Abstract
In China, KSHV seroprevalence varies considerably among different regions and ethnicities. But in Xinjiang province, located in the northwestern China, there is a very high seroprevalence of KSHV in adults of Kazak and Ughur ethnicities. However, KSHV prevalence in children and the risk factors associated with the acquisition of infection are currently not known. The aim of this study was to investigate the prevalence of KSHV infection, identify associated socio-economic or behavioral risk factors and the humoral immune response among children in this population. This is a cross-sectional study (N=178) to screen children and their caregivers from Xinjiang for total KSHV antibodies, KSHV neutralizing antibodies and HIV infection. Structured questionnaires were utilized to investigate risk factors associated with KSHV prevalence. KSHV seroprevalence in children and caregivers in Xinjiang was 48.3% and 64.7%, respectively. Neutralizing antibody was detected in most seropositive caregivers (93.8%) but was detected in only 5.8% of the infected children. Significant association was observed between child KSHV seroprevalence and sharing of food among family members. These results suggest that similar to other endemic areas in Africa, KSHV infection in the minority populations of Xinjiang is likely to be occurring during early childhood likely via horizontal transmission through saliva and results to high seroprevalence in the adult population.
Keywords: Kaposi’s sarcoma-associated herpesvirus, KSHV, HHV8, seroprevalence, Xinjiang, China
INTRODUCTION
Kaposi sarcoma (KS)-associated herpesvirus (KSHV) or Human herpesvirus 8 (HHV8), is the etiological agent associated with KS. [1, 2]. Global seroprevalence of KSHV varies in different geographical regions. It is generally low to moderate in Western countries (3 to 23%) but endemic in the general population (> 50%) in sub-Saharan Africa and even higher in the HIV-positive individuals [3–5].
As in most Asian countries [6], the incidence of KS and seroprevalence of KSHV is low in most provinces of China (7.3 to 16.1% in adults) [7–10]. Xinjiang province, situated in Northwestern China, has a significantly higher incidence of KS (classic and AIDS-associated) and a higher seroprevalence of KSHV in adults [11]. The higher prevalence could be associated with the ethnic makeup of the population. In mainland China, Han is the major ethnic group but in Xinjiang, other ethnicities like Uygur, Kazaks and Hui are in majority [10, 11]. Studies conducted in the Uygur and Kazak ethnic groups have reported KSHV seroprevalence in adults to be as high as 46.6% [10–12]. Interestingly, Xinjiang also has one of the highest prevalence of HIV infection in China, especially among injection drug users in whom prevalence can be as high as 80% [13, 14].
The exact routes of KSHV transmission are unclear and may differ by geographic region and risk group. Sexual transmission, organ transplant and blood transfusion in adults have been reported [15–18]. Saliva is considered to be the major route of transmission from infected adults to children in sub-Saharan Africa, and early childhood infection could be contributing to the high KSHV prevalence in the adult population [19, 20]. The unique lifestyle and culture of the Uyghurs and the Kazakh ethnic groups in Xinjiang could facilitate salivary contact to enhance early childhood KSHV infection, and subsequently high prevalence in the population as seen in KS endemic regions.
Most reports published so far have investigated prevalence and risk factors in adults and not much is known about the prevalence and risk of KSHV infection in children in the Xinjiang region. We hypothesize that early childhood infection in Xinjiang is common and contributes to the high prevalence of KSHV in the population. Therefore, the goal of the current study is to investigate the serological profile and immune response against KSHV in children and their caregivers, and determine the risk factors that may be associated with KSHV prevalence in children.
MATERIAL AND METHODS
Study cohort
Between March and October, 2011, caregivers having children between 6–60 months of age, attending local clinics in Xinyuan and Jiashi Counties in Xinjiang province were approached to participate in this study. Children over six months of age were recruited to avoid the detection of transplacental maternal antibodies. Recruitment occurred from at least three clinics representing different regions of the county to ensure random distribution of the study subjects and reflect the general population of the region where a majority of them are of Uygur and Kazakh ethnicity. The caregivers were educated about the study and signed informed consent was obtained. This study was approved by the institutional review boards at the University of Nebraska and Hangzhou Normal University.
