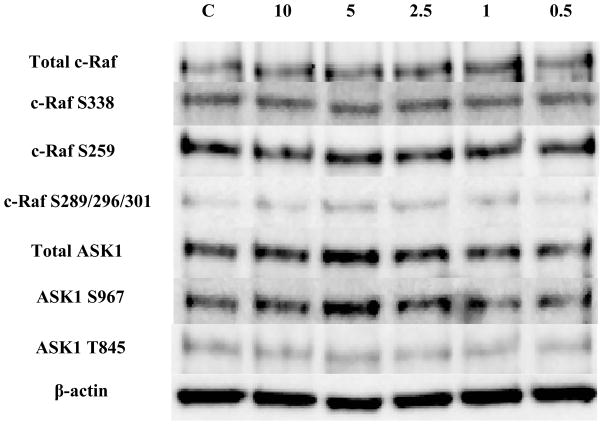
Figure 9. Effect of 1 hour exposures to 10 to 0.5 μM DBT on c-Raf and ASK1 in human NK cells.
Representative western blots for total c-Raf and c-Raf phosphorylation at S338, S259, S289/296/301 and for total ASK1 and ASK1 phosphorylation at S967, T845. Four separate experiments were analyzed using cells from different donors, p>0.05.
The effect of 6 hour exposures to 10, 5, 2.5, 1 and 0.5 μM DBT at concentrations on the phosphorylation state of c-Raf and ASK1 in human NK cells after 6 hour exposure
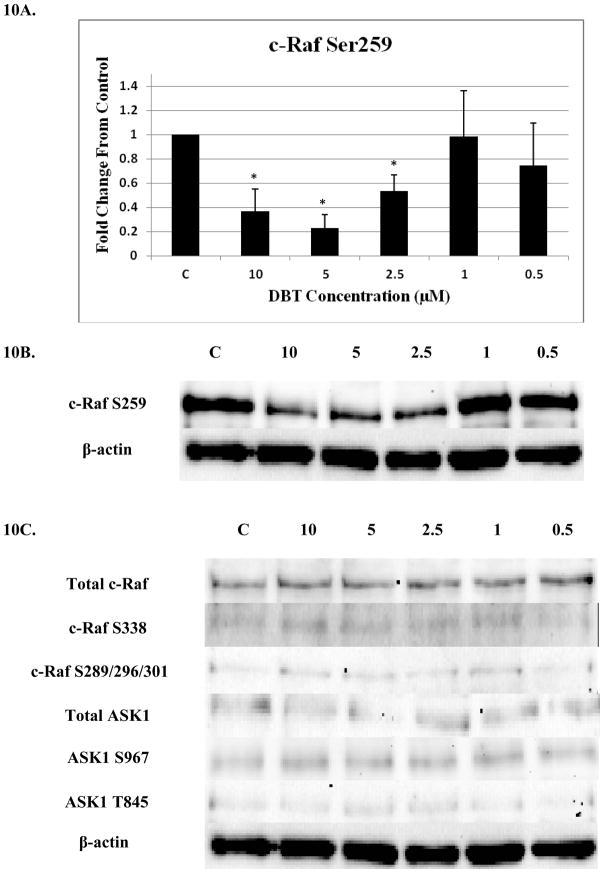
Significant decreases were seen in phosphorylation of c-Raf S259 after 6 h exposures to 10, 5, and 2.5 μM DBT (Figures 10A and B). 10 μM DBT decreased S259 phosphorylation by 63% while 5 μM and 2.5 μM DBT decreased phosphorylation by 77 and 47% respectively. There were no other significant changes in phosphorylation or total levels for either c-Raf or ASK1 in human NK cells after 6 h exposures to DBT (Figure 10C).
Figure 10. Effect of 6 hour exposures to 10 to 0.5 μM DBT on c-Raf and ASK1 in human NK cells.
A) Levels of c-Raf phosphorylated at S259 shown as fold change compared to control. Values are mean ± S.D. from 4 separate experiments using cells from different donors *p<0.05. B) Representative western blot for c-Raf phosphorylation at S259. C) Representative western blots for total c-Raf and c-Raf phosphorylation at S338, S289/296/301 and for total ASK1 and ASK1 phosphorylation at S967, T845. Four separate experiments were analyzed using cells from different donors, p>0.05.
