Abstract
Background
The gene ANK3 is implicated in bipolar disorder and schizophrenia. The present study investigated the influence of this gene on cognitive performance and brain structure among individuals with first-episode psychosis (FEP). The brief illness duration of an FEP sample makes it well suited for studying the effects of genetic variation.
Methods
We genotyped 2 single nucleotide polymorphisms (SNPs; rs1938526 and rs10994336) in ANK3 in patients with FEP. Multivariate analysis of variance compared risk allele carriers and noncarriers on 6 domains of cognition consistent with MATRICS consensus. A subsample of 82 patients was assessed using magnetic resonance imaging. We compared brain structure between carriers and noncarriers using cortical thickness analysis and voxel-based morphometry on white matter.
Results
In the 173 patients with FEP included in our study, rs1938526 and rs10994336 were in very high linkage disequilibrium (d′ = 0.95), and analyses were therefore only carried out on the SNP (rs1938526) with the highest minor allele frequency (G). Allele G of rs1938526, was associated with lower cognitive performance across domains (F6,164 = 2.38, p = 0.030) and significantly lower scores on the domains of verbal memory (p = 0.015), working memory (p = 0.006) and attention (p = 0.019). The significant effects of this SNP on cognition were not maintained when controlling for IQ. Cortical thinning was observed in risk allele carriers at diverse sites across cortical lobes bilaterally at a threshold of p < 0.01, false discovery rate–corrected. Risk-allele carriers did not show any regions of reduced white matter volume.
Limitations
The sample size is modest given that a low-frequency variant was being examined.
Conclusion
The ANK3 risk allele rs1938526 appears to be associated with general cognitive impairment and widespread cortical thinning in patients with FEP.
Introduction
Evidence suggests that psychotic disorders are partly of genetic origin; however, the search for candidate genes has been challenging because these are complex disorders involving the contribution of multiple genes and environmental factors.1–3 Psychotic disorders are characterized by marked phenotypic heterogeneity; conversely, shared genetic risk factors have been observed across disorders. For instance, some of the same genetic variants have been identified as risk factors for both bipolar disorder (BD) and schizophrenia.4 Given this complexity, there has been a movement toward breaking down disorders into component endophenotypes that are more proximal to the biological etiology of the disorder and that are thought to be genetically less complex than the disorder as a whole.1–3 Consistent with the definition of endophenotypes, cognitive deficits, cortical thinning and white matter volume reduction have been associated with both schizophrenia and BD and have been found to be highly heritable.5–11 The presentation of similar endophenotypes in schizophrenia and BD suggests that they may share common pathogenic pathways. Therefore, researchers often use such measures rather than diagnosis when studying the effects of genetic variation.1–3
Numerous variants of the gene ANK3, encoding Ankyrin G, have been associated with BD in genome-wide association studies (GWAS)12,13 and a large genome-wide study combining schizophrenia and BD.14 The evidence for an association with schizophrenia is not as strong, as no genome-wide studies have found a significant effect. Three studies, however, have reported an association with schizophrenia.15–17 One of them was genome-wide and showed near-significant association after correction,15 and another one included a meta-analysis showing significant association of the single nucleotide polymorphisms (SNPs) rs10761482 and rs10994336 with schizophrenia.17 Furthermore, lower levels of ANK3 expression have been observed in multiple cortical areas in patients with schizophrenia.16,18 The risk-associated ANK3 SNPs are intronic, and rs1938526 and other SNPs have been associated with reduced gene expression.16,19
Several studies have examined the role of these genetic variants on neurocognition. A study on BD found significant association between rs10994336 and sustained attention, but no effect on general intelligence, memory or executive functioning.20 In healthy controls, 1 study did not report any effect of this SNP on measures of attention, working memory or executive functioning,21 whereas another study found an association between this SNP and set-shifting, risk-taking behaviour and decreased white matter integrity in the internal capsule.22 A further study found rs9804190 to be associated with poor working memory and executive functioning and higher prefrontal cortical activity during a working memory task.16 A familial mutation in ANK3 is associated with intellectual disability, and knockdown of this gene in drosophila is associated with memory deficits.23
Ankyrin G is found at axon initial segments (AIS) and nodes of Ranvier (NOR). It links voltage-gated sodium channels to the cytoskeleton and promotes their clustering in conjunction with cell adhesion molecules and oligodendrocytes.24 At the AIS, via neurofascin cell adhesion molecules, Ankyrin G promotes formation of GABAergic synapses.25 The reduced expression of ANK3 observed in patients with schizophrenia16,18 is consistent with a large body of research implicating γ-aminobutyric acid (GABA) synapses at the AIS in people with this condition.18,26 Through actions at the NOR, Ankyrin G facilitates the propagation of action potentials.27 Ankyrin G could thus influence the efficiency and synchrony of transmission of neuronal impulses and be necessary for long-range γ oscillations between cortical subfields,28 a process linked to sustained attention.29 In patients with schizophrenia, excessive cortical γ oscillations have been observed during working memory performance.30 A role of Ankyrin G at the NOR is also consistent with a large body of research pointing to deficits in white matter and oligodendrocytes in patients with schizophrenia and BD.31,32
The objective of the present study was to test the association of allelic variation in ANK3 with performance across different domains of cognition and with brain structure in white matter and cortical grey matter in a sample of patients shortly after the onset of a first episode of affective or nonaffective psychosis (FEP). We hypothesized that 2 ANK3 alleles previously associated with psychopathology, rs10994336 (T allele) and rs1938526 (G allele), would be associated with lower performance on measures of attention and working memory. We expected ANK3 risk alleles to be associated with cortical thinning and white matter volume reduction; however, these analyses are more exploratory given the lack of existing research. We used voxel-based morphometry (VBM) to measure white matter volume, but for grey matter we used cortical thickness, a more appropriate endophenotype than cortical grey matter volume, which is confounded by distinct genetic influences on the cortical area.5
Methods
Participants
Participants were treated at the Prevention and Early intervention Program for Psychoses (PEPP-Montréal), a specialized early-intervention service available to all patients with FEP in 1 sector of a Canadian urban centre. The details of the treatment model have been provided elsewhere.33 Admission criteria were age 14–30 years; symptoms consistent with DSM-IV affective (including BD and major depressive disorder with psychosis) or nonaffective psychotic disorder (including schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder and psychosis not otherwise specified); and no antipsychotic therapy for 1 or more months before admission. All patients who met criteria for treatment in the service were eligible to participate in the study. All evaluations reported in this study were approved by the ethics board of the Douglas Institute, and all participants provided written informed consent. All PEPP-Montréal patients are deemed competent and must also provide written informed consent to receive treatment at the research clinic.
