Abstract
Background
Melatonin (MLT) is a pleiotropic neurohormone controlling many physiological processes and whose dysfunction may contribute to several different diseases, such as neurodegenerative diseases, circadian and mood disorders, insomnia, type 2 diabetes and pain. Melatonin is synthesized by the pineal gland during the night and acts through 2 G-protein coupled receptors (GPCRs), MT1 (MEL1a) and MT2 (MEL1b). Although a bulk of research has examined the physiopathological effects of MLT, few studies have investigated the selective role played by MT1 and MT2 receptors. Here we have reviewed current knowledge about the implications of MT2 receptors in brain functions.
Methods
We searched PubMed, Web of Science, Scopus, Google Scholar and articles reference lists for studies on MT2 receptor ligands in sleep, anxiety, neuropsychiatric diseases and psychopharmacology, including genetic studies on the MTNR1B gene, which encodes the melatonin MT2 receptor.
Results
These studies demonstrate that MT2 receptors are involved in the pathophysiology and pharmacology of sleep disorders, anxiety, depression, Alzheimer disease and pain and that selective MT2 receptor agonists show hypnotic and anxiolytic properties.
Limitations
Studies examining the role of MT2 receptors in psychopharmacology are still limited.
Conclusion
The development of novel selective MT2 receptor ligands, together with further preclinical in vivo studies, may clarify the role of this receptor in brain function and psychopharmacology. The superfamily of GPCRs has proven to be among the most successful drug targets and, consequently, MT2 receptors have great potential for pioneer drug discovery in the treatment of mental diseases for which limited therapeutic targets are currently available.
Introduction
Melatonin (MLT) is a neurohormone synthesized from serotonin (5-HT) and secreted foremost by the mammalian pineal gland following a distinct circadian rhythm with the acrophase during the dark phase and the nadir during the light phase in both diurnal and nocturnal species.1 A typical human adult’s average daytime and nighttime levels of blood MLT are approximately 10 pg/mL and 60 pg/mL, respectively.2 Its production is controlled through the suprachiasmatic nucleus (SCN) by the photoperiod, peaking at night and dropping during the day. Melatonin is involved in numerous physiologic processes, including circadian rhythms, mood regulation, anxiety, sleep, appetite, immune responses and cardiac functions.3,4 Most of the effects of MLT in the brain result from the activation of 2 high-affinity G-protein coupled receptors (GPCRs), MT1 and MT2.5 Of note, GPCRs have a proven history of being excellent therapeutic targets. Recent market analyses indicate that 40%–50% of modern drugs and almost 25% of the top 200 best-selling drugs target GPCRs.6
In addition to these high-affinity MLT receptors, another low-affinity MLT binding site, MT3, has recently been characterized as an MLT-sensitive form of the human enzyme quinone reductase 2.7 The functional characterization of MT1 and MT2 receptors in target tissues is hampered by the paucity of selective MT1 and MT2 receptor ligands. Structural determinants that confer MT1 or MT2 binding selectivity have only recently been elucidated.8,9 Therefore, only a few selective ligands have been reported in the literature, especially with respect to MT1 receptors; none of these ligands has been tested in vitro in cells or tissues expressing MLT receptors, and their efficacy in the treatment of neuropsychiatric diseases has been poorly evaluated. Other effects of MLT described in the literature include its neuroprotective,10–12 anti-inflammatory,13,14 pain modulatory,15 retinal,16 vascular,17–19 antitumour20 and antioxidant21 properties.
Melatonin and its receptor: state of the art
Who’s who?
Two subtypes of mammalian MLT receptors have been cloned and characterized, the MT1 (Mel1a) and the MT2 (Mel1b) receptor subtypes. Both subtypes are members of the 7-transmembrane GPCR family (for detailed information on the molecular properties of MLT receptors please refer to the review by Dubocovich and colleagues5). By using recombinant MLT receptors it has been shown that the MT1 receptor is coupled to different G proteins that mediate adenylyl cyclase inhibition by a pertussis toxin-sensitive G protein and phospholipase C β activation.22 The MT2 receptor is also coupled to inhibition of adenylyl cyclase, and it also inhibits the soluble guanylyl cyclase pathway.23 Interestingly, the human MT2 receptor has a lower affinity for 125 I-Mel than the human MT1 receptor.24 Another interesting difference between MT1 and MT2 receptors is that MT2, unlike MT1, desensitizes after exposure to the full agonist MLT,25 likely by an internalization mechanism, but it is not clear whether this occurs after short- (minutes) or long-term (5–8 hours) exposure to MLT in vivo.26 Based on these considerations, it appears reasonable that the use of an MT2 receptor partial agonist might be the best approach to activate a receptor that desensitizes after exposure to its full agonist. Now that selective MT2 receptor partial agonists are available, this hypothesis warrants further investigation.
Where are they?
The distribution of MLT receptors within the central nervous system has not been completely elucidated, primarily owing to the lack of selective antibodies for MLT receptors. The study of MLT receptor localization has mostly been performed using receptor autoradiography with the nonselective 2-[125I]iodomelatonin, or through real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR), marking the MLT receptor mRNA. Messenger RNA expression of MT1 and MT2 receptors has been observed in the retina, SCN, thalamus, hippocampus vestibular nuclei and cerebral and cerebellar cortex.27–30 At the level of the hippocampus, MT2 receptors were detectable in particular on CA3 and CA4 pyramidal neurons, which receive glutamatergic excitatory inputs from the entorhinal cortex, whereas MT1 receptors were predominantly expressed in CA1.31 Recently, using polyclonal antibodies32 we have pointed out the presence of MT2 receptors in the reticular thalamus, substantia nigra (pars reticulata), supraoptic nucleus, red nucleus and CA2 and CA3 areas of the hippocampus.33
Using in situ hybridization histochemistry with selective and specific digoxigenin-labelled antisense and sense oligonucleotide probes, a robust hybridization of MT1 and MT2 receptor mRNA has been observed in the SCN.34
In humans, MT2 receptors have been recently localized at the level of the SCN, the supraoptic nucleus and the para-ventricular nucleus using polyclonal specific antibodies. Importantly, the receptors were confined to neurons and nerve fibres, but not to glial cells.35
What are their roles?
The functional characterization of MLT receptors is still an active matter of research. It is known that MT1 and MT2 receptors could have either opposing or complementary functions. For example, in hippocampal slices, MT1 and MT2 receptor activation appears to differentially modulate γ-aminobutyric acid (GABA)A receptor function, suggesting that MLT, through activation of different receptor subtypes, may exert opposite effects in the same brain area.36 Similarly, MT1 and MT2 receptors have also been shown to act in an opposite manner on the vascular system, producing vasoconstriction or vasodilatation, respectively.18
Controversial results using in vitro and in vivo techniques have been published concerning the role of MT1 and MT2 receptors in circadian regulation and sleep. As mentioned previously, the mammalian SCN is known to be rich in both MT1 and MT2 receptors.37 The SCN is the “master clock” that controls behavioural, metabolic and physiologic rhythms,38 including the light-dependent synthesis and release of MLT from the pineal gland.39
In vitro experiments using ligands toward MLT receptors and SCN slices from wild-type and MLT receptor knockout mice showed that activation of MT1 receptors inhibited the neuronal firing rate of the SCN, whereas activation of MT2 receptors phase-shifted circadian rhythms of neuronal firing rates.26,34,37,40,41 But, in Siberian hamsters in which the MT1 receptor was functional, but the MT2 receptor was not, MLT elicited a clear phase shift of the circadian rhythm of SCN electrical activity.42 In vivo experiments in MLT receptor knockout mice further complicated the scenario. Dubocovich and Markowska,26 injected MLT 2 hours before the onset of activity in mice kept under constant darkness and found a phase shift in the activity onset in wild-type but not in MT1 receptor knockout (MT1−/−) mice. Moreover, MLT accelerated the entrainment to a new light–dark cycle of wild-type, but not of MT1−/− mice. For these reasons, current knowledge does not allow us to rule out a possible role of MT1 receptors in the phase shift of SCN neuronal activity due to MLT.37,43
Selective MT2 receptor ligands used in preclinical psychopharmacological and neurobiological studies
Melatonin, through activation of MT1 and MT2 receptors, modulates and controls several brain functions; however, owing to the lack of selective MT1 and MT2 receptor ligands and a paucity of behavioural studies in MT1−/− and MT2 receptor knockout (MT2−/−) mice, little was known regarding the functional role of these receptors. Recently, some selective MT2 receptor ligands have been designed and synthesized, allowing researchers to explore the role of MT2 receptors in brain functions.