Discussion
Previous studies have shown a significant increase in the activation of the MAPKs p44/42, p38 and JNK and the MAP2Ks MKK3/6 and MEK1/2 by TBT within ten minutes of exposure (Aluoch et al., 2006; 2007). DBT also induces the activation of these same enzymes at brief exposures (Odman-Ghazi et al., 2010). It has been shown that BT-induced loss of NK-lytic function and specific cell-surface proteins needed for lytic function can be attributed to the activation of p44/42 (Dudimah et al., 2010a, b). Additionally, it has been shown that BT-induced alterations of cell-surface protein expression could be prevented if MAPK activation was blocked prior to exposure to BTs (Dudimah et al., 2010b). These results demonstrated the essential role of activation of the MAPK components of the NK signaling pathway in the BT-induced alterations of human NK cell functions. The current study examines whether BTs induce activation of the immediate upstream activators of MAP2Ks, the MAP3Ks. This study specifically examines if TBT and DBT cause the activation of the MAP3Ks c-Raf (also known as Raf1) and ASK1 in human NK cells; and if so, if c-Raf activation is due to BT activation of Ras. Highly purified NK cells were exposed to concentrations of TBT and DBT at which loss of lytic function (Dudimah et al, 2007b; Whalen et al., 1999) and activation of MAPKs and MAP2Ks were seen (Aluoch et al., 2005; 2006; 2007; Odman-Ghazi et al., 2010). These levels approach those found in human blood (Kanan et al., 1999; Whalen et al., 1999).
C-Raf is the upstream regulator of the MAP2Ks, MEK 1 and 2, which in turn activate the MAPKs, p44 and p42. Activated Ras binds to the Ras Binding Domain (RBD) of c-Raf and recruits the protein to the plasma membrane (Vojtek et al., 1993). Activation of c-Raf is also dependent on phosphorylation of Serine 338 (S338) (King et a., 1998; Wellbrock et al., 2004). C-Raf is negatively regulated by phosphorylation of Serine 259 (S259). Phosphorylated S259 serves a needed part of the binding site for 14-3-3 scaffold/adaptor proteins that bind to c-Raf. 14-3-3 prevents the association of c-Raf with its immediate upstream activator Ras-GTP (Matallanas et al., 2011; Molzan et al., 2012). This site is phosphorylated by Akt/protein kinase B (PKB) (Zimmermann et al., 1999). The phosphorylation of Serine 289, 296, and 301 (S289/296/301) in c-Raf are involved in a negative feedback pathway. The three become hyperphosphorylated in response to the incessant activation of c-Raf. The three amino acid residues are phosphorylated by the downstream regulator MAPK. Their phosphorylation decreases the affinity of Ras for c-Raf (Dougherty et al., 2005).
The MAP3K ASK1 is the immediate upstream regulator of the MAP2Ks, MKK3/6 and MKK4. MKK3/6 and MKK4 are responsible for activating p38, but only MKK4 activates JNK (Tobiume et al., 2001). Phosphorylation of threonine 845 (T845) is required for the activation of ASK1; thus, it is an activation site. Phosphorylation on Serine 967 (S967) inactivates ASK1. This phosphorylation is necessary for its association with 14-3-3 proteins (Zhang et al., 1999). ASK1 is activated in response to signals including cellular stress by dephosphorylation of S967 and phosphorylation of T845 (Zhang et al., 1999).
The significant10–15% decrease of activated GTP-Ras observed in NK cells after their exposure to TBT (Figure 1) and 10 to 20 % decrease seen in the NK cells after their exposure to DBT (Figure 2) leads to at least two possible interpretations. First, either exposure of these cells to BTs does not result in the activation of Ras; indicating that an alternative method for BT-induced activation of c-Raf exists. Perhaps an alternate method of activation for c-Raf is via PKC (Abraha et al. 2010) or PAK3 signaling (Corbit et al., 2003; King et al., 1998). The second possibility is that the activation of Ras occurs immediately upon exposure to TBT or DBT (in less than 10 minutes) and at 10 minutes it has already begun to be desensitized and down-regulated. Unfortunately, our techniques do not allow a measurement earlier than 10 min. The second possibility fits with the fact that each of the concentrations that show a decrease in activated Ras are ones where downstream components of the pathway, MEK1/2 and p44/42, are activated by BTs (Aluoch et al., 2006; Odman-Ghazi et al., 2010).