Instruments and assessment
Cognitive measures
The cognitive test battery was administered by a trained professional when patients had reached a stable, but not necessarily asymptomatic, condition. The test battery was administered in either English or French according to the mother tongue of the participant; those whose mother tongue was neither English nor French completed the assessment in 1 of these 2 languages as well. The 6 domains of cognitive performance tested were derived from a standardized neuropsychological test battery, representing 6 of the 7 cognitive domains suggested by the National Institute of Mental Health Measurement and Treatment Research to Improve Cognition in Schizophrenia group.34,35 The 6 domains were verbal memory derived from the Logical Memory subtests of the Wechsler Memory Scale, 3rd Edition36 (WMS-III; immediate recall, delayed recall, recognition); visual memory derived from the Visual Reproduction subtests of the WMS-III (immediate recall, delayed recall, recognition); working memory from the Spatial Span subtests of the WMS-III and the Digit Span subtests of the Wechsler Adult Intelligence Scale (WAIS-III);37 speed of processing from the Digit Symbol subtest of the WAIS-III and the Trail Making Test A;38 reasoning/problem solving from the Block Design subtest of the WAIS-III and the Trail Making Test B;38 and attention from the D2 Test of Attention concentration performance score.39 We measured IQ using the WAIS-III. Standard equivalents (z scores) for all neuropsychological variables were obtained. For each participant, we z-transformed the raw score from each subtest of a given domain using normative data from a group of 34 healthy controls. The z scores were then averaged to obtain a mean z score of performance in each domain. The z score for the reasoning and problem solving domain was transformed to achieve normal distribution by log-transforming all individual task scores for patients and controls and recalculating z scores.
Clinical and demographic measures
The Structured Clinical Interview for DSM-IV (SCID)40 was administered at entry to the program and 1 year later to determine primary diagnoses and lifetime substance-use diagnoses (abuse or dependence, excluding nicotine). We retained the most recent available SCID diagnosis, and diagnoses were confirmed at a consensus meeting attended by a senior research psychiatrist (A.M. or R.J.).
Genetics
We extracted DNA from blood or saliva samples collected from each participant. Genotyping was done at the McGill University and Génome Québec Innovation Centre using Sequenom iPlex Gold Technology.41 The genotyping success rate was 98.3%. We calculated linkage disequilibrium using Haploview 4.0.
Image acquisition
Magnetic resonance imaging (MRI) was carried out at the Montreal Neurological Institute (MNI) on a 1.5 T Siemens Sonata whole body MRI system. Structural T1 volumes were acquired for each participant using a 3-dimensional (3D) gradient echo pulse sequence with sagittal volume excitation (repetition time 22 ms, echo time 9.2 ms, flip angle 30°, 180 contiguous sagittal 1 mm slices). The rectangular field of view for the images was 256 × 204 mm. For analysis of VBM, T1 MRI scans were subsequently combined into 3D volume (NIfTI file format) using SPM8 (Wellcome Institute).
Statistical analysis
Cognition
We investigated the effect of allelic variation in the ANK3 SNP rs1938526 using a dominant model separating the risk allele carriers (GG+ AG) and noncarriers (AA). We used the χ2 statistic and t tests to compare the 2 genotype groups with regard to clinical and demographic characteristics. We then performed multivariate analysis of covariance (MANCOVA) with sex as a covariate given the significant association between female sex and presence of the risk allele. Statistical tests were 2-tailed and performed on SPSS version 15.0 for Windows.