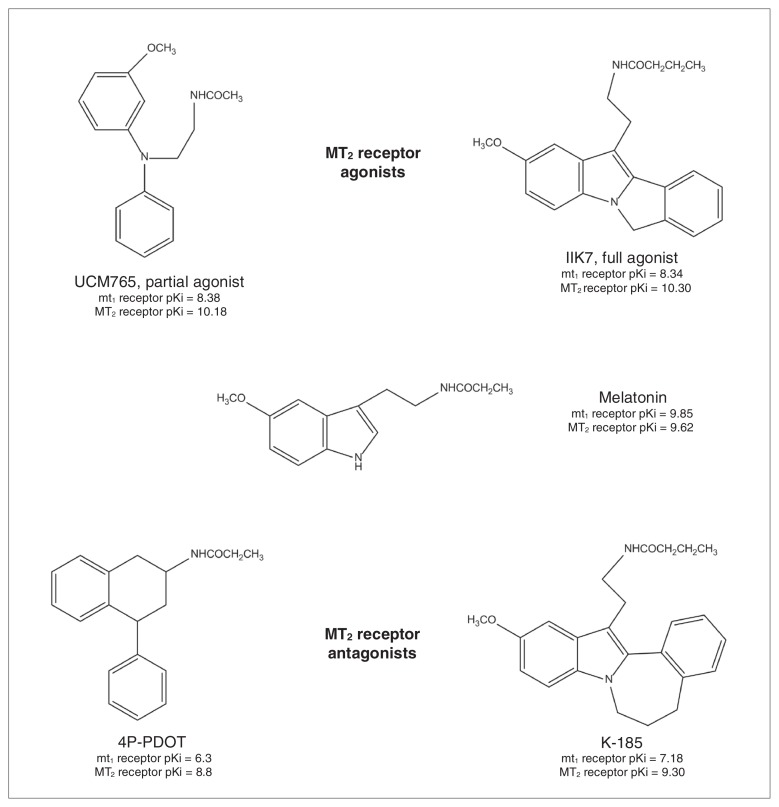
In the last decade, efforts have been made to develop selective MT1 or MT2 receptor agonists.9 A great number of ligands highly selective for the MLT receptors have been recently developed, but these advances have been met with difficulty obtaining compounds with selectivity toward only 1 of the 2 MLT receptor subtypes. Very few selective MT1 receptor agonists have been reported until now.8 On the contrary, since structure–activity relationships for the binding at the MT2 receptor are quite consolidated,44 several MT2 receptor ligands belonging to different chemical classes have been developed (for more details, see the reviews by Mor and colleagues9 and Zlotos45). Only a very limited number of these selective MT2 receptor agonists/antagonists have been tested in preclinical psychopharmacology tests and in neurobiological studies aimed at dissecting the role of MT2 receptors in brain function. Specifically, as revealed by the research we review here, only 4 selective MT2 receptor ligands have been tested: the MT2 partial agonist UCM765,44 the MT2 full agonist IIK7,46 and the MT2 antagonists 4-phenyl-2-propionamidotetralin (4P-PDOT)47 and K-185.46 Figure 1 shows the chemical structure and the binding affinity of these ligands to the human MT1 and MT2 receptors.
Fig. 1.
MT2 receptor ligands used in psychopharmacological studies. Binding affinity constants have been assessed on the recombinant human MT1 and MT2 receptors expressed in NIH3T3 cells for melatonin,44 UCM765,44 IIK7 and K18546 and in COS-7 cells for 4-phenyl-2-propionamidotetralin (4P-PDOT).47
The partial agonist UCM765 belongs to the N-(Substituted-anilinoethyl)amides, a class of compounds that, depending on the type and size of substituents to the aniline nitrogen, may lead to a different degree of selectivity and intrinsic activity toward MT1 or MT2 receptors.44 Even though the indole ring of MLT is not essential for binding to the MT1 and MT2 receptors, IIK7 is a 6H-Isoindolo[2,1-a]indoles derivative whereas K-185 is a 6,7-Dihydro-5H-benzo[c]azepino[2,1-a] indoles derivative. Substituents in ortho position to the indole nitrogen of MLT, such as a phenyl ring, have been shown to increase binding affinity as well as potency to MT1 and MT2 receptors with respect to MLT itself. Interestingly, changes in the ring size that bridges the indole to the phenyl ring greatly affect the potency of these compounds; IIK7 with a 5 atoms ring is a full agonist, whereas K-185 with a 7 atoms ring is an antagonist.46 The antagonist 4P-PDOT is a tetralin derivative with antagonist properties and high selectivity toward MT2 receptors (300-fold higher than the MT1 receptor).47 Of note, even though at the present moment it is the most used MT2 receptor antagonist, it should always be kept in mind that depending on the assay and the concentration, 4P-PDOT may also act as a weak partial agonist.48 Both UCM765 and IIK7 have been studied in vivo for their potential sleep-promoting properties in rats. When compared with MLT, both of these compounds showed higher affinity for the MT2 receptor (Fig. 1). Importantly, while UCM765 is an MT2 partial agonist (α = 0.6) that possesses 100-fold higher affinity for MT2 than MT1 receptors,33,44 IIK7 is an MT2 full agonist with 90-fold higher affinity for MT2 than MT1 receptors.49 Even though experiments comparing the sleep-promoting properties of the 2 compounds have not yet been conducted, it is important to remember that partial agonists, thanks to their “flexible” properties compared with full agonists, are becoming more and more popular in psychopharmacology.50 They are “intelligent drugs” since they can activate receptors to give a desired submaximal response when inadequate amounts of the endogenous ligand are present, or they can reduce the overstimulation of receptors when excess amounts of the endogenous ligand are present, thus acting as competitive antagonists.51
Several other selective MT2 receptor ligands, such as 1,6-Dihydro-2H-indeno[5,4-b]furan,52 N-[3-(3-methoxyphenyl) propyl] amide,53 phenylpropylamide54 and 2-(phenylthio) benzo[b]thiophene55 derivatives, have recently been developed, and according to the literature reviewed herein, they warrant further examination in preclinical psychopharmacology tests.
MT2 receptors and sleep
In mammals, normal sleep is characterized by an orderly progression from wakefulness to non–rapid eye movement sleep (NREMS) and to rapid eye movement sleep (REMS). Wakefulness is characterized by low voltage, fast electroencephalogram (EEG) activity and high muscle tone with phasic electromyogram (EMG) activity. Non–rapid eye movement sleep is characterized by high voltage, slow EEG activity and reduced muscle tone, with characteristic high-voltage slow waveforms (1–4 Hz, Δ waves), sleep spindles, and K-complexes. Rapid eye movement sleep, or paradoxical sleep (PS), is characterized by low-voltage, fast EEG activity with an absence of muscle tone and a pronounced θ rhythm (4–9 Hz). The deep stages of NREMS (stages 3 and 4) are also known as slow-wave sleep (SWS), which is thought to be the most restorative sleep stage.56 During SWS, several physiologic processes, such as memory consolidation,57–59 metabolic regulation,60–62 and drop in blood pressure,63 occur. In people with major depression, secondary insomnia is frequent, with alterations in sleep neurophysiology, notably decreased SWS, reduced REMS latency and increased REMS density. Increased REMS density has also been observed in people with eating disorders, narcolepsy, presenile dementia and other neuropsychiatric diseases.64
Melatonin and sleep
In rats, contradictory effects of MLT in sleep have been reported. Holmes and Sugden65 reported that MLT reduces time to sleep onset and increases both SWS and PS. Others suggest that MLT is involved only in the control of PS regulation since the lesion of the pineal gland or inhibition of MLT synthesis using a β-adrenergic antagonist (propranolol) decreased PS during light and dark periods.66,67 Melatonin (2.5 mg/kg and 5 mg/kg) increased the number of sleep cycles and the total duration of PS,68 and at the dose of 10 mg/kg, MLT decreased the onset to the first episode of SWS and increased the duration of SWS and PS. These latter effects were blocked by the GABAA antagonists flumazenil and picrotoxin.69 In monkeys, MLT (0.3, 1 and 3 mg/kg) does not affect SWS, REMS and light sleep, but does shorten the latency to the first episode of sleep. However, this reduction in the latency to sleep occurred only at the lowest dose.70 In a study using rats,71 MLT affected neither sleep nor circadian rest activity rhythms. In cats, exogenous MLT (0.01–1 mg/kg) significantly increased SWS, but the effect was weak and lasted for only 2 hours.72
There is a large body of research demonstrating hypnotic effects of MLT in clinical studies,73–77 although some studies failed to find significant effects,78,79 and therefore its clinical efficacy is still unclear.80 These discrepant results may originate from the pharmacological constraints of generic MLT, limiting its clinical use: short half-life (< 1 hr), high first-pass metabolism, binding of multiple receptors and effects dependent on time of the day or phase of circadian rhythm.81 For example, the hypnotic effect of MLT in humans varies depending on the time of the administration, consistent with the circadian phase resetting properties of MLT.82 Although several studies suggest that MLT treatment may be appropriate for sleep disorders, it is not established whether MLT acts directly on sleep regulation or on circadian rhythms associated with sleep. However, the sleep promoting effect of the MT1/MT2 receptor agonist ramelteon (TAK-375) strongly suggests that MLT compounds facilitate the induction of sleep rather than modify all sleep architecture or circadian rhythms. Ramelteon has been approved by the U.S. Food and Drug Administration (July 2005) for the treatment of insomnia characterized by difficulty with sleep onset.83 Given that ramelteon is an MLT receptor agonist with high affinity for both MT1 and MT2 receptors, it is difficult to dissect the receptor subtype(s) involved in its sleep-promoting properties.
MT2 receptor ligands (UCM765 and IIK7) and sleep
The pharmacological studies using selective MT2 receptor ligands have allowed researchers to better identify the role of the MT2 receptor in sleep function. Since MLT synthesis, and thus its circulating levels as well as the expression of its receptors, follow circadian daily variations, sleep experiments with UCM765 were performed across the entire 24-hour sleep–wake cycle to examine its effects across the light–dark period.33 Given the short half-life of the compound (T1/2 = 44 min) due to an extensive first-pass metabolism,33,84 UCM765 was injected every 4 hours to keep the concentration of the drug within the steady state range. A subcutaneous injection of UCM765 at a dose of 40 mg/kg significantly reduced the latency to NREMS by 59% while increasing the amount of NREMS during the 24-hour period by 38%. The increase in NREMS was mainly evident during the light/inactive phase of the 24-hour light–dark cycle. The amount and latency of REMS were not altered by UCM765. The amount of wakefulness was instead significantly decreased (−22%) due to the increase in NREMS paralleled by no changes in REMS (Fig. 2).