Sample collection
Blood samples were collected in EDTA tubes from children and their caregivers and plasma was separated. Specimens collected from children and caregivers were coded by a unique identification number. All specimens were stored at −70°C until testing.
Data collection
A standardized format was used to collect information on study participants and the data included socio-economic, home living conditions, life-style risk factors and child care. A trained interviewer conducted field-based intake interviews with the child’s primary caregiver. Data collection instruments that was used in the study represent modified versions of the data forms used by our ongoing household study in Zambia, but were field tested and adapted to local setting [21].
Serological testing
KSHV serological test
All plasma samples were tested using monoclonal-enhanced immunofluorescence assay (mIFAs) as described [22]. Briefly, BC3 cells were stimulated with tetradecanoyl phorbol acetate (TPA), then fixed with 4% paraformaldehyde and incubated with patient plasma. The signal was enhanced using murine monoclonal anti-human immunoglobulin and then incubated with fluorescein-isothiocyanate conjugated anti-mouse antibody. Slides were read independently by two experienced laboratory workers. All positive plasma were diluted further and tested by mIFA to estimate the KSHV antibody titer of each sample.
HIV-1 Test
All plasma samples were tested by a standard HIV-1 test kit [1 +2 type antibody diagnostic kits, Wantai, China] and confirmed by a second kit (Colloidal Gold Device, Wantai, China).
KSHV Neutralization assay
Neutralizing antibodies against KSHV was detected for all seropositive children and caregivers by a flow cytometry based neutralization assay as described earlier with minor modifications [23]. Plasma was diluted and mixed with recombinant KSHV expressing GFP (rKSHV.219) and incubated for 1 hr at 37 °C. The virus and plasma mixture was added into a 96-well plate seeded with 293 cells. After incubation for 72 hours, green cells were counted by flow cytometry. Results were confirmed by counting at least 100 cells under a fluorescence microscope. A positive neutralizing antibody outcome was defined as ≥ 50% reduction in infectivity as compared to the sero-negative control serum. Positive plasma samples were diluted further to determine the neutralizing antibody titer.
Statistical analysis
All data was analyzed using the statistical software packages, SPSS version 17 (SPSS, Chicago, IL) and SAS v9.2 (SAS Institute, NC). Descriptive analyses and distribution of all key variables was conducted. Risk factor analysis was conducted using logistic regression. Multivariable analysis included all variables that had a p-value ≤0.20 to control for possible confounders and identify independent associations. We also checked the correlation between all variables to identify interactions and multi-collinearity. Correlation analysis was evaluated by Pearson’s correlation coefficient.
RESULTS
Study participants
A total of 178 children and 150 caregivers (15 caregivers had two children, 13 caregiver samples had insufficient volume for laboratory testing) were recruited to participate in the study. Table 1 summarizes the characteristics of the study cohort and the unadjusted odds ratio for the child being KSHV seropositive. Briefly, only 7 participants were of Han ethnicity, the rest (171 children) belonged to ethnic minority. All the participants were recruited from either Jiashi (89.9%) or from Xinyuan counties (10.1%). None of the demographic characteristics associated with increased risk for KSHV prevalence in children.
Table 1.