Cortical thickness
We submitted MRI scans to the CIVET processing pipeline version 1.1.9 (http://wiki.bic.mni.mcgill.ca/index.php/CIVET).42,43 Native T1-weighted images were first registered to the ICBM152 template using linear transformation44,45 and simultaneously corrected for nonuniformity artifacts using N3.46 We then segmented the transformed images into grey matter, white matter, cerebrospinal fluid (CSF) and background using a neural net classifier (INSECT).42 Grey and white matter surfaces were extracted using CLASP algorithm.47–49 We used a spherical-mesh deformation algorithm to produce a surface mesh of 81 920 polygons (40 962 nodes or vertices) for each hemisphere. Nonlinear registration of both cortical surfaces to a high-resolution average surface template generated from the ICBM152 data set was performed to establish interparticipant correspondence of vertices.50,51 Reverse linear transformation of volumes was performed to allow vertex-based corticometric measurements in native space for each participant’s MRI scan.52 The deformation algorithm first fits the white matter surface and then expands to the outer grey matter and CSF intersection. From these surfaces, we computed cortical thickness in native space using the t-link method,53 which determines the linked distance between the inner and outer cortical surfaces at each of 40 962 vertices. Each participant’s cortical thickness map was subsequently blurred using a 20 mm full-width at half-maximum (FWHM) surface-based diffusion smoothing kernel.54
Cortical thickness maps of t statistics for effect of group (carrier v. noncarrier) at 40 962 surface points per hemisphere were projected onto an average brain template, revealing vertices that differed significantly between groups. We did not include total intracranial volume as a covariate, because cortical thickness and brain volume are poorly correlated;52,55 however, age, sex and handedness were included as covariates in the analysis. Statistical maps were thresholded and multiple comparisons were taken into account using the false discovery rate (FDR) procedure, with q = 0.01.56 We considered results to be significant at t = 2.65 (p < 0.01, FDR-corrected).
Voxel-based morphometry
We analyzed structural T1 images using VBM57,58 with VBM8 software version 414 (http://dbm.neuro.uni-jena.de/vbm/). First, the T1 images were normalized to a template space using high-dimensional (DARTEL) spatial normalization and then segmented into grey matter, white matter and CSF. After preprocessing, the resulting modulated images were smoothed with a 8 mm FWHM Gaussian kernel. We explored white matter differences between carriers and noncarriers using a t test with total intracranial volume (estimated during preprocessing), age at the time of scanning, sex and handedness as covariates.
Contrasts were explored using a statistical threshold of p < 0.05, family-wise error–corrected for multiple comparisons. Using the MNI coordinates of the peak voxel(s) for significant clusters, we identified white matter tract(s) based on structures identified using the automated anatomic labelling toolbox.59 Finally, we estimated whole brain grey matter, white matter and CSF volumes during preprocessing for each participant, which we summed for an estimation of total intracranial volume (TIV). We then compared the 4 volumes between the 2 groups using a 2-way analysis of variance (ANOVA) with genotype and sex as between-subjects factors.
Results
Of the 380 patients accepted in the PEPP-Montréal clinic during our study period, 173 (26 risk allele carriers and 147 non-carriers) agreed to participate in both cognitive and genetic testing. As shown in Table 1, the genotype distribution of the 2 ANK3 SNPs did not depart from Hardy–Weinberg equilibrium. Since the 2 SNPs are in strong linkage disequilibrium (d′ = 0.95), results are only presented for rs1938526 given evidence of an effect of this SNP on gene expression.19 Analyses on the effects of rs10994336 on neurocognition (not shown) yielded similar but slightly less significant results.
Table 1.
Genotype distribution of ANK3 single nucleotide polymorphisms
| Marker | Genotype | MAF | p value* | d′ | ||
|---|---|---|---|---|---|---|
| rs1938526 | AA, n = 147 | AG, n = 23 | GG, n = 3 | 0.086 | 0.20 | 0.952 |
| rs10994336 | CC, n = 153 | CT, n = 17 | TT, n = 3 | 0.066 | 0.06 |
MAF = minor allele frequency.
Hardy–Weinberg equilibrium.
Table 2 and Table S1 in the Appendix, available at cma.ca/jpn, indicate the clinical and demographic characteristics of participants included in both the cognitive and imaging portions of the study. Carriers of the G allele of rs1938526 did not differ from noncarriers on any of these measures except that there was a higher proportion of female participants and a lower proportion of participants who were tested in their mother tongue among the carriers than the noncarriers (all p < 0.05; Table 2). Regarding lifetime substance dependence, cannabis use was most common, with 91% of participants who had a substance-use diagnosis using cannabis. Regarding DNA extraction, blood samples were available from most participants (n = 125 samples, 72%), and saliva samples were available for the remaining participants.
Table 2.
Clinical and demographic characteristics of study samples
| Group; no. (%)* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Study sample, cognitive data n = 173 | Study sample, imaging data n = 82 | rs1938526 AA n = 147 | rs1938526 G carrier n = 26 |
| Male sex | 132 (76) | 61 (74) | 119 (80) | 14 (54)† |
| Age at cognitive testing, mean (range) yr | 22.8 (16–31) | 23.7 (18–31) | 22. (16–31) | 23. (18–28) |
| Education, mean (SD) yr | 11.3 (2.4) | 11.9 (2.4) | 11.3 (2.3) | 10.8 (2.7) |
| Diagnosis | ||||
| Nonaffective psychosis | 138 (80) | 69 (84) | 118 (80) | 20 (77) |
| Schizophrenia | 102 (59) | 50 (61) | 89 (61) | 13 (50) |
| Bipolar disorder | 24 (14) | 10 (12) | 22 (15) | 2 (8) |
| Age at onset of psychosis, median (range) yr | 21.2 (13–31) | 22.1 (15–31) | 21.1 (13–31) | 21.3 (14–27) |
| White race | 102 (59) | 55 (68) | 90 (61) | 13 (50) |
| Cognition tested in mother tongue | 136 (79) | 67 (83) | 120 (82) | 16 (62)† |
| Lifetime substance-use disorder diagnosis | 116 (67) | 53 (65) | 101 (69) | 15 (60) |
| Taking antipsychotics at cognitive testing | 139 (95) | 69 (97) | 114 (94) | 24 (92) |
| Chlorpromazine equivalents at cognitive testing, mean (SD) | 237 (241) | 210 (185) | 235 (252) | 246 (186) |
| Full scale IQ, mean (SD) | 92.6 (15.7) | 95.3 (14.5) | 94.1 (15.8) | 84.5 (13.2)‡ |
SD = standard deviation.