There was increased phosphorylation of c-Raf at the activating site S338 when NK cells were exposed to TBT for 10 min. Those concentrations of TBT that caused activation of c-Raf also showed activation of MEK1/2 and p44/42 (Aluoch et al., 2006). Thus the data from this study would indicate that the TBT-induced activation of p44/42 seen in previous studies was due to TBT-induced activation of c-Raf (Figures 3C and D). Increased phosphorylation of S289/296/301 after 10 minute (Figures 3G and H) and after 1 hour (Figures 5E and F) exposures also corroborates the activation of c-Raf in TBT-exposed NK cells, since these sites are part of a negative feedback mechanism that help “shut-down” c-Raf in response to subsequent activation events (Dougherty et al., 2005). In addition, though not statistically significant due to donor variability, all of the donors showed an increase in phosphorylation of c-Raf at S289/296/301 after 10 minute exposure to TBT at the 50 nM concentration (Table 1) and three out of four donors showed an increase ranging up to 3 fold at the 200, 50 and 25 nM concentrations after NK cell exposure to 1 hour of TBT. The activation of c-Raf in the NK cells after as little as 10 minute exposure to TBT but no activation after 6 hour exposure (Figure 7C) implies that c-Raf has already been desensitized by 6 hours. Activation of c-Raf by TBT would lead to activation of the p44/42 MAPK pathway and ultimately to its “shut-down.” As this pathway is essential to the normal function of the NK cell, its activation by TBT will leave the cell unable to respond to appropriate stimuli such as tumor cell or virally infected cells (Wei et al., 1998).
In addition to the TBT-induced activation of c-Raf by phosphorylation of S338, the total amount of c-Raf present within the NK cells was significantly increased at several concentrations after 10 minute exposures (Figure 3A and B). Although, data from the remaining three concentrations- 300, 50 and 25 nM- after 10 minute exposure and data from the 200, 100 and 25 nM concentrations after 6 hour exposure did not yield statistically significant results, at least three out of the five donors per concentration showed an increase ranging up to 4.3 fold of total c-Raf after 10 minutes and half of the donors exposed to 6 hours of TBT also showed an increase ranging up to 2.4 fold in the total amount of c-Raf. As with all human-related studies, there are many factors that contribute to donor variability. For example, diet, weight, genetic make-up, environment (among other things) can all contribute to donor variation. The increases seen in the total amount of c-Raf readily available within the NK cells after their exposure to TBT suggests that c-Raf is being upregulated in response to the chemical at least in some cases. This may indicate that TBT is interfering with the normal processes involved in regulating c-Raf synthesis and destruction either by decreasing or increasing ubiquitination of the protein (Hayes et al, 2012).
Although exposures of NK cells to 300-50 nM TBT caused significant increases in activated MKK3/6 and p38 after 10 min, there was no activation of the MAP3K for that pathway, ASK1. This indicates that we may be missing the activation of this MAP3K due to the fact that the phosphorylation sites that we examined not being the sole mechanism of regulation of this MAP3K. It may also be that the phosphatases that regulate ASK1 are more active than those for Raf. In addition, it may be that another MAP3K or mechanism is responsible for the activation of MKK3/6.
Ten minute, one hour, and six hour exposures of human NK cells to DBT yielded no significant increases in phosphorylation of either c-Raf or ASK1 (Figures 8C, 9 and 10C). After only 10 minutes of DBT exposure, there were significant decreases in the amount of total c-Raf protein levels at the lowest concentration, 25 μM (Figure 8A and B). This suggests that c-Raf may be down-regulated in response to its prior constant activation, as it was in the NK cells that were exposed to 1 hour of TBT. This observation also supports the notion that DBT-induced activation of c-Raf occurs before 10 minutes- i.e. immediately- and that higher concentrations of DBT have induced a significant down regulation due to a very fast intense activation. C-Raf activation prior to 10 minutes explains why the enzyme is unresponsive or desensitized after 10 minute, 1 or 6 hour exposure to DBT (Figures 8C, 9 and 10C). C-Raf’s downregulation, again, could be due to DBT inhibition of c-Raf synthesis or increase in its destruction.
Significant decreases in phosphorylation of c-Raf at S259 (Figure 10A and B) were seen after 6 h exposures to DBT. S259 is the site that when phosphorylated causes association of c-Raf with 14-3-3 and inhibition of c-Raf catalytic activity. The fact that this site is not being phosphorylated after 6 h exposures to DBT may be due to DBT-induced changes in levels or catalytic activity of PI3K/Akt signaling pathway. Akt, also known as PKB, is responsible for phosphorylating S259 and thereby inactivating c-Raf (Zimmermann et al., 1999). Therefore, if Akt is being inhibited, for example, it would render it incapable of phosphorylating c-Raf at S259; hence, the levels of phosphorylated S259 would decrease. The decreased inhibiton of c-Raf by decreased inhibitory phosphorylation of S259 is consistent with the fact that after 6 h there is an activation of both MEK1/2 and p44/42 in DBT exposed cells (Odman-Ghazi et al., 2010).