Characteristics of the study population and KSHV seroprevalence in Xinjiang, China
| Characteristic | N (%) | Child KSHV number positive (%) | Unadjusted Odds ratio | P value | |
|---|---|---|---|---|---|
| Ethnicity | |||||
| Han | 7 (3.9) | 1 (14.3) | |||
| Minority | 171 (96.1) | 85 (49.7) | ND | ||
| County of residence | |||||
| Jiashi | 160 (89.9) | 80 (50.0) | 2.00 (0.72 – 5.59) | 0.19 | |
| Xinyuan | 18 (10.1) | 6 (33.3) | 1.00 | ||
| Gender | |||||
| Male | 108 (60.7) | 47 (43.5) | 0.61 (0.33 – 1.12) | 0.11 | |
| Female | 70 (39.3) | 39 (48.3) | 1.00 | ||
| Child age, Median (Range) | 3.5 (0.6–5.5) | 86 (48.3) | 1.16 (0.90 – 1.50) | 0.27 | |
| Caregiver HIV Positive | 2 (1.3) | ND | |||
| Caregiver Education | |||||
| No schooling | 5 (50) | 1.41 (0.38 – 5.33) | 0.61 | ||
| Primary school | 51 (53.1) | 1.60 (0.86 – 2.98) | 0.14 | ||
| Junior school and beyond | 29 (41.4) | 1.00 | |||
|
| |||||
| Total | 178 | 86 (48.3) | |||
ND: Not done. The number of HIV positive caregivers was too small for reliable statistical analysis.
KSHV seroprevalence and antibody titers
The overall seroprevalence of KSHV among children in this cohort was 48.3% and was significantly higher among caregivers (64.7 %) (Figure 1A). The end point titer of all KSHV positive children and caregiver plasma was determined, and we observed that the geometric mean titer (GMT) of caregivers was significantly higher than the children (p= 0.002) (Figure 1B). Also, the interquartile (IQR) range was wider for the caregivers. Since the antibody titer in caregivers is significantly higher, we hypothesized that KSHV titers could correlate with age. The scatter plots in Figure 2 show that there was no significant relationship between age and KSHV titers for caregivers (correlation coefficient = 0.14) (Figure 2A) and children (correlation coefficient = 0.09) (Figure 2B).
Figure 1.
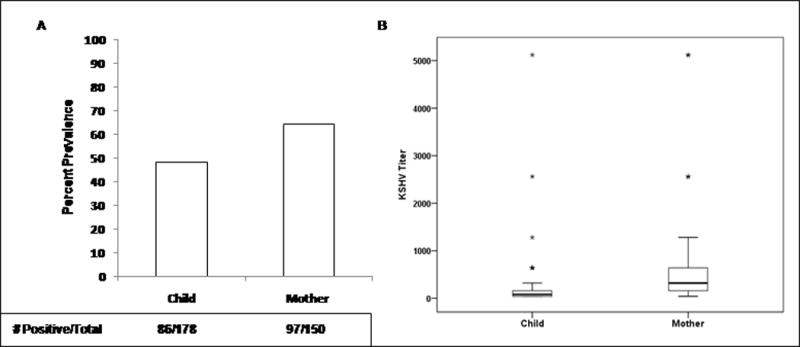
(A) Prevalence of KSHV infection in children and caregivers and (B) Comparison of KSHV antibody titers in children and caregivers in the study population in Xinjiang, China.
Figure 2.
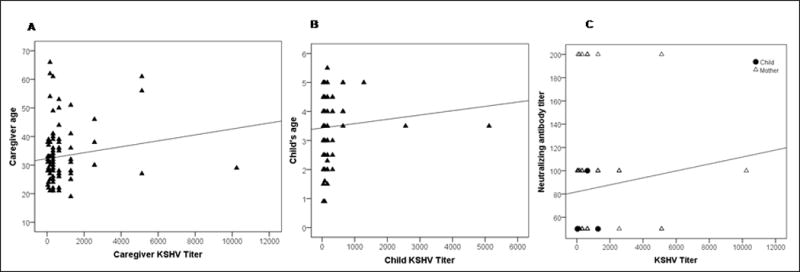
Scatter plot to demonstrate the correlation between KSHV antibody titer and age in (A) caregivers and; (B) children. (C) Scatter plot to demonstrate the correlation between KSHV antibody titer and neutralizing antibody titers in caregivers and children in the study population in Xinjiang, China.