Unless otherwise indicated.
p < 0.05.
p < 0.01.
Eighty percent of patients (n = 139) were tested within 4 months of program entry. The genotype groups did not differ on time of testing after treatment entry (mean 78 d in G allele carriers v. 93 d in noncarriers, t = 0.67, p = 0.46). All scans were carried out within the first 16 months following treatment entry; most occurred within the first 6 months (n = 66, 82%). The genotype groups did not differ on time of scanning after treatment entry (mean 5.6 mo for G allele carriers v. 4.9 mo for noncarriers, t = 0.85, p = 0.41).
Cognitive performance
The MANCOVA including sex as a covariate revealed a main effect of the rs1938526 SNP on cognitive performance across all domains. The overall performance of G allele carriers was weaker than that of noncarriers (F6,164 = 2.4, partial η2 = 0.080, p = 0.030). There were significant differences between the genotype groups on working memory (F1,171 = 7.6, partial η2 = 0.043, p = 0.006), verbal memory (F1,171 = 6.0, partial η2 = 0.034, p = 0.015), and attention (F1,171 = 5.5, partial η2 = 0.032, p = 0.019) and a borderline effect on reasoning and problem solving (F1,171 = 2.8, partial η2 = 0.016, p = 0.09). There was no significant effect of genotype on visual memory (partial η2 = 0.011, p = 0.17) or speed of processing (partial η2 = 0.002, p = 0.58). In addition, the risk allele carriers had lower full-scale IQ scores than noncarriers (ANOVA controlling for sex: F1,172 = 8.9, p = 0.003). Adding diagnosis (affective, nonaffective) and mother tongue (English, French, other) as covariates in the MANCOVA yielded an identical pattern of significant results with minimal reduction in effect size (partial η2 for overall cognition, working memory, verbal memory and attention were 0.077, 0.036, 0.032 and 0.030, respectively). Adding full-scale IQ as a covariate eliminated all significant effects of ANK3 on cognition.
Cortical thickness
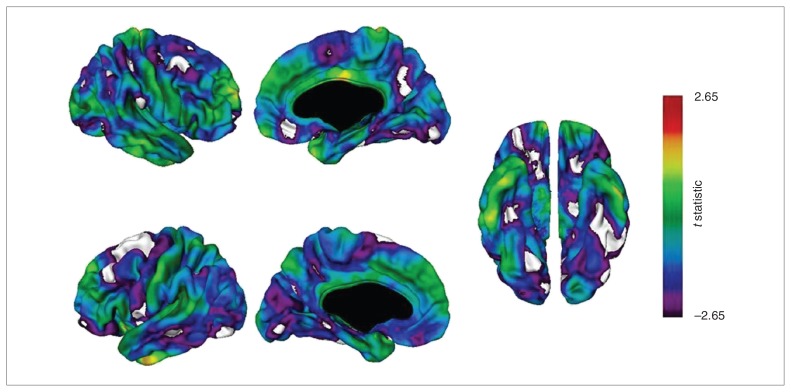
Carriers of the ANK3 rs1938526 risk allele (n = 14) had a significantly thinner cortex at numerous regions than noncarriers (n = 68), as shown in Table 3 and Figure 1. At p < 0.01, FDR-corrected, carriers had a thinner cortex in the right hemisphere at regions with maxima in the medial frontal gyrus, lingual gyrus, parahippocampal gyrus, inferior parietal lobule, superior frontal gyrus, cingulate gyrus, inferior frontal gyrus, supramarginal gyrus, precuneus, middle frontal gyrus, middle occipital gyrus, superior frontal gyrus and middle temporal gyrus (descending order of significance) and in the left hemisphere at regions with maxima in the superior frontal gyrus, inferior parietal lobule, precuneus, fusiform gyrus, inferior frontal gyrus, middle temporal gyrus, precentral gyrus, middle occipital gyrus, cingulate gyrus, middle frontal gyrus and lingual gyrus. Carriers had no regions of cortical thickening.
Table 3.