The MAP3K c-Raf is responsible for activating the MAP2Ks MEK 1 and 2, which in turn activate the MAPKs p44 and p42. This pathway ultimately leads to the release of the NK cell’s cytotoxic proteins. ASK1, another MAP3K, activates MKK3/6 (MAP2Ks) which in turn activate p38 and JNK (MAPKs). This pathway regulates other aspects of NK function that are important to NK cytotoxic function (Trotta et al., 2000). Previous studies have shown a significant increase in the activation of the MAPKs, p38, p44/42, JNK, and the MAP2Ks, MKK3/6 and MEK1/2, by TBT within ten minutes of exposure (Aluoch et al., 2005; 2006; 2007). DBT also induces the activation of p38 and p44/42 and their respective MAP2K (Odman-Ghazi et al., 2010). The intent of the current study was to examine if compound induced activation of MAP3K was responsible for MAP2K and thus, MAPK activation. Here we show that significant activation of c-Raf in human NK cells also occurs within 10 minutes of TBT exposure. ASK1 is also being activated after one hour exposure of human NK cells to TBT. In addition, our total c-Raf results suggest that both TBT and DBT are impacting regulation of c-Raf.
Acknowledgments
This work was supported by 1U54CA163066-02 from the National Institutes of Health
References
- Abraha A, Rana K, Whalen MM. Role of protein kinase C in the TBT-induced inhibition of lytic function and MAPK activation in human natural killer cells. Arch Environ Cont Toxicol. 2010;59:661–669. doi: 10.1007/s00244-010-9520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluoch A, Whalen M. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells. Toxicology. 2005;209:263–277. doi: 10.1016/j.tox.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–237. doi: 10.1016/j.tox.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Pattern of MAP kinases p44/42 and JNK activation by non-lethal doses of tributyltin in human natural killer cells. Archives of Toxicology. 2007;81:271–277. doi: 10.1007/s00204-006-0155-4. [DOI] [PubMed] [Google Scholar]
- Antizar-Ladislao B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environmental International. 2008;34:292–308. doi: 10.1016/j.envint.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Azenha M, Vasconcelos MT. Butyltin compounds in Portuguese wines. Journal of Agricultural and Food Chemistry. 2002;50:2713–2716. doi: 10.1021/jf0115544. [DOI] [PubMed] [Google Scholar]
- Belfroid AC, Purperhart M, Ariese F. Organotin Levels in Seafood. Marine Pollution Bulletin. 2000;40:226–232. [Google Scholar]
- Boyer IJ. Toxicity of dibutyltin, tributyltin and other organotin compounds to humans and to experimental animals. Toxicology. 1989;55:253–298. doi: 10.1016/0300-483x(89)90018-8. [DOI] [PubMed] [Google Scholar]
- Catlin R, Shah H, Bankhurst AD, Whalen MM. Dibutyltin exposure decreases granzyme B and perforin in human natural killer cells. Environmental Toxicology and Pharmacology. 2005;20:395–403. doi: 10.1016/j.etap.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Clark EA, Sterritt RM, Lester JN. The fate of tributyltin in the aquatic environment. Environmental Science and Technology. 1988;22:600–604. [Google Scholar]
- Corbit KC, Trakul N, Eves EM, Diaz B, Marshall M, Rosner MR. Action of Raf-1 Signaling by Protein Kinase C through a Mechanism Involving Raf Kinase Inhibitory Protein. The Journal of Biological Chemistry. 2003;278:13061–13068. doi: 10.1074/jbc.M210015200. [DOI] [PubMed] [Google Scholar]
- Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, Conrades TP, Veenstra TD, Lu KP, Morrison DK. Regulation of Raf-1 by Direct Feedback Phosphorylation. Molecular Cell. 2005;17:215–224. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effects of tributyltin (TBT) on ATP levels in human natural killer (NK) cells: Relationship to TBT-induced decreases in NK function. Journal of Applied Toxicology. 2007a;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Gibson C, Whalen MM. Effect of dibutyltin on ATP levels in human natural killer cells. Environmental Toxicology. 2007b;22:117–23. doi: 10.1002/tox.20252. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Griffey D, Wang X, Whalen MM. Activation of p44/42 MAPK plays a role in the TBT-induced loss of human natural killer (NK) cell function. Cell Biology and Toxicology. 2010a;20:544–555. doi: 10.1007/s10565-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudimah FD, Abraha A, Wang X, Whalen MM. Activation of p44/42 in human natural killer cells decreases cell-surface protein expression: Relationship to tributyltin-induced alterations of protein expression. Toxicology Mechanisms and Methods. 2010b;20:544–555. doi: 10.3109/15376516.2010.518174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RL, Phillippo ET, Harr R, Koscinski W, Vosco G. Organotin residue determination in poultry and turkey sample survey in the United States. Journal of Agricultural and Food Chemistry. 1991;39:917–921. [Google Scholar]