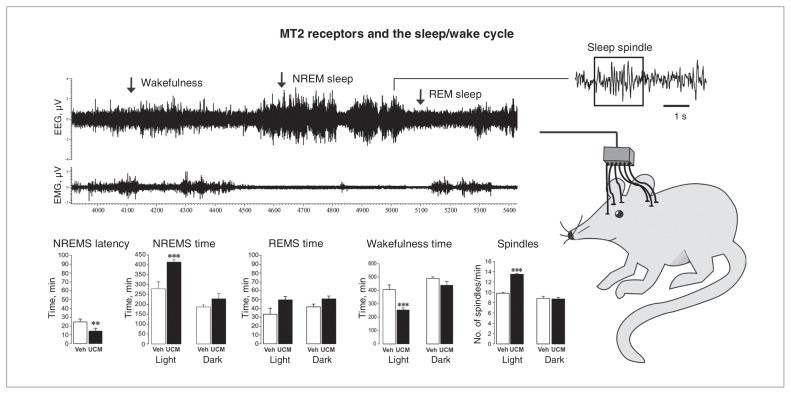
Fig. 2.
The MT2 receptor partial agonist UCM765 promotes non–rapid eye movement sleep (NREMS; modified from Ochoa-Sanchez and colleagues,33 with permission). (Top right) Schematic depiction of a rat with electrodes implanted for electroencephalography (EEG) and electromyography (EMG) recordings. (Top left) Example of an EEG/EMG recording highlighting a period of wakefulness, NREMS and rapid-eye movement sleep (REMS). (Bottom) The effect of UCM765 (40 mg/kg, subcutaneous) on NREMS latency, NREMS time, REMS time, wakefulness time and number of sleep spindles during the light and dark phases of the 12:12 hour light–dark cycle. **p < 0.01 and ***p < 0.001 versus vehicle, 2-way mixed-design analysis of variance plus Newman–Keuls test for post hoc comparison.
Similar effects were replicated after intraperitoneal injection of IIK7 (10 mg/kg):49 the injection led to a 38% decrease in the latency to NREMS and a 250% increase in the amount of NREMS during the first hour after injection. Like UCM765, no effects on REMS amount and latency were reported with IIK7.
Remarkably, the effects of UCM765 on NREMS as well as on NREMS latency were prevented by the MT2 antagonist 4P-PDOT (10mg/kg), suggesting an MT2 receptor–dependent mechanism. Administration of 4P-PDOT (10 mg/kg) alone did not significantly modify sleep parameters;33 however, we cannot rule out that higher or lower doses of the MT2 antagonist may influence sleep parameters.
Until now, it was hypothesized that MT1 receptors control sleep, whereas MT2 receptors are involved in the time shift mainly by controlling the neural activity of the SCN.43 Contrary to this common belief, pharmacological studies have pointed out that MT2 receptors modulate sleep, particularly NREMS.
Possible changes in sleep spindles and power spectra were also evaluated after the injection of UCM765 and IIK7. According to Steriade,85 sleep spindles are considered the epitome of EEG synchronization at sleep onset (NREMS stage 2) and are defined by the association of 2 distinct rhythms: the waxing and waning spindle waves at 7–14 Hz with sequences lasting for 1–2 seconds, and the periodic recurrence of spindle sequences with a slow rhythm of 0.1–0.2 Hz.
Administration of UCM765 significantly increased the number of sleep spindles per minute of NREMS (+16%),33 whereas no information was reported for IIK7. When we analyzed the effects of UCM765 on the power spectra across the 24-hour period, we found that, unlike the benzodiazepine diazepam, the EEG power of the Δ band of NREMS was significantly enhanced (+14%), whereas REMS θ power was not altered.33 Neither Δ nor θ powers were affected after IIK7 injection.
Of note, the same 24-hour EEG/EMG experiment carried out with the nonselective MT1/MT2 receptor ligand UCM793 (40 mg/kg), which belongs to the same class of N-(substituted-anilinoethyl)amides, and MLT (40 mg/kg) failed to reproduce similar results. Administration of UCM793 slightly decreased the onset to the first NREMS episode (−10.7%) and did not increase the total amount of NREMS.33 On the other hand, MLT produced a slight but nonsignificant increase of NREMS duration during the inactive phase (unpublished data, 2012). These data confirmed that the MT2 receptor ligands possess more potent hypnotic properties than the nonselective MT1-MT2 ligands.
In our labaratory, we also examined whether MT2 receptors were present in brain areas or nuclei, other than the SCN, that are known to be involved in the process of sleep. Immunocytochemical, pharmacological and in vivo electrophysiology studies answered these questions. Using specific MT2 receptor antibodies, we found that basically all neurons in the reticular thalamus bear MT2 receptors33 and, as previously reported, most of these neurons (if not all) are GABAergic.86 The reticular thalamus is an important nuclei in the neural network controlling sleep; it is implicated in the generation of Δ waves during deep SWS87 and also of spindle waves during light SWS, thus being called the spindle “pacemaker.”88 During NREMS, the neurons of the reticular thalamus change their activity, from single spikes firing during waking and REMS to rhythmic spike bursts and increased firing mode, which is transmitted to thalamic relay nuclei and modulated by corticothalamic inputs, resulting in a widespread synchronization across neuronal assemblies.89–91 For detailed information on how the reticular thalamus controls the cortical oscillations and on its role in the thalamocortical networks, see the reviews by Steriade92 and by Linás and Steriade.93
Since MT2 receptors are highly expressed in the reticular thalamic nucleus, and given that UCM765 acts to promote NREMS, we decided to test whether UCM765 was able to enhance the activity of reticular thalamic neurons by using in vivo electrophysiology (Fig. 3, top). Intravenous administration of UCM765 (20 mg/kg) significantly increased the firing (+91%) and burst activities of reticular thalamic neurons. In addition, to demonstrate that the increased activity of the reticular thalamus relies on MT2 receptors present within the nucleus, we microinfused the selective MT2 receptor antagonist 4P-PDOT94 directly into the nucleus before intravenous injection of UCM765. When 4P-PDOT was injected before UCM765, there was no change in the firing pattern of reticular thalamic neurons. These findings allowed us to conclude that MT2 receptor agonists promote sleep through the activation of reticular thalamic neurons.
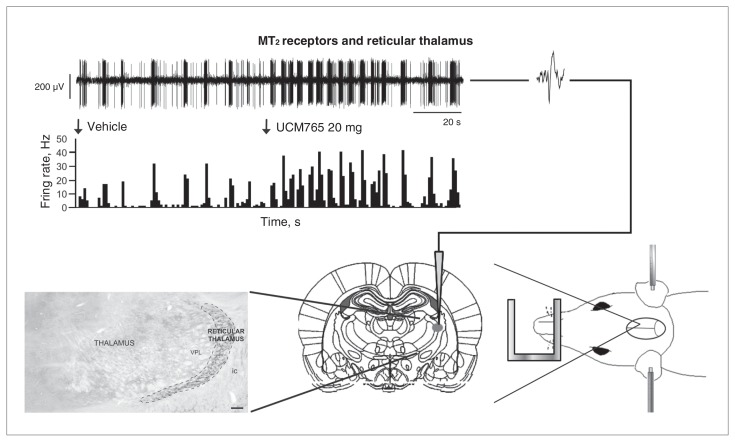
Fig. 3.
The MT2 receptor partial agonist UCM765 increases the neural activity of the reticular thalamus, enhancing the rhythmic burst activity (modified from Ochoa-Sanchez and colleagues,33 with permission). (Right) A rat under anesthesia and restrained in a stereotaxic apparatus. An electrode is lowered into the reticular thalamus according to the Paxinos and Watson atlas.193(Left) Reticular thalamic neurons bear MT2 receptors. (Top) An injection of UCM765 (20 mg/kg, intravenous) increases the firing activity of a reticular thalamic neuron.
We then examined whether the selective activation of MT2 receptors into the reticular thalamus was sufficient to promote sleep, particularly NREMS. We bilaterally implanted 2 cannula into the reticular thalamus. Microinfusion of UCM765 into the reticular thalamus produced results that mirrored the subcutaneous administration of the drug: decreased latency to NREMS, increased amount of NREMS and no effects on REMS. Remarkably, the same dose of UCM65 microinfused into the substantia nigra, a brain area not involved in NREMS, but with MT2 receptors, did not alter the vigilance states. A summary of the effects of UCM765 on vigilance states and reticular thalamic activity is reported in Figure 3.
MT2 knockout mice and sleep
To support the proof of the concept that MT2 receptors may represent a novel target for treating sleep abnormalities, the sleep–wake cycle in MT2−/− mice has been examined. Knockout mice represent an excellent tool to study the involvement of different neurotransmitters, receptors and enzymes in sleep regulation, as demonstrated for orexins;95 histamine deficient L-histidine decarboxylase;96 corticotropin-releasing hormone receptor type 1;97 dopamine (DA) D2 receptor;98 and 5-HT1A, 5-HT1B, 5-HT2A and 5-HT2C receptors.99 Compared with wild-type controls, MT2−/− mice40 displayed a significant decrease in the amount of NREMS during the 24-hour period (−17%), with the decrease mainly due to changes occurring during the light/inactive phase of the 12-hour light–dark cycle.33 No variation in the amount of REMS was found. The decline in the amount of NREMS is likely attributed to the decrease of NREMS Δ and ∑ powers found after analyzing the 24-hour power spectra. Even though some issues remain to be considered, NREMS ∑ power is considered a valid measure of sleep spindle activity.100 To date, no research has reported on visually scored sleep spindles in MT2−/− mice, but the decrease in NREMS ∑ power observed in MT2−/− mice suggests that the number of sleep spindles is likely reduced.