Regions of cortical thinning in rs1938526 risk allele carriers at p < 0.01, false discovery rate–corrected
| Hemisphere; region | BA | Stereotaxic coordinates (MNI space)* | t value at peak vertex | ||
|---|---|---|---|---|---|
|
| |||||
| x | y | z | |||
| Right hemisphere | |||||
| Medial frontal gyrus/rectal gyrus | 11/10/25/32 | 4 | 34 | −16 | −3.8 |
| Lingual gyrus/fusiform gyrus/culmen | 19/18 | 25 | −67 | −5 | −3.63 |
| Parahippocampal gyrus/fusiform gyrus | 36/20 | 39 | −29 | −26 | −3.49 |
| Inferior parietal lobule/postcentral gyrus | 40/3/5 | 35 | −39 | 54 | −3.48 |
| Inferior parietal lobule/postcentral gyrus | 40/2 | 65 | −25 | 28 | −3.3 |
| Lingual gyrus/inferior occipital gyrus | 18/17 | 3 | −92 | −7 | −3.28 |
| Superior and middle frontal gyri | 11 | 34 | 52 | −13 | −3.25 |
| Cingulate gyrus/posterior cingulate/precuneus | 31/7 | 3 | −64 | 27 | −3.25 |
| Inferior and medial frontal gyri/rectal gyrus/orbital gyrus | 11/47/25 | 20 | 27 | −23 | −3.21 |
| Supramarginal gyrus | 40 | 58 | −54 | 33 | −3.18 |
| Medial frontal gyrus/precentral gyrus | 6 | 38 | −6 | 58 | −3.13 |
| Precuneus | 7/31 | 5 | −58 | 44 | −3.08 |
| Middle frontal gyrus | 6 | 35 | 0 | 59 | −3.02 |
| Inferior parietal lobule | 40 | 45 | −52 | 49 | −2.97 |
| Precuneus/superior occipital gyrus | 19 | 36 | −81 | 38 | −2.94 |
| Middle and superior frontal gyri | 10/11 | 22 | 58 | −6 | −2.93 |
| Precuneus | 19/7 | 27 | −74 | 29 | −2.93 |
| Medial frontal gyrus | 32 | 7 | 8 | 51 | −2.78 |
| Middle and inferior occipital gyri | 18 | 34 | −88 | −7 | −2.78 |
| Superior frontal gyrus | 6 | 21 | 17 | 65 | −2.76 |
| Middle temporal gyrus | 37 | 53 | −65 | 0 | −2.76 |
| Left hemisphere | |||||
| Superior and middle frontal gyri/precentral gyrus | 6/8/9 | −21 | 8 | 54 | −4.09 |
| Inferior parietal lobule | 40 | −46 | −42 | 46 | −4.07 |
| Precuneus | 31/7 | −4 | −68 | 28 | −4.07 |
| Fusiform gyrus/parahippocampal gyrus | 36/37/20 | −41 | −37 | −23 | −3.92 |
| Inferior and medial frontal gyri/orbital gyrus | 47/11/25 | −20 | 21 | −21 | −3.69 |
| Middle and inferior temporal gyri | 20/21/37 | −59 | −41 | −17 | −3.21 |
| Precentral gyrus | 6/43/4/44 | −58 | −1 | 11 | −3.20 |
| Middle and inferior occipital gyri/fusiform gyrus | 18/19 | −45 | −79 | −14 | −3.16 |
| Cingulate gyrus/precuneus | 31/7 | −8 | −47 | 42 | −2.97 |
| Middle temporal gyrus | 37 | −55 | −64 | −3 | −2.74 |
| Middle frontal gyrus | 10 | −42 | 55 | −7 | −2.68 |
| Middle temporal gyrus | 21 | −61 | −30 | −6 | −3.26 |
| Lingual gyrus/parahippocampal gyrus/culmen | 30/19 | −17 | −43 | −5 | −2.85 |
| Lingual gyrus | 18 | −2 | −81 | −4 | −2.89 |
BA = Brodmann area; MNI = Montreal Neurological Institute.
MNI coordinates refer to the maxima.
Fig. 1.
t Statistical maps of cortical thickness comparing carriers and noncarriers of the G allele at rs1938526. Negative values of the t statistic correspond to thinner cortex in rs1938526 risk (G) allele carriers. Carriers (n = 14) had a significantly thinner cortex than noncarriers (n = 68) at vertices shown in white at p < 0.01, false discovery rate (FDR)–corrected, and at vertices shown in violet at p < 0.05, FDR-corrected.
Voxel-based morphometry: differences in white matter and whole brain volumes
The “carrier > noncarrier” and the “noncarrier > carrier” contrasts revealed no significant white matter differences. There was less whole brain grey matter in carriers than noncarriers (p = 0.06); groups did not significantly differ on whole brain white matter, TIV, or CSF volumes (Table 4).
Table 4.
Whole brain tissue volumes estimated during voxel-based morphometry preprocessing
| Group, volume, mL; mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Brain area | rs1938526 AA n = 70 |
rs1938526 G carrier n = 14 |
Statistic | p value |
| Grey matter | 683 ± 59 | 633 ± 66 | F1,83 = 3.6 | 0.06 |
| White matter | 569 ± 66 | 555 ± 65 | F1,83 = 0.06 | 0.80 |
| Cerebrospinal fluid | 215 ± 34 | 202 ± 33 | F1,83 = 0.34 | 0.56 |
| Total intracranial | 1467 ± 127 | 1390 ± 153 | F1,83 = 0.84 | 0.36 |
SD = standard deviation.
Discussion
The present investigation found the G allele of rs1938526 to be associated with poor performance across 6 domains of cognition and with widespread cortical thinning and grey matter volume reduction in patients with FEP, but this variant had no significant effect on white matter volume.