KSHV neutralizing antibody prevalence and titers
Given the high prevalence of KSHV infection in both children and caregivers, and high KSHV antibody titer in caregivers, we investigated the prevalence of neutralizing antibodies in KSHV positive participants. We found that 91/97 seropositive caregivers (93.8%) have detectable levels of neutralizing antibodies. In contrast, only 4/86 (4.7 %) KSHV seropositive children have detectable neutralizing antibody. Even though there were a small number of children with detectable neutralizing antibody response, we further investigated if there is a significant difference between neutralizing antibodies titers among children and caregivers (data not shown). Further, we also investigated whether there is a correlation between neutralizing antibodies titers and KSHV antibody titers in both caregivers and children. Figure 2C shows the scatter plot of KSHV antibody titers against neutralizing antibody titers for caregivers and children, and did not find a strong positive correlation between them (correlation coefficient = 0.10).
Risk factors associated with KSHV prevalence
To analyze whether any behavior risk factors could have contributed to the high KSHV seroprevalence in the children in this study cohort, a wide range of data pertaining to socioeconomic status, household living conditions and various behavioral risks factors was collected. We specifically focused on factors that may contribute to horizontal transmission of KSHV to children. Tables 1 and 2 summarize the results of univariate analysis to investigate the association between KSHV prevalence and various risk factors. None of the socio-economic status factors such as living conditions and their dwellings was found to be associated with KSHV infection of the children in the household. Other factors involving the child’s hygiene and interactions with other children and family members were also not found to be significant.
Table 2.
Univariate analysis of KSHV seroprevalence in the study population
|
|
|||
|---|---|---|---|
| Socio-economic Status | Odds Ratio (95% CI) | p-value | |
| Total living area | 1.00 (0.99 – 1.00) | 0.28 | |
| Per capita living space | 1.01 (0.98 – 1.05) | 0.56 | |
| Number of rooms in house | 0.87 (0.72 – 1.06) | 0.17 | |
| Number of sleeping areas in house | 1.08 (0.86 – 1.36) | 0.50 | |
| Number of members in each sleeping area | 0.84 (0.62 – 1.14) | 0.27 | |
| Temporary house vs Brick house (Ref) | 0.73 (0.37 – 1.41) | 0.34 | |
| No running water vs running water (Ref) | 0.79 (0.26 – 2.37) | 0.67 | |
| Farmer vs Other (Ref) | 0.61 (0.30 – 1.26) | 0.19 | |
| Shared kitchen vs private kitchen (Ref) | 1.20 (0.48 – 2.99) | 0.69 | |
| Ventilation in kitchen absent vs present (Ref) | 1.09 (0.54 – 2.20) | 0.81 | |
|
| |||
| Breast Feeding and Eating Practices | |||
|
| |||
| Premastication by caregiver* | 0.82 (0.39 – 1.74) | 0.61 | |
| Premastication by others* | 0.92 (0.37 – 2.29) | 0.86 | |
| Caregiver tests temperature of food* | 0.77 (0.43 – 1.39) | 0.39 | |
| Family tests temperature of food* | 0.95 (0.51 – 1.77) | 0.87 | |
| Others test temperature of food* | 1.10 (0.46 – 2.59) | 0.83 | |
| Caregiver cools food by blowing* | 0.87 (0.48 – 1.57) | 0.65 | |
| Others cool food by blowing* | 0.83 (0.42 – 1.63) | 0.58 | |
| Caregivers chews food for child* | 0.98 (0.41 – 2.35) | 0.96 | |
| Family chews food for child* | 1.60 (0.58 – 4.41) | 0.37 | |
| Others chew food for child* | 1.38 (0.36 – 5.30) | 0.64 | |
| Share food with family* | 2.48 (1.36 – 4.54) | 0.003 | |
| Share food with neighbors* | 1.44 (0.61 – 3.41) | 0.41 | |
| Exchange of food/candy among children* | 1.20 (0.61 – 2.35) | 0.60 | |
| Eat with hands from common bowl* | 0.79 (0.43 – 1.46) | 0.45 | |
| Caregiver shares tableware* | 1.16 (0.58 – 2.34) | 0.67 | |