- Fent K. Worldwide occurrence of organotins from antifouling paints and effects in the aquatic environment. The Handbook of Environmental Chemistry. 2006;5:71–100. [Google Scholar]
- Forsyth DS, Weber D, Cldroux C. Determination of butyltin, cyclohexyltin and phenyltin compounds in beers and wines. Food Additives and Contaminants. 1992;9:161–169. doi: 10.1080/02652039209374058. [DOI] [PubMed] [Google Scholar]
- Forsyth DS, Jay B. Organotin leachates in drinking water from chlorinated poly (vinyl chloride) (CPVC) pipe. Applied Organometallic Chemistry. 1997;11:551–558. [Google Scholar]
- Gipperth L. The legal design of the international and European Union ban on tributyltin antifouling paint: Direct and indirect effects. J Environ Management. 2009;90:S86–S95. doi: 10.1016/j.jenvman.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Hayes SD, Liu H, MacDonald E, Sanderson CM, Coulson JM, Clague MJ, Urbe S. Direct and Indirect Control of Mitogen-activated Protein Kinase Pathway-associated Components, BRAP/IMP E3 Ubiquitin Ligase and CRAF/RAF1 Kinase, by the Deubiquitylating Enzyme USP15. The Journal of Biological Chemistry. 2012;287:43007–43018. doi: 10.1074/jbc.M112.386938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch M. Organotin compounds in the environment. Applied Geochemistry. 2001;16:719–743. [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R. Occurrence of Butyltin Residues in Certain Foodstuffs. Bulletin of Environmental Contamination and Toxicology. 1995;55:510–516. doi: 10.1007/BF00196029. [DOI] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. Occurance of butyltin compounds in human blood. Environmental Science and Technology. 1999;33:1776–1779. [Google Scholar]
- King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, Marshall MS. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature. 1998;396:180–183. doi: 10.1038/24184. [DOI] [PubMed] [Google Scholar]
- Lowin B, Beermann F, Schmidt A, Tschopp J. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell- mediated cytotoxicity. Proc Natl Acad Sci USA. 1994;91:11571–11575. doi: 10.1073/pnas.91.24.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, Kriegsheim AV, Kolch W. Raf Family Kinases Old Dogs Have Learned New Tricks. Genes and Cancer. 2011;2:232–260. doi: 10.1177/1947601911407323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molzan M, Ottmann C. Synergistic binding of the phosphorylated S233- and S259-binding sites of C-RAF to one 14-3-3 dimer. Journal of Molecular Biology. 2012;423:486–495. doi: 10.1016/j.jmb.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Mora SJ, King NG, Miller MC. Tributyltin and total tin in marine sediments: Profiles and the apparent rate of TBT degradation. Environmental Technology. 1989;10:901–908. [Google Scholar]
- Nakanishi T, Hiromori Y, Yokoyama H, Koyanagi M, Itoh N, Nishikawa J, Tanaka K. Organotin compounds enhance 17β-hydroxysteroid dehydrogenase type I activity in human choriocarcinoma JAr cells: Potential promotion of 17β-estradiol biosynthesis in human placenta7. Biochemical Pharmacology. 2006;71:1349–1357. doi: 10.1016/j.bcp.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Nakanishi T. Potential toxicity of organotin compound via nuclear receptor signaling in mammals. Journal of Health Science. 2007;53:1–9. [Google Scholar]
- Nielsen JB, Strand J. Butyltin Compounds in Human Liver. Environmental Research. 2002;88:129–133. doi: 10.1006/enrs.2001.4321. [DOI] [PubMed] [Google Scholar]