To our knowledge, this is the first study of the effects of the rs1938526 SNP on neurocognition. This SNP was associated with lower global cognitive performance and there were no significant effects of the SNP on any particular cognitive domain when controlling for IQ. Thus we conclude that it may be promoting a general intellectual impairment; however, the effect of genotype was stronger for certain domains, particularly working memory, which is consistent with a role of ANK3 in this domain.16 Although there has been a great deal of interest in deficits in specific domains of cognition in psychotic illness, it is also well established that a general intellectual deficit is often comorbid with psychotic disorders and is a risk factor for such disorders.60–62
Our results suggest that the rs1938526 risk variant of ANK3 is associated with significant and widespread reduction in cortical thickness and grey matter volume across diverse regions of the cortex. The thinning was so extensive that we increased our FDR-corrected threshold from p < 0.05 to p < 0.01 to highlight the regions most affected. Some regions of thinning, such as the orbitofrontal cortex, parahippocampal gyrus, dorsolateral prefrontal cortex and cingulate gyrus, are highly implicated in psychotic disorders.8,10,11,31,63,64 Previous studies looking specifically at cortical thinning in patients with schizophrenia have reported widespread thinning across diverse cortical areas even at the time of a first episode.8–10 Cortical thinning in patients with BD may be more restricted, with robust findings in prefrontal areas7,8,64 and in the left ACC.7,64 A recent twin study merging schizophrenia and BD found genetic liability for these disorders to be associated with thinning of the right parahippocampal gyrus and right orbitofrontal cortex.11
It is not surprising that carriers of the risk allele had both poor cognition and cortical thinning, since these characteristics have shown strong association.65 The mechanism through which ANK3 could impact cortical thickness is not fully clear. ANK3 promotes the propagation of action potentials,27 and some researchers have posited that decreased ANK3 expression in patients with schizophrenia could reduce pyramidal cell activation in the cortex.26 Reduced cognitive activation has been associated with cortical thinning in individuals with schizophrenia.66 Furthermore, it has been shown that cortical thinning in the frontal lobes of these patients is due to thinning of pyramidal cell layers.67 Testing prospectively at multiple time points could verify if the risk allele is associated with progressive cortical thinning over time. However, Ankyrin G plays an important role in early development,26 which could implicate developmental rather than progressive effects on cortical thickness.
Since schizophrenia and BD have been associated with decreased white matter31,32 and ANK3 is highly expressed in this tissue, we wondered whether the rs1938526 risk variant could decrease white matter volume. According to these data, white matter volume does not seem a likely mechanism through which rs1938526 affects cognition or risk for psychotic illness. Future studies could verify if variation in ANK3 affects white matter integrity using diffusion tensor imaging.
Limitations
One important limitation of this study was the lack of genetic information on a control sample. It would have been informative to test whether variation in ANK3 would be associated with cognitive impairment and cortical thinning in controls in a similar manner as we observed in patients. Another limitation was the sample size. Carriers of the risk allele of rs1938526 made up only 15% of our sample, which is relatively small, especially for the brain structural analyses. Also significantly fewer ANK3 carriers had their cognition tested in their mother tongue; however, controlling for this factor had little impact on the effect of genotype.
These limitations are mitigated by several factors. First, ANK3 is a strong candidate gene implicated in BD and schizophrenia. Second, the role of this gene in axon physiology provides a plausible mechanism through which it could affect neurocognition and brain structure. Third, it was encouraging that our findings of reduced grey matter in ANK3 carriers were confirmed using 2 very different methods of analysis (cortical thickness and whole brain grey matter). Finally, although many studies examining the genetics of endophenotypes in psychiatric illness look only at healthy controls, there is a need to complement this literature with more studies examining these associations in clinical populations to ensure that variants are behaving in the same fashion in people who can be assumed to have a high degree of other risk factors and who actually have the disorder of interest, thus exposing the interplay between the effects of the SNP and the neural pathology of the disorder. It is possible that a variant associated with worse cognition in controls can be associated with better cognition in patients with schizophrenia.68 In populations with chronic illness, studying the genetics of endophenotypes may have limited usefulness owing to numerous confounds, such as long-term exposure to the effects of illness and its associated morbidity (long-term use of psychotropic medication, social deprivation, metabolic disorders and prolonged substance use), which introduce heterogeneity and reduce the power of genetic association studies. Although it is more challenging to study patients within the restricted timeframe of an FEP, taking the trouble to do so may be necessary to minimize such confounds.
Conclusion
The G allele of rs1938526 located in ANK3 was associated with widespread cortical thinning and reduced cognitive performance across domains, but not with decreased white matter in patients with FEP. These findings suggest that cognitive deficits and cortical thinning could be mechanisms through which ANK3 confers risk for psychotic disorders, as observed in GWAS.
Acknowledgements
We acknowledge the contributions of Dr. Mallar Chakravarty, Dr. Norbert Schmitz and Dr. Aurelie Labbé for statistical advice and Aldanie Rho, Anastasia Lezos and Connie Lee for assistance with sample collection. This work was supported by grants from the Canadian Institutes of Health Research to A. Malla, M. Lepage and R. Joober. R. Joober has salary support from Fonds de la recherche en santé du Québec. The funding sources had no role in the design or conduct of the study, nor in the collection, interpretation of data, nor in the preparation, review, or approval of the manuscript.