In our labaratory, we have also tested UCM765 in MT2−/− mice, and we found that it was ineffective at modifying vigilance states. The lack of effect in MT2−/− mice concurred with the evidence indicating that the NREMS promoting effect of UCM765 is selectively mediated by MT2 receptors. In keeping with the findings in rats, in wild-type mice UCM765 significantly increased NREMS duration (wild-type plus vehicle = 64.4 ± 4.0 min, n = 6; wild-type plus UCM765 = 89.3 ± 5.5 min, n = 6, p = 0.004) and decreased wakefulness (wild-type plus vehicle = 110.3 ± 5.6 min, n = 6; wild-type plus UCM765 = 83.82 ± 5.6 min, n = 6, p = 0.005), without affecting REMS duration.
To verify whether the reduction in NREMS observed in MT2−/− mice was due to the lack of MT2 receptors rather than to impairments in other sleep-related neurotransmitter systems, MT2−/− mice were treated with the GABAA agonist diazepam. As observed in wild-type mice, diazepam decreased the latency to the first episode of NREMS (−64.4%), enhanced NREMS duration (+49.1%), and tended to decrease REMS duration (−20.6%) in MT2−/− mice, suggesting that the GABAergic system regulating sleep was intact in these mice.
We have also performed EEG/EMG studies in MT1−/− mice and found that these mice have a selective perturbation of REMS. In particular, during the light phase, REMS duration was decreased compared with wild-type mice (−46%) and NREMS duration was increased compared with MT2−/− mice (+33%).101 This particular phenotype induced by the lack of the MT1 receptor could explain the reason why a selective MT2 receptor ligand is a more effective hypnotic agent than a nonselective MLT receptor ligand. We have indeed hypothesized that stimulation of the MT2 receptor only increases NREMS, whereas MT1 receptor activation increases REMS, while slightly decreasing NREMS.
General consideration
Pharmacological and genetic studies targeting MT2 receptors have revealed a new important function of this MLT receptor subtype: the modulation of NREMS. Pharmacological activation of MT2 receptors using selective partial and full agonists promotes NREMS without affecting REMS. On the other hand, genetic inactivation of MT2 receptors leads to a decrease in the amount of NREMS without affecting REMS (Table 1). Our results33 demonstrated that the local pharmacological activation of MT2 receptors within the reticular thalamus is sufficient to induce and promote NREMS, whereas MT2 receptor activation in brain nuclei not intimately involved in the neural circuit of sleep, such as the substantia nigra, produces no effects on NREMS. These experiments thus support the concept that MT2 receptors may be considered a novel target for hypnotic agents.
Table 1.
Summary of sleep changes occurring after pharmacological activation and genetic inactivation of MT2 receptors
| Factor | Pharmacological studies with MT2 receptor agonists | Genetic inactivation of MT2 receptors |
|---|---|---|
| NREMS amount | ↑ | ↓ |
| REMS amount | ↔ | ↔ |
| NREMS Δ power | ↑ | ↓ |
| NREMS β power | ↔ | ↓ |
| Number of spindles | ↑ | ↓* |
↑= increase; ↔ = no change; ↓= decrease; NREMS = non–rapid eye movement sleep; REMS = rapid eye movement sleep.
Empirically based on the reduction of NREMS ∑ power.
Since we found MT2 receptors at the level of the hippocampus33 and since neocortical and hippocampal activity is coupled on both long and short time scales during SWS,102 we cannot rule out the possibility that the involvement of these areas may also participate in the promotion of SWS.
Further research needs to be undertaken to determine whether these MT2 receptor agonists affect the phase shift. Our experiments seem to suggest that at the hypnotic doses, MT2 receptor agonists do not affect time shifting. However, we cannot discount the possibility that the activation of MT2 receptors in the SCN, which is involved in time shifting of tSCN firing activity,40 may also contribute indirectly to the NREMS process. The development of selective MT1 or MT2 receptor agonists will offer the opportunity to better understand and distinguish the role of each receptor in the phase shifting from the sleep promoting effects of MLT. This will ultimately allow for the development of selective pharmacotherapeutic treatment strategies in patients with sleep and time shifting disorders. To date, structure–activity relationships conferring selectivity for the MT1 receptor subtype are still a work in progress;8 therefore, no selective MT1 receptor agonists have been tested in sleep and time shifting studies.
Insomnia is a common public health problem, with a prevalence ranging from 11% to 16%.103,104 Primary insomnia is characterized by a difficulty to initiate or maintain sleep for at least 1 month. Currently available drugs to treat insomnia include benzodiazepine receptor agonists (benzodiazepines and nonbenzodiazepines), antidepressants, antipsychotics, antihistamines and ramelteon, a nonselective MT1/MT2 receptor agonist. Unfortunately, these hypnotics cannot reproduce a physiologic sleep,105 and their use, especially chronic use, is associated with adverse effects, such as tolerance to the drug, rebound insomnia, physical withdrawal symptoms when discontinued, sedation, anorexia, anxiety, agitation, tremors, convulsion, and physical and psychological dependence. Therefore, the sleep disorders market has substantial unmet needs,106 and based on the preclinical evidence, MT2 receptors may be a promising novel target deserving consideration. Our experiments indicate that MT2 receptor agonists may have a more favourable pharmacological profile than classical benzodiazepines, such as diazepam. Indeed, in comparison with diazepam, UCM765 showed similar NREMS promoting properties, but unlike the benzodiazepine, it did not produce sedation in the rotorod test (unpublished data, 2012) and open field test (OFT) at the hypnotic dose.107 Moreover, the typical reduction of NREMS Δ and θ powers and REMS θ power108 observed with benzodiazepines was not observed with UCM765, which instead increases the Δ power during NREMS and REMS. In addition, UCM765, unlike benzodiazepines, does not increase the peak of β frequency.109 These pharmacological studies have proven that targeting MT2 receptors may become a novel pharmacological strategy in sleep research and therapy, representing a valid alternative to benzodiazepines and benzodiazepine derivatives.
MT2 receptors and anxiety
Melatonin and anxiety
Anxiety disorder (or generalized anxiety, as per DSM-IV110) is characterized by excessive, uncontrollable and often irrational worry about everyday things that is disproportionate to the actual source of worry lasting for more than 6 months. The psychological symptoms are often associated with physical symptoms, including fatigue, fidgeting, headaches, nausea, numbness and sweating.
Melatonin and the nonselective MT1/MT2 receptor agonist agomelatine have displayed anxiolytic-like properties in classical animal paradigms of anxiety.111–113 For instance, MLT increased the activity within the central area of the OFT112 and the time spent in the open arms of the elevated plus maze test (EPMT).111,113 Agomelatine increased the number of punished responses in the Vogel test, increased open arm exploration in the EPMT and decreased vocalization time in the conditioned footshock-induced ultrasonic vocalization test.113
In humans, MLT has been used to reduce or eliminate benzodiazepine administration in elderly patients.114 Still the receptor mediating the anxiety-like effect of MLT was unknown. The recent development of selective MT2 receptor agonists and the use of selective antagonists have shed light on the distinct role of each MLT receptor subtype in the regulation of anxiety.
The MT2 receptor ligand UCM765 and anxiety
Using UCM765, we recently made the first attempt, to our knowledge, to investigate the possible role of MT2 receptors in anxiety.107 We used 3 well-established animal paradigms to study anxiety: the EPMT, OFT and the novelty-suppressed feeding test (NSFT). The first experiment in the EPMT and NSFT was designed to obtain a dose-response curve using lower doses of UCM765 than those active in promoting NREMS. Administration of UCM765 at a dose of 10 mg/kg, displayed anxiolytic properties by increasing the time spent in the open arms (+208%) of the EPMT and by decreasing the latency to eat in a new environment (−46%) without affecting the latency to eat and the amount of food intake per animal body weight in the home cage. Melatonin was also tested, and it produced results similar to UCM765. By using a nonselective MT1/MT2 receptor antagonist (luzindole) and a selective MT2 receptor antagonist (4P-PDOT), we investigated whether both MT1 and MT2 receptors were implicated in the anxiolytic properties of MLT. Administration of UCM765 or MLT did not show anxiolytic effects in the EPMT and in the NSFT in animals pretreated with luzindole (10 mg/kg) or 4P-PDOT (10 mg/kg). These results collectively demonstrate that the anxiolytic effects of UCM765 and MLT are both pharmacologically mediated by MT2 receptors. Notably, 4P-PDOT or luzindole alone did not affect anxiety levels at the doses used to block the effects of UCM765 and MLT. Accordingly, Nava and Carta115 found that luzindole administered in higher doses than those in our experiments (30 and 60 mg/kg v. 10 mg/kg) did not modify anxiety levels. Administration of UCM765 and MLT was also tested in the OFT, but they did not alter measures of anxiety behaviour in this paradigm. However, unlike diazepam, UCM765 and MLT did not reduce locomotor activity107 or increase the number of falls in the rotoroad tests (unpublished data, 2012), 2 measures related to the sedative properties of a putative psychotropic compound.
These findings are the first to examine the role of MLT receptors in anxiety, and they indicate that MT2 receptors mediate the anxiolytic effects of the neurohormone.
MT2 knockout mice and anxiety
To support the pharmacological studies showing that MT2 receptors are involved in anxiety, we investigated whether MT2−/− mice display altered levels of anxiety in the EPMT, NSFT and OFT (unpublished data, 2012). In the EPMT, the number of entries and the time spent in the open arm was not modified, but interestingly MT2−/− mice spent more time in the centre than wild-type controls, suggesting a cognitive impairment at the level of goal-oriented behaviour. In the EPMT, the time spent by animals in the open arm is the most important variable to consider when establishing whether or not a treatment affects anxiety. In MT2−/− mice, open arm entries and time spent in the open arm were not affected, but they spent more time than controls in the centre of the maze. On the other hand, the time spent in the central platform of the EPMT has been linked to the decision-making process rather than to anxiety itself.116 In agreement with the notion of possible decision-making impairments in MT2−/− mice, Larson and colleagues117 found that these animals, when tested in the EPMT on 2 consecutive days, failed to shorten the transfer latencies to enter a closed arm on the second day, which is an index of learning and/or memory impairments.