| Others share tableware* | 0.93 (0.43 – 2.01) | 0.86 | |
| Caregiver shares packaged drinks* | 0.82 (0.45 – 1.50) | 0.51 | |
| Members share packaged drinks* | 0.93 (0.49 – 1.79) | 0.83 | |
| Others share packaged drinks* | 0.62 (0.28 – 1.38) | 0.24 | |
| Neighbors share packaged drinks* | 0.81 (0.34 – 1.89) | 0.62 | |
| Number of people who share, 1 to 3 vs 4 or more (Ref) | 0.82 (0.35 – 1.91) | 0.64 | |
| Child spends night at else’s house* | 0.65 (0.20 – 2.06) | 0.46 | |
| Share toothbrush* | 1.34 (0.65 – 2.76) | 0.43 | |
| Use pacifier vs not use (Ref) | 1.05 (0.58 – 1.90) | 0.87 | |
| Share pacifier vs not share (Ref) | 0.85 (0.25 – 2.91) | 0.80 | |
| Child being breastfed vs not breastfed (Ref) | 1.12 (0.47 – 2.66) | 0.79 | |
| Child being breastfed now vs not being breastfed (Ref) | 0.82 (0.29 – 2.30) | 0.70 | |
| Child eating solid food vs not eating (Ref) | 0.74 (0.35 – 1.57) | 0.43
|
|
| Child hygiene and interactions | |||
|
| |||
| Ease teething pain with (Ref - No aid used) | |||
| Teething ring | 0.81 (0.30 – 2.15) | 0.67 | |
| Other | 1.28 (0.63 – 2.61) | 0.49 | |
| Bathing infrequently vs at least once or more/day (Ref) | 1.36 (0.57 – 3.26) | 0.49 | |
| Number of children who play with child (Ref - No child) | |||
| 1 to 2 | 0.92 (0.31 – 2.74) | 0.88 | |
| 3 to 4 | 0.89 (0.30 – 2.64) | 0.83 | |
| 5 or more | 0.66 (0.22 – 2.01) | 0.46 | |
| Number of children who play with child (< 5 years old) (Ref - No child) | |||
| 1 to 2 | 1.24 (0.54 – 2.85) | 0.62 | |
| 3 to more | 1.11 (0.49 – 2.52) | 0.81 | |
Always/sometimes vs Infrequently/never categories were used as the reference category
Since saliva has been implicated to be a major source of early childhood infection in KSHV endemic regions, a number of risk factors associated with feeding habits, including those that may involve saliva exchange between household members and the children were analyzed. The only risk factor that was significantly associated with KSHV infection in the children was the sharing of food with family members (OR=2.48, 95% Confidence Interval (CI) = 1.36–4.54, P=0.003). None of the other feeding practices has significant association with KSHV infection of children.
A multivariate analysis was carried out to further identify independent risk factors for KSHV infection after controlling for potential confounding factors (Table 3). Sharing food between the child and family members remained significant in a multivariate model after adjusting for gender, ethnicity of children, per capita living space, occupation and the number of rooms in the house (OR=2.19, 95% CI=1.17–4.13, P=0.015).
Table 3.
Multivariate logistic regression analysis of determinants of KSHV seropositivity
| Characteristic | Odds Ratio (95% Confidence Interval) | p-value |
|---|---|---|
| Gender of Child | 0.63 (0.33 – 1.20) | 0.157 |
| Ethnicity | 5.38 (0.60 – 48.45) | 0.133 |
| Per capita living space | 0.76 (0.40 – 1.45) | 0.406 |
| Occupation | 0.64 0.30 – 1.39) | 0.259 |
| Number of rooms in the house | 0.85 (0.69 – 1.04) | 0.105 |
| Share food with family members | 2.19 (1.17 – 4.13) | 0.015 |
DISCUSSION
This is the first study conducted among children in Xinjiang, an autonomous region in Northwestern China to determine the humoral immune response against KSHV and identify risk factors associated with KSHV prevalence. Xinjiang has a unique mix of various ethnicities and has been reported to be an endemic area for KS and KSHV infection. More than 95% of KS cases were observed in minority groups, particularly in Uygurs [24]. Several studies have investigated KSHV seroprevalence in adults, but none in children to investigate the seroprevalence of KSHV, the prevalence of neutralizing antibodies and identify risk factors associated with KSHV seroprevalence.