Footnotes
Competing interests: None declared.
Contributors: A. Malla, M. Lepage and R. Joober designed the study. C. Cassidy, M. Bodnar, J. Dell’Elce, F. Ferid, R. Fox, S. Iyer and R. Joober acquired the data, which C. Cassidy, L. Buchy, M. Bodnar, J. Dell’Elce, Z. Choudhry, S. Sengupta, R. Fox, A. Malla, M. Lepage and R. Joober analyzed. C. Cassidy, M. Bodnar, J. Dell’Elce, R. Fox, S. Iyer and R. Joober wrote the article, which all authors reviewed and approved for publication.
References
- 1.Snitz BE, Macdonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–94. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savitz JB, Solms M, Ramesar RS. Neurocognitive function as an endophenotype for genetic studies of bipolar affective disorder. Neuromolecular Med. 2005;7:275–86. doi: 10.1385/NMM:7:4:275. [DOI] [PubMed] [Google Scholar]
- 3.Kaymaz N, van Os J. Heritability of structural brain traits an endophenotype approach to deconstruct schizophrenia. Int Rev Neurobiol. 2009;89:85–130. doi: 10.1016/S0074-7742(09)89005-3. [DOI] [PubMed] [Google Scholar]
- 4.Williams HJ, Craddock N, Russo G, et al. Most genome-wide significant susceptibility loci for schizophrenia and bipolar disorder reported to date cross-traditional diagnostic boundaries. Hum Mol Genet. 2011;20:387–91. doi: 10.1093/hmg/ddq471. [DOI] [PubMed] [Google Scholar]
- 5.Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Haren NE, Rijsdijk F, Schnack HG, et al. The genetic and environmental determinants of the association between brain abnormalities and schizophrenia: the schizophrenia twins and relatives consortium. Biol Psychiatry. 2012;71:915–21. doi: 10.1016/j.biopsych.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 8.Rimol LM, Hartberg CB, Nesvag R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Schultz CC, Koch K, Wagner G, et al. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010;116:204–9. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 10.van Haren NE, Schnack HG, Cahn W, et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–80. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- 11.Hulshoff Pol HE, van Baal GC, Schnack HG, et al. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry. 2012;69:349–59. doi: 10.1001/archgenpsychiatry.2011.1615. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira MA, O’Donovan MC, Meng YA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–8. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith EN, Bloss CS, Badner JA, et al. Genome-wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755–63. doi: 10.1038/mp.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ripke S, Sanders AR, Kendler KS, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–76. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athanasiu L, Mattingsdal M, Kahler AK, et al. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res. 2010;44:748–53. doi: 10.1016/j.jpsychires.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roussos P, Katsel P, Davis KL, et al. Molecular and genetic evidence for abnormalities in the nodes of ranvier in schizophrenia. Arch Gen Psychiatry. 2012;69:7–15. doi: 10.1001/archgenpsychiatry.2011.110. [DOI] [PubMed] [Google Scholar]
- 17.Yuan A, Yi Z, Wang Q, et al. ANK3 as a risk gene for schizophrenia: new data in han Chinese and meta analysis. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:997–1005. doi: 10.1002/ajmg.b.32112. [DOI] [PubMed] [Google Scholar]
- 18.Cruz DA, Weaver CL, Lovallo EM, et al. Selective alterations in postsynaptic markers of chandelier cell inputs to cortical pyramidal neurons in participants with schizophrenia. Neuropsychopharmacology. 2009;34:2112–24. doi: 10.1038/npp.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rueckert EH, Barker D, Ruderfer D, et al. Cis-acting regulation of brain-specific ANK3 gene expression by a genetic variant associated with bipolar disorder. Mol Psychiatry. 2012 Jul 31; doi: 10.1038/mp.2012.104. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruberto G, Vassos E, Lewis CM, et al. The cognitive impact of the ANK3 risk variant for bipolar disorder: initial evidence of selectivity to signal detection during sustained attention. PLoS ONE. 2011;6:e16671. doi: 10.1371/journal.pone.0016671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roussos P, Giakoumaki SG, Georgakopoulos A, et al. The CACNA1C and ANK3 risk alleles impact on affective personality traits and startle reactivity but not on cognition or gating in healthy males. Bipolar Disord. 2011;13:250–9. doi: 10.1111/j.1399-5618.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- 22.Linke J, Witt SH, King AV, et al. Genome-wide supported risk variant for bipolar disorder alters anatomical connectivity in the human brain. Neuroimage. 2012;59:3288–96. doi: 10.1016/j.neuroimage.2011.10.083. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal Z, Vandeweyer G, van der Voet M, et al. Homozygous and heterozygous disruptions of ANK3: at the crossroads of neurodevelopmental and psychiatric disorders. Hum Mol Genet. 2013;22:1960–70. doi: 10.1093/hmg/ddt043. [DOI] [PubMed] [Google Scholar]
- 24.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–80. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 25.Ango F, di Cristo G, Higashiyama H, et al. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–72. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118–27. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou D, Lambert S, Malen PL, et al. Ankyrin G is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nave KA. Myelination and support of axonal integrity by glia. Nature. 2010;468:244–52. doi: 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- 29.Gregoriou GG, Gotts SJ, Zhou H, et al. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–10. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barr MS, Farzan F, Tran LC, et al. Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr Res. 2010;121:146–52. doi: 10.1016/j.schres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd AM, Laurens KR, Matheson SL, et al. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–56. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Vita A, De Peri L, Sacchetti E. Gray matter, white matter, brain, and intracranial volumes in first-episode bipolar disorder: a meta-analysis of magnetic resonance imaging studies. Bipolar Disord. 2009;11:807–14. doi: 10.1111/j.1399-5618.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 33.Malla A, Norman R, McLean T, et al. A Canadian programme for early intervention in non-affective psychotic disorders. Aust N Z J Psychiatry. 2003;37:407–13. doi: 10.1046/j.1440-1614.2003.01194.x. [DOI] [PubMed] [Google Scholar]