In the NSFT, the latency to eat in the new environment was significantly longer in MT2−/− mice than wild-type mice (+80%), whereas the latency to eat and the amount of food intake per animal body weight in the home cage were unchanged. In the OFT, the number of entries and the time spent in the centre of the arena were significantly higher in MT2−/− mice than wild-type mice, whereas locomotion was unchanged. The results gleaned from these experiments suggest that the genetic manipulation of MT2 receptors interferes in a complex manner with some paradigms of anxiety behaviour. Anxiety disorders, by definition, are complex and heterogeneous;118 nonetheless, different animal models could be tapping different aspects of anxiety,119 and studies of different mouse strains and different anxiolytic treatments have previously yielded contrasting results, depending on the behavioural test (Table 2).116,119
Table 2.
Summary of changes in anxiety levels occurring after pharmacological activation and genetic inactivation of MT2 receptors
| Factor | Pharmacological studies with UCM765 | Genetic inactivation of MT2 receptors |
|---|---|---|
| Elevated plus maze test | ||
| Open arm entries | ↔ | ↔ |
| Open arm time | ↑ | ↔ |
| Novelty suppressed feeding test | ||
| Latency to eat in a new environment | ↓ | ↑ |
| Latency to eat in the home cage | ↔ | ↔ |
| Open field test | ||
| Locomotion | ↔ | ↔ |
| Number of entries in the central area | ↔ | ↑* |
| Time spent in the central area | ↔ | ↑* |
↑ = increase; ↔ = no change; ↓= decrease.
Only during the first 5 minutes of the test session.
General consideration
Pharmacological and genetic studies targeting MT2 receptors have shed new light into the neurobiological mechanism through which the MLT system modulates anxiety. Although further studies are necessary, the research undertaken until now shows that MT2 receptors modulate anxiety levels and consequently this receptor may become a novel target for the treatment of anxiety disorders. Common anxiolytic drugs, such as benzodiazepines, targeting the GABAergic system are also used as hypnotic agents at higher dosages. Targeting MT2 receptors seems to provide similar pharmacological outcomes. Administration of UCM765 at a dose of 40 mg/kg promotes sleep,33 whereas a lower dose (10 mg/kg) reduces anxiety.107 These similar pharmacological effects occurring after the activation of the GABAergic and the MLT systems are likely due to the mutual interaction between these 2 systems. We found that almost all of the GABAergic reticular thalamic neurons express MT2 receptors.33 Moreover, MLT administration increased GABA levels in several brain regions, such as the hypothalamus, cerebellum and cerebral cortex,120 and the administration of flumazenil, a benzodiazepine receptor antagonist, blocked the anxiolytic-like effect of MLT in the EPMT.111 However, given that the MT2 receptor–mediated anxiolytic properties are also associated with low sedation and a lack of abuse potential, as demonstrated by the use of nonselective melatoninergic compounds,121 targeting this MLT receptor subtype may offer better anxiolytic pharmacological strategies over the GABAergic compounds, especially when treating mild levels of anxiety.107
Further studies will need to investigate the neurobiological mechanism through which MT2 receptors modulate anxiety, the effects of MT2 receptor full agonists and the pharmacological and genetic blockade of MT2 receptors. Further study is also needed to determine whether MT2 receptors are localized only on GABAergic neurons or also on other systems, such as 5-HT and glutamate, known to be implicated in anxiety.
MT2 receptor, memory function and Alzheimer disease
Memory is the process by which new information is encoded, stored and retrieved.122,123 Synaptic plasticity is a critical component of the neural mechanisms underlying learning and memory. In 1949, Donald Hebb hypothesized that the storage of information relies on changes in the strength of synaptic connections between neurons, suggesting that if 2 neurons are active at the same time, the synaptic efficiency of the appropriate synapse will be strengthened. Therefore, plastic changes are crucial in the mechanism of learning by increasing the synaptic efficiency that allows strengthening between neurons.124 These changes increase not only the number of synapses in specific brain areas, but also the number of receptors located on a synapse, resulting in modified synaptic neurotransmission.125,126 The most widely studied electrophysiological models of memory formation in the mammalian brain127,128 that explains these changes in the synapse during learning and memory processes is called long-term potentiation (LTP); its opposite process is long-term depression (LTD).128 Long-term potentiation is a measure that results from simultaneous activity between 2 neurons, enhancing the signal neurotransmission after electrical stimulation.128
As reviewed by Bob and Fedor-Freybergh,129 MLT regulates memory formation, acting directly on hippocampal neurons,29,130 which are involved in the processes of memory acquisition and consolidation.131–133 In particular, MLT enhances the firing rate of action potentials in CA1 neurons of hippocampal slices29 and promotes synaptic transmission.130 Accordingly, expression of MT1 and MT2 receptors has been observed in the hippocampus.33,134,135
Wang and colleagues136 showed that MLT produces a concentration-dependent inhibition of LTP in hippocampal slices in mice, and this effect is due to the action of MLT on the postsynaptic nitric oxide signalling pathway.137 Other studies suggested that the inhibitory actions of MLT on LTP in the hippocampus in mice were blocked by the MT1/MT2 receptor antagonist luzindole and by the selective MT2 receptor antagonist 4P-PDOT, thus suggesting an MT2 receptor–mediated effect. Such inhibition was also observed in MT1−/−, but not in MT2−/− mice.136 Importantly, using in situ pharmacology, subtype expression and transfection studies, Wan and colleagues36 found that MLT inhibits GABAA receptor–mediated current in the rat hippocampus via MT2 receptors.
Interestingly, memory and LTP maintenance are impaired in MT2−/− mice.117 Hippocampal slices from MT2−/− mice have revealed the magnitude of LTP to be smaller than slices from wild-type mice, and when MT2−/− mice were tested in the EPMT on 2 consecutive days, they showed learning and/or memory impairments. Specifically, MT2−/− mice failed to shorten the transfer latencies to enter a closed arm on the second day, an experience-dependent behaviour observed in wild-type mice.117 In addition, we found that MT2−/− mice spent more time than wild-type mice in the central platform of the elevated plus maze (unpublished data, 2012), which, as mentioned before, could be a symptom of impaired decision making and cognitive flexibility. In the hippocampus, MLT is also able to modulate evoked potentials, and, in particular, the attenuation of synaptic transmission observed during the first phase of a biphasic ligand-induced effect has been shown to be mediated by MT2 receptors.138 Given these contrasting results, more studies are required to clarify the role of melatonergic agonists versus antagonists in the modulation of LTP and LTD as well as the role of each MLT receptor subtype.
Alzheimer disease is an age-associated, progressive neurodegenerative disease characterized by a loss of cognitive function, dementia and other neurobiological manifestations.139 Using specific antibodies, MT2 receptors have been localized to pyramidal and granular neurons of the hippocampus in patients with Alzheimer disease and controls,140 but the intensity of MT2 immunoreactivity in single cells and the number of MT2 immunoreactive neurons were significantly reduced in the hippocampus of patients with Alzheimer disease. Similarly, the overall intensity of MT2 receptor staining was clearly decreased in the retina, particularly at the level of ganglion and bipolar cells in the inner nuclear layer, the inner segments of the photoreceptor cells,141 the pineal gland and in pyramidal and nonpyramidal cells of cortical layers II to V.142 In agreement with the possible involvement of MT2 receptors in the etiology of Alzheimer disease, MT2−/− mice express a phenotype relevant for Alzheimer disease, including impaired learning and/or memory as well as reduction of hippocampal LTP maintenance.117
One of the challenges in neuroscience is to identify the neurobiological mechanisms underlying the process of learning and memory formation, and accumulating evidence suggests that MLT, via MT2 receptors, is likely a contributing factor. However, some central issues still warrant further clarification. Is MLT, via MT2 receptors, enhancing or inhibiting hippocampal LTP? Is the reduction in MT2 receptors observed in many brain areas of patients with Alzheimer disease the cause or the consequence of the disease? The development of selective brain imaging ligands targeting MT2 receptors may help to provide in vivo evidence on receptor expression levels throughout the progression of the disease. Nonetheless, selective MT2 receptor ligands should be tested in behavioural models of learning and memory, such as the T-maze, the radial arm maze and the Morris water maze (for details see the reviews by Dudchenko143 and by Paul and colleagues144).
MT2 receptors and depression
Major depression is a mental disorder characterized by low mood accompanied by low self-esteem and loss of interest or pleasure in normally enjoyable activities (anhedonia), fatigue, anxiety and changes in sleep and weight.110 Substantial evidence suggests that people with major or seasonal depression show impaired MLT secretion associated with a dysfunction of circadian rhythms.145
In animals, chronic, but not acute, administration of the MLT receptor agonist S20394 induced antidepressant-like effects in Flinder Sensitive Line rats, which display high innate levels of immobility in the forced swim test (FST), indicative of enhanced behavioural despair.146 Studies have demonstrated that the antidepressant-like effects of MLT likely occur via 5-HT147 and DA148 systems. Similarly, the treatment of stressed mice with MLT has been shown to reverse some stress-induced behavioural disturbances.149 Accordingly, recent studies suggest that the MT2 receptor is implicated in antidepressant activity.150 In mice, the nonselective MT1/MT2 receptor antagonist luzindole displayed antidepressant-like activity by antagonizing the action of endogenous MLT, and this effect was MT2 receptor–mediated since luzindole was not able to decrease the duration of immobility during the FST in MT2−/− mice. However, the genetic removal of MT2 receptors did not modify immobility time.150
In humans, MLT alone is not effective as an antidepressant even though in combination with other antidepressants it seems to increase the efficacy of the pharmacological treatment, especially when depression is correlated with sleep disturbance.151 Similarly, the combination of low doses of MLT and buspirone has antidepressant effects.152 In November 2008, the nonselective MT1/MT2 receptor agonist and 5-HT2C receptor antagonist agomelatine was approved by the European Medicines Agency for the treatment of major depression in Europe (for details on agomelatine and depression please refer to the study by de Bodinat and colleagues153). The antidepressant mechanism of action of agomelatine has not yet been elucidated, but preclinical studies suggest a multimodal mechanism, including theinvolvement of both 5-HT2C and MLT receptors.154
In agreement with a role for the MT2 receptor in depression, a study in Polish patients with recurrent depressive disorder revealed that variability in MTNR1B, the gene responsible for the encoding of the MT2 receptor, may be associated with a risk for recurrent depressive disorders.155 In particular, depressed patients with the rs794837 AT heterozygote had increased mRNA levels compared with healthy controls, and the presence of the rs4753426 C allele increased while the presence of the T allele decreased the risk of recurrent depressive disorders.