In this study, we observed high KSHV seroprevalence in children (48.3%), supporting our hypothesis that KSHV infection occurs in early childhood, which may contribute to high KSHV prevalence in the adult population. We have observed similarly high KSHV prevalence in Zambia, another endemic area for KSHV infection [25]. As shown by other studies we also observed high prevalence of KSHV in adult caregivers (64.7%) in this population demonstrating that Xinjiang is a unique region where KSHV infection is significantly higher than other provinces in China [7, 10]. The high prevalence and high KSHV antibody titers in adults is likely leading to transmission of KSHV to children. Therefore, we were interested in identifying specific child-rearing behaviors that may be associated with transmssion. No study to date has examined the association of KSHV infection in children with common child-rearing behavior, socio-economic indicators, breast feeding, and other child hygiene practices in China. In this study we did not find any significant association between KSHV infection of children and various demographic and socio-economic indicators. We observed that sharing food with family members was highly associated with KSHV seropositivity in children, indicating that horizontal transmission via saliva could be an important factor for early childhood infection. This is consistent with our previous findings that KSHV DNA can be easily detected in the saliva of infected individuals and more frequently in those who had higher KSHV antibody titers [20, 27–29]. We did not find an increased risk of KSHV seropositivity in breastfed children. These results are also consistent with our previous study in Zambian children, where we observed that KSHV cannot by readily detected and transmitted via breast milk [20].
The observed high seroprevalence among caregivers seem to be higher than other studies and could be attributed to differences in study population. Also, most KSHV assays differ in their sensitivity and specificity to detect KSHV antibodies. We chose to conduct IFA to screen for KSHV antibodies because stimulated BC3 cells provide the entire range of KSHV antigens to detect anti-KSHV antibodies, and is more sensitive than other KSHV serological assays. We believe that the observed high seroprevalence, even though higher than other reports, is specific because we titered the KSHV antibodies by serial dilution for all positive plasma samples. We observed a wide range of titers with some samples showing specific fluorescence at 10,000 fold plasma dilution. Further, we also observed a high prevalence of neutralizing antibodies in most caregivers and in some children indicating that the observed high seroprevalence in children and caregivers is specific for KSHV. Our results also show that GMT for KSHV was significantly higher in caregivers as compared to children though we did not observe any relationship between age and KSHV titer. It is likely that these caregivers have been infected for a longer time resulting in higher titers.
The neutralizing antibody response is known to play an important role in preventing viral infection [26] and may be protective against the development of KS [23]. Dilnur et al have reported that Xinjiang province is an endemic area for classic KS [11]. We observed that the prevalence of neutralizing antibodies in KSHV positive children (5.8%) was significantly lower as compared to KSHV positive caregiver adults (93.8%) with a median titer of 1:100 in caregivers. Taken together, these results do not indicate that the high prevalence of neutralizing antibodies in adults correlate with KS endemicity, but longitudinal studies are needed to investigate the association between the presence of neutralizing antibodies and the development of KS in this population.
A number of studies have documented that HIV-1 infection is a significant risk factor for KSHV infection [25, 30]. However, we could not evaluate HIV-1 as a risk factor since only 2 caregivers were found to be HIV-1 positive. Interestingly, these results also suggest that in contrast to other high HIV-1 and KSHV endemic regions, KSHV infection in this population is independent of HIV both in adults and children. Other potential risk factors may be involved and need to be further investigated. This study has several limitations. We could not evaluate KSHV DNA in saliva or peripheral blood cells to determine viremia as these samples were not collected. Also, the cross-sectional design could not examine when primary infection occurred and investigate the risk factors that may be associated with KSHV incidence in early childhood.
In summary, we observed that KSHV infection was highly prevalent in early childhood in Xinjiang region in China and horizontal transmission appears to be the major route of transmission through sharing food with family members. Childhood infection appears to be the major contributing factor to the high KSHV prevalence in the population. A better understanding of the risk factors that are associated with KSHV transmission to children would contribute to the design and implementation of intervention strategies for prevention of KSHV infection.
