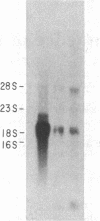
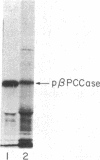
Abstract
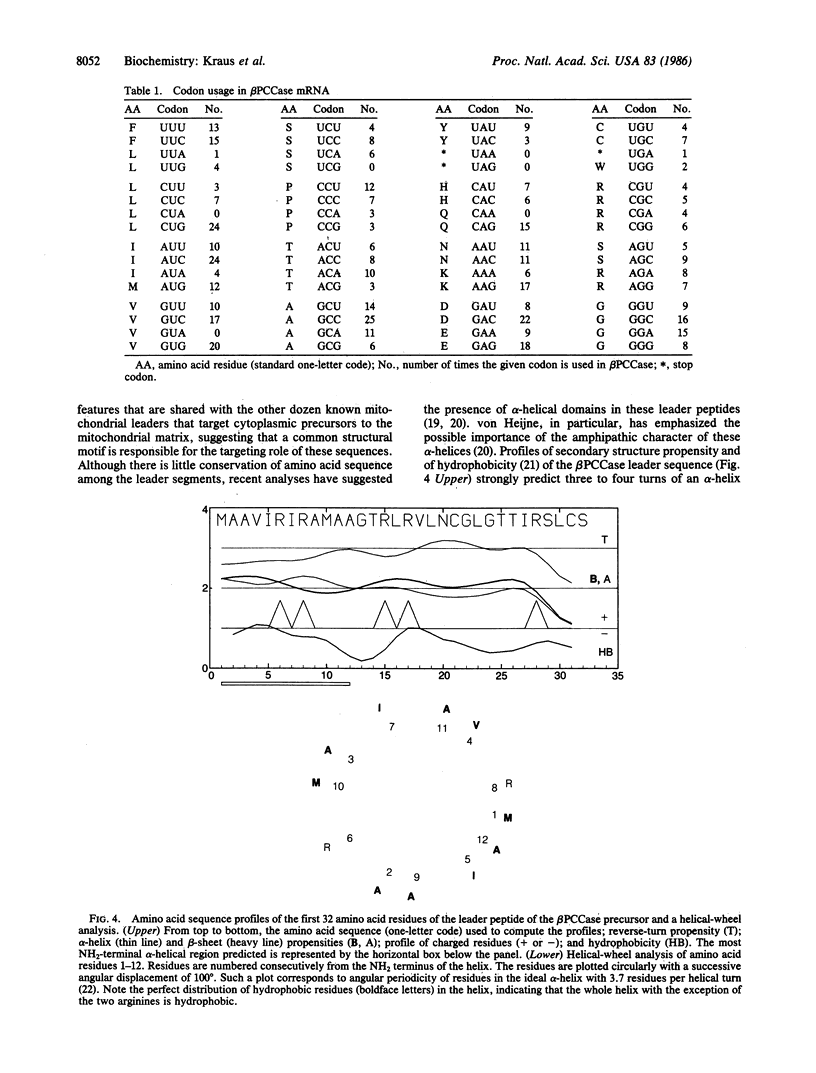
A cDNA encoding the cytoplasmic precursor of the beta subunit of the mitochondrial enzyme propionyl-CoA carboxylase (EC 6.4.1.3) was cloned and sequenced. The DNA sequence of 2070 nucleotides is almost identical in size to the major hybridizing mRNA from rat liver (2000 +/- 50 nucleotides), suggesting that the cloned DNA represents nearly all of the mRNA sequence. A polypeptide expressed in vitro from an mRNA transcript of this cDNA is indistinguishable in size from the beta subunit precursor (58,500 Da). An open reading frame of 1623 nucleotides, flanked by stop codons, encodes a polypeptide of 541 amino acids; the predicted amino acid sequence was confirmed as that of the beta subunit of propionyl-CoA carboxylase by matching it to the amino acid sequences of five peptides derived from pure mature rat enzyme. Although the exact length of the cleavable, NH2-terminal leader peptide has not been determined because the NH2-terminal residue of the mature subunit is blocked, the leader is most likely 40-42 amino acids in length and is highly positively charged. Computer-aided analysis of secondary structure suggests that the leader peptide consists of two alpha-helical segments, with the two most NH2-terminal arginine residues occupying opposite sites of the first helix; this helix has no apparent hydrophobic moment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The helical hydrophobic moment: a measure of the amphiphilicity of a helix. Nature. 1982 Sep 23;299(5881):371–374. doi: 10.1038/299371a0. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Lam K. F., Mahuran D., Kronis A. Purification of human liver propionyl-CoA carboxylase by carbon tetrachloride extraction and monomeric avidin affinity chromatography. Arch Biochem Biophys. 1980 May;201(2):669–673. doi: 10.1016/0003-9861(80)90557-3. [DOI] [PubMed] [Google Scholar]
- Gravel R. A., Lam K. F., Scully K. J., Hsia Y. Genetic complementation of propionyl-CoA carboxylase deficiency in cultured human fibroblasts. Am J Hum Genet. 1977 Jul;29(4):378–388. [PMC free article] [PubMed] [Google Scholar]
- Haase F. C., Beegen H., Allen S. H. Propionyl-coenzyme A carboxylase of Mycobacterium smegmatis. An electron microscopic study. Eur J Biochem. 1984 Apr 2;140(1):147–151. doi: 10.1111/j.1432-1033.1984.tb08078.x. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Kalousek F., Darigo M. D., Rosenberg L. E. Isolation and characterization of propionyl-CoA carboxylase from normal human liver. Evidence for a protomeric tetramer of nonidentical subunits. J Biol Chem. 1980 Jan 10;255(1):60–65. [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Kalousek F., Rosenberg L. E. Biosynthesis and mitochondrial processing of the beta subunit of propionyl coenzyme A carboxylase from rat liver. J Biol Chem. 1983 Jun 25;258(12):7245–7248. [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus J. P., Williamson C. L., Firgaira F. A., Yang-Feng T. L., Münke M., Francke U., Rosenberg L. E. Cloning and screening with nanogram amounts of immunopurified mRNAs: cDNA cloning and chromosomal mapping of cystathionine beta-synthase and the beta subunit of propionyl-CoA carboxylase. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2047–2051. doi: 10.1073/pnas.83.7.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamhonwah A. M., Barankiewicz T. J., Willard H. F., Mahuran D. J., Quan F., Gravel R. A. Isolation of cDNA clones coding for the alpha and beta chains of human propionyl-CoA carboxylase: chromosomal assignments and DNA polymorphisms associated with PCCA and PCCB genes. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4864–4868. doi: 10.1073/pnas.83.13.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mueckler M. M., Pitot H. C. Sequence of the precursor to rat ornithine aminotransferase deduced from a cDNA clone. J Biol Chem. 1985 Oct 25;260(24):12993–12997. [PubMed] [Google Scholar]
- Myerowitz R., Piekarz R., Neufeld E. F., Shows T. B., Suzuki K. Human beta-hexosaminidase alpha chain: coding sequence and homology with the beta chain. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7830–7834. doi: 10.1073/pnas.82.23.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotný J., Auffray C. A program for prediction of protein secondary structure from nucleotide sequence data: application to histocompatibility antigens. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):243–255. doi: 10.1093/nar/12.1part1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell I. J., Inglis A. S. Amino acid sequence of a feather keratin from silver gull (Larus novae-hollandiae) and comparison with one from emu (Dromaius novae-hollandiae). Aust J Biol Sci. 1974 Aug;27(4):369–382. doi: 10.1071/bi9740369. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Correlation of amino acid sequence and conformation in tobacco mosaic virus. Biophys J. 1968 Jan;8(1):29–39. doi: 10.1016/S0006-3495(68)86472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. R., LoPresti M. B., Setoguchi M., Konigsberg W. H. Amino acid sequence of the T4 DNA helix-destabilizing protein. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4614–4617. doi: 10.1073/pnas.77.8.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]