The evidence of the role of MT2 receptors in depression is still limited; therefore, no sound conclusions can be drawn yet. However, future research employing the selective agonists for the MT2 receptor as well as larger genetic studies in patients with depression may partially answer this question. Research should also examine whether dysfunctional MT2 receptors are a common denominator of all type of depressive disorders or whether they are only correlated with certain subtypes of depression, such as unipolar, bipolar or melancholic depression.
MT2 receptors and pain
Experimental and clinical evidence have indicated that MLT possesses analgesic properties and plays a role in pain regulation through several mechanisms, such as activation of MLT and opioid receptors and ion channels (K+ and Ca2+) (for more information, see the review by Ambriz-Tututi and colleagues156 and by Wilhelmsen and colleagues157). In rodents, autoradiography studies have identified MLT receptors in cerebral structures related with pain, such as the thalamus, hypothalamus, trigeminal tract, trigeminal nucleus and pituitary gland.158,159 Moreover, they have been found in the spinal cords of chickens and rabbits.160
Studies aimed at assessing the role of MLT receptors in the analgesic effect of MLT have shown that MT2 and not MT1 receptors are likely involved.161–164 Yoon and colleagues161 found that an intrathecal injection of MLT dose-dependently attenuated the flinching response during phases 1 and 2 of the formalin test in rats. The analgesic effect of MLT was blocked by the intrathecal administration of luzindole or 4P-PDOT, and consequently, it was mediated by the MT2 receptor. Yu and colleagues163 showed that an intraperitoneal injection of MLT dose-dependently increased the pain threshold of rats in the hot water tail-flick test, an effect that was blocked by luzindole. The authors claimed that the antinociceptive effect of MLT was consequently mediated by the MT2 receptor. Even though luzindole has 16- to 26-fold greater affinity for the MT2 than the MT1 receptor,5 it is a relatively nonselective antagonist; therefore, the conclusion by Yu and colleagues163 needs further support, for instance, with the use of the selective MT2 receptor antagonists 4P-PDOT or K-185.
In a rat model of neuropathic pain, the tactile allodynia induced by L5/L6 spinal nerve ligation was reversed by oral or intrathecal administration of MLT.164 This effect was the result of the activation of spinal MT2 receptors, since it was blocked by an intrathecal injection of luzindole or 4P-PDOT. However, it is important to note that the effect of MLT was also blocked by the subcutaneous and intrathecal administration of naltrexone, thus indicating that opioids are also likely involved in the analgesic properties of MLT.164
Arreola-Espino and colleagues162 found that the oral administration of MLT dose-dependently reduced flinching behaviour in the 0.5% formalin test and tactile allodynia in diabetic rats. Remarkably, the MT2 receptor antagonist K-185 blocked the effects due to MLT treatment.
Altogether these studies support a role for MT2 receptors in mediating analgesic properties of MLT, but no studies have yet specifically looked at the neurobiological mechanism through which the MT2 receptor activation induces analgesia.
MT2 receptors in other diseases
There are a number of examples in which naturally occurring mutations and polymorphisms of known GPCRs are directly related to human diseases, thus providing direct evidence for their therapeutic relevance.6 Given that MT2 receptors are part of GPCRs, it is not surprising that during the last decade several investigations have discovered an association between variations in the gene encoding the MT2 receptor (MTNR1B) and genetic diseases. For example, genome-wide association studies have found associations between variations in MTNR1B and insulin and glucose levels, and thus diabetes. A common variant in the MTNR1B single nucleotide polymorphism (SNP) rs10830963 was found to be associated with an increase in fasting glucose and insulin levels165–168 and was also predictive of future development of type 2 diabetes.165 Interestingly, this genetic variant was also found to be associated with increased risk for gestational diabetes mellitus169 and with diabetes-related traits in overweight and obese children and adolescents.170 Two other MTNR1B SNP variants, rs1387153171 and rs2166706,172 were also found to be associated with plasma glucose levels and increased risk of type 2 diabetes. A recent genetic study has further supported the firm functional link between MT2 receptors and type 2 diabetes risk. Sequencing of 2 exons of MTNR1B in 7632 unrelated European individuals with known glycemic status (among whom 2186 participants had type 2 diabetes), revealed that the rarest variants strongly contributed to increased type 2 diabetes risk.173 Of note, among the rare variants, only those leading to the loss of function of MT2 receptors were strongly associated with type 2 diabetes risk.
A study by Staiger and colleagues174 investigated the possible pathophysiological mechanism through which the common genetic variations within MTNR1B were linked to increased risk of type 2 diabetes, showing that they markedly impacted β cell function.
The possible link between MT2 receptors and type 2 diabetes deserves further investigation, especially considering that type 2 diabetes is one of the most common chronic diseases in both developed and developing countries and that its prevalence is expected to markedly increase in the coming years.175
The MT2 receptors have also been studied in several other physiologic and pathophysiological processes, such as Parkinson disease,176 adolescent idiopathic scoliosis,177 rheumatoid arthritis,178 cell-mediated and humoural immunity,179 cardiovascular functions180 and ischemic neuronal damage.181 Adi and colleagues,176 in an RT-PCR–based study in postmortem tissue, found that patients with sporadic Parkinson disease showed decreased expression of MT2 receptors in brain areas, such as the substantia nigra and the amygdala, that are heavily involved in the etiopathology of the disease.
The MT2 receptors also appear to mediate the neuroprotective effect of MLT after ischemic brain injury.181 In this study, 5 minutes of transient cerebral ischemia was applied to gerbils; MT2 receptor immunoreactivity and protein levels were increased in the CA1 region of the hippocampus after ischemic damage, and the receptors were colocalized with astrocytes and not microglia.
These findings open up many potential avenues for research on MT2 receptors as the primary mediator of the physiologic and pathophysiological effects of MLT. Unfortunately, these studies are still limited and sometimes controversial. Consequently, they do not allow general conclusions to be drawn. Nevertheless, the social burden and lack of effective treatments for diseases such as Parkinson disease, myocardial infarction and rheumatoid arthritis will hopefully provide the necessary impetus for extending research efforts and investing further resources into trying to establish whether MT2 receptors are associated with or linked to these debilitating pathologies.
Conclusion
Melatonin is an important mediator of a wide range of physiologic functions, and alterations in the MLT systems may be implicated in the etiopathogenesis of several diseases. This neurohormone acts on its targets at cellular and intracellular levels through receptor-dependent (but also receptor-independent) mechanisms.1,3 In this review, we focused our attention on the role of the G-protein coupled MT2 receptor in mediating the effects of MLT on brain function. We were also interested in examining the role of MT2 receptors in some physiologic/pathological conditions, such as diabetes, stroke and myocardial infarction, that exhibit a higher grade of co-morbidity with neurologic/psychiatric diseases.182–185 It can be hypothesized that MT2 receptors may represent a common etiological determinant whose dysfunction can lead to several comorbid conditions. Further investigation is needed to understand how MT2 receptors can be implicated in these comorbid diseases. For example, pharmacological and genetic studies in rodents corroborated the involvement of MT2 receptors in the modulation of sleep, particularly NREMS, and a relationship between sleep disorders and glucose metabolism has been extensively demonstrated by several studies. In particular, sleep disorders are a risk factor for obesity and type 2 diabetes.186–189
Interestingly, one genome-wide association study recently revealed a possible association between sleep disorders and diabetes due to the SNP rs10830963 in the MTNR1B gene.190
Similarly, depression, pain and insomnia are closely related in fibromyalgia, a medical condition estimated to affect 5 million adults in the United States191 and for which few treatments are currently available. It is possible that MT2 receptor agonists or partial agonists may represent a novel therapeutic approach for this multisymptomatic disease. The literature reviewed herein points to the MT2 receptor as a common etiological factor, and experimental efforts in this direction may lead to the development of novel synergic therapeutic strategies.
Several investigations have been conducted on MT2 receptors in different psychiatric/neurologic diseases, and these preliminary positive findings with respect to disease pathology and treatment mark only the beginning of a fruitful new era in psychopharmacology. Historically, the super family of GPCRs has proven to be among the most successful drug targets, such as the muscarinic receptors, the adrenoreceptors, DA and 5-HT receptors, opioids, angiotensine, prostglandine, histamine, leukotriene and several hormones. In the past 30 years several hundred new drugs targeting GPCRs as agonists or antagonists have been registered,192 and many of them reached the market.6 Consequently, being part of this super family, MT2 receptors have great potential for pioneer drug discovery. The recent development of novel selective MT1 and MT2 receptor ligands will undoubtedly help researchers attain this goal. Thanks to this advancement in drug discovery, the role of the MT2 receptor may be further elucidated, and selective MT1 and MT2 receptor ligands may be tested in clinical settings to target diseases that are in dire need of more effective therapeutic strategies and better treatment outcomes.