Acknowledgments
We would like to thank all the study subjects for their participation in the study. We thank Dr Pankaj Kumar for his help with the KSHV neutralization assay. This project was supported by Public Health Service grants from National Cancer Institute (grant number CA-75903) and National Institute of General Medical Sciences (grant number P30 GM103509) at the National Institutes of Health and Fogarty International Training grant (grant number D43TW01492) to CW and National Science Foundation of China grant (grant number 81161120420) to YL.
Footnotes
This was presented in part at the International Congress on Oncogenic Herpesviruses and Associated Diseases, Philadelphia, Pennsylvania, August 1th to August 4th, 2012.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest
References
- 1.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Schalling M, Ekman M, Kaaya EE, et al. A role for a new herpes virus (KSHV) in different forms of Kaposi’s sarcoma. Nat Med. 1995;1:707–708. doi: 10.1038/nm0795-707. [DOI] [PubMed] [Google Scholar]
- 3.Blauvelt A, Sei S, Cook PM, et al. Human herpesvirus 8 infection occurs following adolescence in the United States. J Infect Dis. 1997;176:771–774. doi: 10.1086/517298. [DOI] [PubMed] [Google Scholar]
- 4.Gao MY, Wang S. Participatory communication and HIV/AIDS prevention in a Chinese marginalized (MSM) population. AIDS Care. 2007;19:799–810. doi: 10.1080/09540120601114832. [DOI] [PubMed] [Google Scholar]
- 5.Martro E, Bulterys M, Stewart JA, et al. Comparison of human herpesvirus 8 and Epstein-Barr virus seropositivity among children in areas endemic and non-endemic for Kaposi’s sarcoma. J Med Virol. 2004;72:126–131. doi: 10.1002/jmv.10548. [DOI] [PubMed] [Google Scholar]
- 6.Fujii T, Taguchi H, Katano H, et al. Seroprevalence of human herpesvirus 8 in human immunodeficiency virus 1-positive and human immunodeficiency virus 1-negative populations in Japan. J Med Virol. 1999;57:159–162. doi: 10.1002/(sici)1096-9071(199902)57:2<159::aid-jmv12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, He B, Zhang Z, et al. Human herpesvirus-8 in northwestern China: epidemiology and characterization among blood donors. Virol J. 2010;7:62. doi: 10.1186/1743-422X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, He N, Ding Y, et al. Prevalence of human herpesvirus 8 and hepatitis C virus in a rural community with a high risk for blood-borne infections in central China. Clin Microbiol Infect. 2011;17:395–401. doi: 10.1111/j.1469-0691.2010.03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mei Q, Ming ZW, Ping YX, et al. HHV-8 seroprevalence in blood donors and HIV-positive individuals in Shandong area, China. J Infect. 2007;55:89–90. doi: 10.1016/j.jinf.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 10.Fu B, Sun F, Li B, et al. Seroprevalence of Kaposi’s sarcoma-associated herpesvirus and risk factors in Xinjiang, China. J Med Virol. 2009;81:1422–1431. doi: 10.1002/jmv.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dilnur P, Katano H, Wang ZH, et al. Classic type of Kaposi’s sarcoma and human herpesvirus 8 infection in Xinjiang, China. Pathol Int. 2001;51:845–852. doi: 10.1046/j.1440-1827.2001.01293.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Liu J, Dilimulati, et al. Seroprevalence and risk factors of Kaposi’s sarcoma-associated herpesvirus infection among the general Uygur population from south and north region of Xinjiang, China. Virol J. 2011;8:539. doi: 10.1186/1743-422X-8-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Zhang C. Phylogeographic analyses reveal a crucial role of Xinjiang in HIV-1 CRF07_BC and HCV 3a transmissions in Asia. PLoS One. 2011;6:e23347. doi: 10.1371/journal.pone.0023347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Shan H, Trizzino J, et al. HIV incidence, retention rate, and baseline predictors of HIV incidence and retention in a prospective cohort study of injection drug users in Xinjiang, China. Int J Infect Dis. 2007;11:318–323. doi: 10.1016/j.ijid.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Engels EA, Atkinson JO, Graubard BI, et al. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007;196:199–207. doi: 10.1086/518791. [DOI] [PubMed] [Google Scholar]