- Odman-Ghazi SO, Abrah A, Isom ET, Whalen MM. Dibutyltin activates MAP kinases in human natural killer cells, in vitro. Cell Biology and Toxicology. 2010;26:469–479. doi: 10.1007/s10565-010-9157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odman-Ghazi SO, Hatcher F, Whalen MM. Expression of functionally relevant cell surface markers in dibutyltin-exposed human natural killer cells. Chemico-Biological Interact. 2003;146:1–18. doi: 10.1016/s0009-2797(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Roper WL. Toxicological profile for tin. U.S department of health and human services, agency for toxic substances and disease registry; 1992. [Google Scholar]
- Sadiki AI, Williams DT, Carrier R, Thomas B. Pilot Study on the Contamination of Drinking Water by Organotin Compounds from PVC Materials. Chemosphere. 1996;32:2389–2398. doi: 10.1016/0045-6535(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Yanase T, Morinaga H, Tanabe M, Mu YM, Nishi Y, Nomura M, Okabe T, Gotu K, Takayanagi R, Nawata H. Tributyltin or Triphenyltin Inhibits Aromatase Activity in the Human Granulosa-Like Tumor Cell Line KGN. Biochemical and Biophysical Research Communications. 2001;289:198–204. doi: 10.1006/bbrc.2001.5952. [DOI] [PubMed] [Google Scholar]
- Shresta S, MacIvor DM, Heusel JW, Russell JH, Ley TJ. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc Natl Acad Sci USA. 1995;92:5679–5683. doi: 10.1073/pnas.92.12.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeij NJ, Penninks AH, Seinen W. Thymus atrophy and immunosuppression induced by organotins compounds. Arch Toxicol Suppl. 1989;13:171–174. doi: 10.1007/978-3-642-74117-3_23. [DOI] [PubMed] [Google Scholar]
- Snoeij NJ, Penninks AH, Seinen W. Biological activity or organotins compounds- An overview. Environ Res. 1987;44:335–353. doi: 10.1016/s0013-9351(87)80242-6. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Mukai H, Tanabe S, Sakayama K, Miyazaki T, Masuno H. Butyltin resides in livers of humans and wild terrestrial mammals and in plastic products. Environmental Pollution. 1999;106:213–218. doi: 10.1016/s0269-7491(99)00068-8. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. European Molecular Biology Organization. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta R, Fettucciari K, Azzoni L, Abebe B, Puorro KA, Eisenlohr LC, Perussia B. Differential role of p38 and c-Jun N-terminal kinase 1 mitogen-activated protein kinases in NK cell cytotoxicity. J Immunol. 2000;165:1782–1789. doi: 10.4049/jimmunol.165.4.1782. [DOI] [PubMed] [Google Scholar]
- Veltman K, Huijbregts MAJ, van den Heuvel-Greve MJ, Vethaak AD, Hendriks AJ. Organotin accumulation in an estuarine food chain: comparing field measurements with model estimations. Marine Environmental Research. 2006;61:511–530. doi: 10.1016/j.marenvres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- Vos JG, DeKlerk A, Krajnc EI, Van Loveren V, Rozing J. Immunotoxicity of bis(tri-n-butyltin)oxide in the rat: Effects on thymus- dependent immunity and on nonspecific resistance following long-term exposure in young versus aged rats. Toxicol Appl Pharmacol. 1990;105:144–155. doi: 10.1016/0041-008x(90)90366-3. [DOI] [PubMed] [Google Scholar]
- Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. Journal of Innate Immunity. 2011;3:258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]
- Wei S, Camero AM, Liu JH, Daulton AA, Valkov NI, Trapani JA, Larner AC, Weber MJ, Djeu JY. Control of Lytic Function by Mitogen-activated Protein Kinase/Extracellular Regulatory Kinase 2 (ERK2) in a Human Natural Killer Cell Line: Identification of Perforin and Granzyme B Mobilization by Functional ERK2. Journal of Experimental Medicine. 1998;187:1753–1765. doi: 10.1084/jem.187.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellbrock C, Karasarides M, Marais R. The RAF proteins take center stage. Nature Reviews Molecular Cell Biology. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of Environmentally Relevant Concentrations of Buytltins on Human Natural Killer Cells in Vitro. Environmental Research. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Ghazi S, Loganathan BG, Hatcher F. Expression of CD16, CD18 and CD56 in tributyltin-exposed human natural killer cells. Chemico-Biological Interactions. 2002;139:159–176. doi: 10.1016/s0009-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen J, Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proceeding of the National Academy of Sciences. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and Regulation of Raf by Akt (Protein Kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]