Acknowledgments
This work was supported by grants from the Fonds de la Recherche en Santé du Québec (FRSQ), the Canadian Institutes of Health Research (CIHR), the Canadian Foundation for Innovation (CFI), MSBi Valorisation, the McGill University Health Center (MUHC).
Footnotes
Competing interests: None declared by S. Comai. G. Gobbi has received payment for lectures from Lilly and AstraZeneca. She is also the inventor of the patent Pub. No. WO/2007/079593, International Application No. PCT/CA2007/000055).
Contributors: Both authors contributed equally to this work.
References
- 1.Arendt J. Melatonin. Clin Endocrinol (Oxf) 1988;29:205–29. doi: 10.1111/j.1365-2265.1988.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 2.Waldhauser F, Dietzel M. Daily and annual rhythms in human melatonin secretion: role in puberty control. Ann N Y Acad Sci. 1985;453:205–14. doi: 10.1111/j.1749-6632.1985.tb11811.x. [DOI] [PubMed] [Google Scholar]
- 3.Reiter RJ. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab. 2003;17:273–85. doi: 10.1016/s1521-690x(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 4.Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin — a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011;93:350–84. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Dubocovich ML, Delagrange P, Krause DN, et al. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–80. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomsen WJ, Behan DP. 2.20 — G protein-coupled receptors. In: John BT, David JT, editors. Comprehensive Medicinal Chemistry II. Oxford: Elsevier; 2007. pp. 771–826. [Google Scholar]
- 7.Nosjean O, Ferro M, Coge F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000;275:31311–7. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 8.Rivara S, Pala D, Lodola A, et al. MT(1) — Selective melatonin receptor ligands: synthesis, pharmacological evaluation, and molecular dynamics investigation of N-{[(3-O-Substituted)anilino]alkyl} amides. ChemMedChem. 2012 Aug 27; doi: 10.1002/cmdc.201200303. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Mor M, Rivara S, Pala D, et al. Recent advances in the development of melatonin MT(1) and MT(2) receptor agonists. Expert Opin Ther Pat. 2010;20:1059–77. doi: 10.1517/13543776.2010.496455. [DOI] [PubMed] [Google Scholar]
- 10.Kondoh T, Uneyama H, Nishino H, et al. Melatonin reduces cerebral edema formation caused by transient forebrain ischemia in rats. Life Sci. 2002;72:583–90. doi: 10.1016/s0024-3205(02)02256-7. [DOI] [PubMed] [Google Scholar]
- 11.Liu RY, Zhou JN, van Heerikhuize J, et al. Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J Clin Endocrinol Metab. 1999;84:323–7. doi: 10.1210/jcem.84.1.5394. [DOI] [PubMed] [Google Scholar]
- 12.Zisapel N. Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol Neurobiol. 2001;21:605–16. doi: 10.1023/a:1015187601628. [DOI] [PubMed] [Google Scholar]
- 13.Genovese T, Mazzon E, Muia C, et al. Attenuation in the evolution of experimental spinal cord trauma by treatment with melatonin. J Pineal Res. 2005;38:198–208. doi: 10.1111/j.1600-079X.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 14.Maestroni GJ, Sulli A, Pizzorni C, et al. Melatonin in rheumatoid arthritis: synovial macrophages show melatonin receptors. Ann N Y Acad Sci. 2002;966:271–5. doi: 10.1111/j.1749-6632.2002.tb04226.x. [DOI] [PubMed] [Google Scholar]
- 15.Peres MF. Melatonin, the pineal gland and their implications for headache disorders. Cephalalgia. 2005;25:403–11. doi: 10.1111/j.1468-2982.2005.00889.x. [DOI] [PubMed] [Google Scholar]
- 16.Iuvone PM, Tosini G, Pozdeyev N, et al. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–56. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Cagnacci A, Arangino S, Angiolucci M, et al. Potentially beneficial cardiovascular effects of melatonin administration in women. J Pineal Res. 1997;22:16–9. doi: 10.1111/j.1600-079x.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 18.Doolen S, Krause DN, Dubocovich ML, et al. Melatonin mediates two distinct responses in vascular smooth muscle. Eur J Pharmacol. 1998;345:67–9. doi: 10.1016/s0014-2999(98)00064-8. [DOI] [PubMed] [Google Scholar]
- 19.Sewerynek E. Melatonin and the cardiovascular system. Neuroendocrinol Lett. 2002;23(Suppl 1):79–83. [PubMed] [Google Scholar]
- 20.Blask DE, Sauer LA, Dauchy RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113–32. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 21.Sofic E, Rimpapa Z, Kundurovic Z, et al. Antioxidant capacity of the neurohormone melatonin. J Neural Transm. 2005;112:349–58. doi: 10.1007/s00702-004-0270-4. [DOI] [PubMed] [Google Scholar]
- 22.Reppert SM, Godson C, Mahle CD, et al. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995;92:8734–8. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petit L, Lacroix I, de Coppet P, et al. Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′-5′-monophosphate pathway. Biochem Pharmacol. 1999;58:633–9. doi: 10.1016/s0006-2952(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 24.von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–62. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 25.Witt-Enderby PA, Bennett J, Jarzynka MJ, et al. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 2003;72:2183–98. doi: 10.1016/s0024-3205(03)00098-5. [DOI] [PubMed] [Google Scholar]
- 26.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 27.Mazzucchelli C, Pannacci M, Nonno R, et al. The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res. 1996;39:117–26. doi: 10.1016/0169-328x(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 28.Sallinen P, Saarela S, Ilves M, et al. The expression of MT1 and MT2 melatonin receptor mRNA in several rat tissues. Life Sci. 2005;76:1123–34. doi: 10.1016/j.lfs.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Musshoff U, Riewenherm D, Berger E, et al. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002;12:165–73. doi: 10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- 30.Ahn SK, Khalmuratova R, Hah YS, et al. Immunohistochemical and biomolecular identification of melatonin 1a and 1b receptors in rat vestibular nuclei. Auris Nasus Larynx. 2012;39:479–83. doi: 10.1016/j.anl.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother. 2006;60:97–108. doi: 10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Angeloni D, Longhi R, Fraschini E. Production and characterization of antibodies directed against the human melatonin receptors Mel-1a (mt1) and Mel-1b (MT2) Eur J Histochem. 2000;44:199–204. [PubMed] [Google Scholar]
- 33.Ochoa-Sanchez R, Comai S, Lacoste B, et al. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31:18439–52. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunt AE, Al-Ghoul WM, Gillette MU, et al. Activation of MT2 melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–8. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- 35.Wu YH, Ursinus J, Zhou JN, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. doi: 10.1016/j.jad.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Wan Q, Man HY, Liu F, et al. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999;2:401–3. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Weaver DR, Jin XW, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 38.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 39.Axelrod J, Wurtman RJ, Winget CM. Melatonin synthesis in the hen pineal gland and its control by light. Nature. 1964;201:1134. doi: 10.1038/2011134a0. [DOI] [PubMed] [Google Scholar]
- 40.Jin X, von Gall C, Pieschl RL, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003;23:1054–60. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeffer M, Rauch A, Korf HW, et al. The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol Int. 2012;29:415–29. doi: 10.3109/07420528.2012.667859. [DOI] [PubMed] [Google Scholar]
- 42.Weaver DR, Liu C, Reppert SM. Nature’s knockout: the Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol Endocrinol. 1996;10:1478–87. doi: 10.1210/mend.10.11.8923472. [DOI] [PubMed] [Google Scholar]
- 43.Dubocovich ML. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007;8:34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Rivara S, Lodola A, Mor M, et al. N-(substituted-anilinoethyl) amides: design, synthesis, and pharmacological characterization of a new class of melatonin receptor ligands. J Med Chem. 2007;50:6618–26. doi: 10.1021/jm700957j. [DOI] [PubMed] [Google Scholar]
- 45.Zlotos DP. Recent progress in the development of agonists and antagonists for melatonin receptors. Curr Med Chem. 2012;19:3532–49. doi: 10.2174/092986712801323153. [DOI] [PubMed] [Google Scholar]
- 46.Faust R, Garratt PJ, Jones R, et al. Mapping the melatonin receptor. 6. Melatonin agonists and antagonists derived from 6H-isoindolo[2,1-a]indoles, 5,6-dihydroindolo[2,1-a]isoquinolines, and 6,7-dihydro-5H-benzo[c]azepino[2,1-a]indoles. J Med Chem. 2000;43:1050–61. doi: 10.1021/jm980684+. [DOI] [PubMed] [Google Scholar]
- 47.Dubocovich ML, Masana MI, Iacob S, et al. Melatonin receptor antagonists that differentiate between the human Mel(1a), and Mel(1b) recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML(1) presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:365–75. doi: 10.1007/pl00004956. [DOI] [PubMed] [Google Scholar]
- 48.Audinot V, Mailliet F, Lahaye-Brasseur C, et al. New selective ligands of human cloned melatonin MT1 and MT2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:553–61. doi: 10.1007/s00210-003-0751-2. [DOI] [PubMed] [Google Scholar]
- 49.Fisher SP, Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett. 2009;457:93–6. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohlsen RI, Pilowsky LS. The place of partial agonism in psychiatry: recent developments. J Psychopharmacol. 2005;19:408–13. doi: 10.1177/0269881105053308. [DOI] [PubMed] [Google Scholar]