- 34.Green MF, Nuechterlein KH, Gold JM, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–7. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Nuechterlein KH, Barch DM, Gold JM, et al. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Weschler Memory Scale. 3rd ed. Toronto (ON): The Psychological Corporation; 1997. [Google Scholar]
- 37.Wechsler D. Weschler Adult Intelligence Scale. 3rd ed. Toronto (ON): The Psychological Corporation; 1997. [Google Scholar]
- 38.Reitan R. Trail Making Test: Manual for Administration and Scoring. Tuscon (AZ): Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 39.Brickencamp RZE. The D2 test of attention. Seattle (WA): Hogrefe & Huber; 1998. [Google Scholar]
- 40.First M, Spitzer R, Miriam G, et al. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition (SCID-I/P) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 41.Ehrich M, Bocker S, van den Boom D. Multiplexed discovery of sequence polymorphisms using base-specific cleavage and MALDI-TOF MS. Nucleic Acids Res. 2005;33:e38. doi: 10.1093/nar/gni038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 43.Ad-Dab’bagh YED, Lyttelton O, Muehlboeck JS, et al. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research [presentation]. 12th annual meeting of the Organization for Human Brain Mapping (OHBM); 2006; Florence, Italy. [Google Scholar]
- 44.Collins DP, Peters MT, Evans TA. An automated 3D non-linear deformation procedure for determination of gross morphometric variability in the human brain. In: Robb RA, editor. Visualization in Biomedical Computing. Rochester (MN): SPIE; 1994. Oct 4, 1994. pp. 180–94. [Google Scholar]
- 45.Grabner G, Janke AL, Budge MM, et al. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Assist Interv. 2006;9:58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- 46.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 47.Kabani N, Le Goualher G, MacDonald D, et al. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13:375–80. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- 48.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–21. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 49.MacDonald D, Kabani N, Avis D, et al. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–56. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 50.Lyttelton O, Boucher M, Robbins S, et al. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–44. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 51.Robbins SM. Anatomical standardization of the human brain in euclidean 3-space and on the cortical 2-manifold. Montréal (QC): McGill University; 2004. [Google Scholar]
- 52.Ad-Dab’bagh YSV, Robbins S, Lerch J, et al. Native space cortical thickness measurement and the absence of correlation to cerebral volume [presentation]. 11th annual meeting of the Organization for Human Brain Mapping (OHBM); 2005; Toronto (ON). [Google Scholar]
- 53.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–73. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 54.Chung MK, Worsley KJ, Robbins S, et al. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003;18:198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- 55.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–60. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 57.Ashburner J, Friston KJ. Voxel-based morphometry — the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 58.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 59.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-participant brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 60.Owen MJ. Intellectual disability and major psychiatric disorders: a continuum of neurodevelopmental causality. Br J Psychiatry. 2012;200:268–9. doi: 10.1192/bjp.bp.111.105551. [DOI] [PubMed] [Google Scholar]
- 61.Morgan VA, Croft ML, Valuri GM, et al. Intellectual disability and other neuropsychiatric outcomes in high-risk children of mothers with schizophrenia, bipolar disorder and unipolar major depression. Br J Psychiatry. 2012;200:282–9. doi: 10.1192/bjp.bp.111.093070. [DOI] [PubMed] [Google Scholar]
- 62.Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–71. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 63.Bodnar M, Malla AK, Joober R, et al. Neural markers of early remission in first-episode schizophrenia: a volumetric neuroimaging study of the parahippocampus. Psychiatry Res. 2012;201:40–7. doi: 10.1016/j.pscychresns.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Foland-Ross LC, Thompson PM, Sugar CA, et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am J Psychiatry. 2011;168:530–9. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hartberg CB, Lawyer G, Nyman H, et al. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 2010;182:123–33. doi: 10.1016/j.pscychresns.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Schultz CC, Koch K, Wagner G, et al. Reduced anterior cingulate cognitive activation is associated with prefrontal-temporal cortical thinning in schizophrenia. Biol Psychiatry. 2012;71:146–53. doi: 10.1016/j.biopsych.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Williams MR, Chaudhry R, Perera S, et al. Changes in cortical thickness in the frontal lobes in schizophrenia are a result of thinning of pyramidal cell layers. Eur Arch Psychiatry Clin Neurosci. 2013;263:25–39. doi: 10.1007/s00406-012-0325-8. [DOI] [PubMed] [Google Scholar]
- 68.Zhu X, Gu H, Liu Z, et al. Associations between TCF4 gene polymorphism and cognitive functions in schizophrenia patients and healthy controls. Neuropsychopharmacology. 2013;38:683–9. doi: 10.1038/npp.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]