- 16.Martin JN, Ganem DE, Osmond DH, et al. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 17.Melbye M, Cook PM, Hjalgrim H, et al. Risk factors for Kaposi’s-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543–548. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Cannon MJ, Dollard SC, Smith DK, et al. Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N Engl J Med. 2001;344:637–643. doi: 10.1056/NEJM200103013440904. [DOI] [PubMed] [Google Scholar]
- 19.Mantina H, Kankasa C, Klaskala W, et al. Vertical transmission of Kaposi’s sarcoma-associated herpesvirus. Int J Cancer. 2001;94:749–752. doi: 10.1002/ijc.1529. [DOI] [PubMed] [Google Scholar]
- 20.Brayfield BP, Kankasa C, West JT, et al. Distribution of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 in maternal saliva and breast milk in Zambia: implications for transmission. J Infect Dis. 2004;189:2260–2270. doi: 10.1086/421119. [DOI] [PubMed] [Google Scholar]
- 21.Minhas V, Brayfield BP, Crabtree KL, et al. Primary gamma-herpesviral infection in Zambian children. BMC Infect Dis. 2010;10:115. doi: 10.1186/1471-2334-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minhas V, Crosby LN, Crabtree KL, et al. Development of an immunofluorescence assay using recombinant proteins expressed in insect cells to screen and confirm presence of human herpesvirus 8-specific antibodies. Clin Vaccine Immunol. 2008;15:1259–1264. doi: 10.1128/CVI.00487-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimball LE, Casper C, Koelle DM, et al. Reduced levels of neutralizing antibodies to Kaposi sarcoma-associated herpesvirus in persons with a history of Kaposi sarcoma. J Infect Dis. 2004;189:2016–2022. doi: 10.1086/386371. [DOI] [PubMed] [Google Scholar]
- 24.He F, Wang X, He B, et al. Human herpesvirus 8: serovprevalence and correlates in tumor patients from Xinjiang, China. J Med Virol. 2007;79:161–166. doi: 10.1002/jmv.20730. [DOI] [PubMed] [Google Scholar]
- 25.Minhas V, Crabtree KL, Chao A, et al. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am J Epidemiol. 2008;168:311–320. doi: 10.1093/aje/kwn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida M, Torigoe S, Ikeue K, et al. Neutralizing antibody responses to human herpesviruses 6 and 7 do not cross-react with each other, and maternal neutralizing antibodies contribute to sequential infection with these viruses in childhood. Clin Diagn Lab Immunol. 2002;9:388–393. doi: 10.1128/CDLI.9.2.388-393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcelin AG, Gorin I, Morand P, et al. Quantification of Kaposi’s sarcoma-associated herpesvirus in blood, oral mucosa, and saliva in patients with Kaposi’s sarcoma. AIDS Res Hum Retroviruses. 2004;20:704–708. doi: 10.1089/0889222041524689. [DOI] [PubMed] [Google Scholar]
- 28.Mbulaiteye SM, Pfeiffer RM, Engels EA, et al. Detection of kaposi sarcoma-associated herpesvirus DNA in saliva and buffy-coat samples from children with sickle cell disease in Uganda. J Infect Dis. 2004;190:1382–1386. doi: 10.1086/424489. [DOI] [PubMed] [Google Scholar]
- 29.Taylor MM, Chohan B, Lavreys L, et al. Shedding of human herpesvirus 8 in oral and genital secretions from HIV-1-seropositive and -seronegative Kenyan women. J Infect Dis. 2004;190:484–488. doi: 10.1086/421466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–355. doi: 10.1097/QAI.0b013e31802f12ea. [DOI] [PubMed] [Google Scholar]