- 51.Zhu BT. Mechanistic explanation for the unique pharmacologic properties of receptor partial agonists. Biomed Pharmacother. 2005;59:76–89. doi: 10.1016/j.biopha.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 52.Koike T, Hoashi Y, Takai T, et al. 1,6-Dihydro-2H-indeno[5,4-b] furan derivatives: design, synthesis, and pharmacological characterization of a novel class of highly potent MT(2)-selective agonists. J Med Chem. 2011;54:3436–44. doi: 10.1021/jm200221q. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y, Ho MK, Chan KH, et al. Synthesis of substituted N-[3-(3-methoxyphenyl)propyl] amides as highly potent MT(2)-selective melatonin ligands. Bioorg Med Chem Lett. 2010;20:2582–5. doi: 10.1016/j.bmcl.2010.02.084. [DOI] [PubMed] [Google Scholar]
- 54.Chan KH, Hu Y, Ho MK, et al. Characterization of substituted phenylpropylamides as highly selective agonists at the melatonin MT2 receptor. Curr Med Chem. 2013;20:289–300. doi: 10.2174/092986713804806649. [DOI] [PubMed] [Google Scholar]
- 55.Mésangeau C, Fraise M, Delagrange P, et al. Preparation and pharmacological evaluation of a novel series of 2-(phenylthio)benzo[b] thiophenes as selective MT2 receptor ligands. Eur J Med Chem. 2011;46:1835–40. doi: 10.1016/j.ejmech.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 56.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–33. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 57.Born J. Slow-wave sleep and the consolidation of long-term memory. World J Biol Psychiatry. 2010;11(Suppl 1):16–21. doi: 10.3109/15622971003637637. [DOI] [PubMed] [Google Scholar]
- 58.Marshall L, Helgadottir H, Molle M, et al. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 59.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 60.Madsen PL, Schmidt JF, Wildschiodtz G, et al. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. J Appl Physiol. 1991;70:2597–601. doi: 10.1152/jappl.1991.70.6.2597. [DOI] [PubMed] [Google Scholar]
- 61.Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during stage 2 sleep in man. Brain Res. 1992;571:149–53. doi: 10.1016/0006-8993(92)90522-b. [DOI] [PubMed] [Google Scholar]
- 62.Tasali E, Leproul R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sayk F, Teckentrup C, Becker C, et al. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R191–7. doi: 10.1152/ajpregu.00368.2009. [DOI] [PubMed] [Google Scholar]
- 64.Thase ME. Depression, sleep, and antidepressants. J Clin Psychiatry. 1998;59:55–65. [PubMed] [Google Scholar]
- 65.Holmes SW, Sugden D. Effects of melatonin on sleep and neurochemistry in the rat. Br J Pharmacol. 1982;76:95–101. doi: 10.1111/j.1476-5381.1982.tb09194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendelson WB, Gillin JC, Dawson SD, et al. Effects of melatonin and propranolol on sleep of the rat. Brain Res. 1980;201:240–4. doi: 10.1016/0006-8993(80)90793-3. [DOI] [PubMed] [Google Scholar]
- 67.Mouret J, Coindet J, Chouvet G. Effect of pinealectomy on sleep stages and rhythms of male rat. Brain Res. 1974;81:97–105. doi: 10.1016/0006-8993(74)90480-6. [DOI] [PubMed] [Google Scholar]
- 68.Mailliet F, Galloux P, Poisson D. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats. Psychopharmacology (Berl) 2001;156:417–26. doi: 10.1007/s002130100769. [DOI] [PubMed] [Google Scholar]
- 69.Wang F, Li J, Wu C, et al. The GABA(A) receptor mediates the hypnotic activity of melatonin in rats. Pharmacol Biochem Behav. 2003;74:573–8. doi: 10.1016/s0091-3057(02)01045-6. [DOI] [PubMed] [Google Scholar]
- 70.Yukuhiro N, Kimura H, Nishikawa H, et al. Effects of ramelteon (TAK-375) on nocturnal sleep in freely moving monkeys. Brain Res. 2004;1027:59–66. doi: 10.1016/j.brainres.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 71.Tobler I, Jaggi K, Borbely AA. Effects of melatonin and the melatonin receptor agonist S-20098 on the vigilance states, EEG spectra, and cortical temperature in the rat. J Pineal Res. 1994;16:26–32. doi: 10.1111/j.1600-079x.1994.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 72.Miyamoto M, Nishikawa H, Doken Y, et al. The sleep-promoting action of ramelteon (TAK-375) in freely moving cats. Sleep. 2004;27:1319–25. doi: 10.1093/sleep/27.7.1319. [DOI] [PubMed] [Google Scholar]
- 73.Zhdanova IV. Melatonin as a hypnotic: pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Zhdanova IV, Wurtman RJ, Lynch HJ, et al. Sleep-inducing effects of low doses of melatonin ingested in the evening. Clin Pharmacol Ther. 1995;57:552–8. doi: 10.1016/0009-9236(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 75.Luthringer R, Muzet M, Zisapel N, et al. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int Clin Psychopharmacol. 2009;24:239–49. doi: 10.1097/YIC.0b013e32832e9b08. [DOI] [PubMed] [Google Scholar]
- 76.Brzezinski A, Vangel MG, Wurtman RJ, et al. Effects of exogenous melatonin on sleep: a meta-analysis. Sleep Med Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Nave R, Herer P, Haimov I, et al. Hypnotic and hypothermic effects of melatonin on daytime sleep in humans: lack of antagonism by flumazenil. Neurosci Lett. 1996;214:123–6. doi: 10.1016/0304-3940(96)12899-8. [DOI] [PubMed] [Google Scholar]
- 78.James SP, Mendelson WB, Sack DA, et al. The effect of melatonin on normal sleep. Neuropsychopharmacology. 1987;1:41–4. doi: 10.1016/0893-133x(87)90008-x. [DOI] [PubMed] [Google Scholar]
- 79.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mendelson WB. Efficacy of melatonin as a hypnotic agent. J Biol Rhythms. 1997;12:651–6. doi: 10.1177/074873049701200621. [DOI] [PubMed] [Google Scholar]
- 81.Turek FW, Gillette MU. Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists. Sleep Med. 2004;5:523–32. doi: 10.1016/j.sleep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 82.Tzischinsky O, Lavie P. Melatonin possesses time-dependent hypnotic effects. Sleep. 1994;17:638–45. doi: 10.1093/sleep/17.7.638. [DOI] [PubMed] [Google Scholar]
- 83.McGechan A, Wellington K. Ramelteon. CNS Drugs. 2005;19:1057–65. doi: 10.2165/00023210-200519120-00007. [DOI] [PubMed] [Google Scholar]
- 84.Rivara S, Vacondio F, Fioni A, et al. N-(Anilinoethyl)amides: design metabolically stable, selective and synthesis of melatonin receptor ligands. ChemMedChem. 2009;4:1746–55. doi: 10.1002/cmdc.200900240. [DOI] [PubMed] [Google Scholar]
- 85.Steriade M. Cellular substrates of brain rhythms. In: Niedermeyer E, Lopes Da Silva FH, editors. Electroencephalography: basic principles, clinical applications, and related fields. Philadelphia (PA): Lippincott Williams & Wilkins; 1993. [Google Scholar]
- 86.Houser CR, Vaughn JE, Barber RP, et al. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–54. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- 87.Bal T, McCormick DA. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993;468:669–91. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steriade M, Deschenes M, Domich L, et al. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985;54:1473–97. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 89.Steriade M, Dossi RC, Nunez A. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta-waves — cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11:3200–17. doi: 10.1523/JNEUROSCI.11-10-03200.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nuñez A, Dossi RC, Contreras D, et al. Intracellular evidence for incompatibility between spindle and delta-oscillations in thalamocortical neurons of cat. Neuroscience. 1992;48:75–85. doi: 10.1016/0306-4522(92)90339-4. [DOI] [PubMed] [Google Scholar]
- 91.Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005;28:317–24. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 92.Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22:337–45. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- 93.Llinás RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 94.Dubocovich ML, Yun K, Al-Ghoul WM, et al. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–20. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 95.Diniz Behn CG, Klerman EB, Mochizuki T, et al. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 2010;33:297–306. doi: 10.1093/sleep/33.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thakkar MM. Histamine in the regulation of wakefulness. Sleep Med Rev. 2011;15:65–74. doi: 10.1016/j.smrv.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Romanowski CPN, Fenzl T, Flachskamm C, et al. Central deficiency of corticotropin-releasing hormone receptor type 1 (CRH-R1) abolishes effects of CRH on NREM but not on REM sleep in mice. Sleep. 2010;33:427–36. doi: 10.1093/sleep/33.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Qu WM, Xu XH, Yan MM, et al. Essential role of dopamine D-2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci. 2010;30:4382–9. doi: 10.1523/JNEUROSCI.4936-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Monti JM. The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep. Sleep Med Rev. 2010;14:319–27. doi: 10.1016/j.smrv.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 100.De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003;7:423–40. doi: 10.1053/smrv.2002.0252. [DOI] [PubMed] [Google Scholar]
- 101.Comai S, Ochoa-Sanchez R, Gobbi G. Sleep-wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav Brain Res. 2013;243:231–8. doi: 10.1016/j.bbr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 102.Sirota A, Csicsvari J, Buhl D, et al. Communication between neo-cortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–9. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gershell L. Insomnia market. Nat Rev Drug Discov. 2006;5:15–6. doi: 10.1038/nrd1932. [DOI] [PubMed] [Google Scholar]
- 104.Morin CM, LeBlanc M, Daley M, et al. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 105.Wisor JP, Morairty SR, Huynh NT, et al. Gene expression in the rat cerebral cortex: comparison of recovery sleep and hypnotic-induced sleep. Neuroscience. 2006;141:371–8. doi: 10.1016/j.neuroscience.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ockert W. A new dawn in the sleep disorders pipeline? Nat Rev Drug Discov. 2012;11:595–6. doi: 10.1038/nrd3789. [DOI] [PubMed] [Google Scholar]
- 107.Ochoa-Sanchez R, Rainer Q, Comai S, et al. Anxiolytic effects of the melatonin MT(2) receptor partial agonist UCM765: comparison with melatonin and diazepam. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:318–25. doi: 10.1016/j.pnpbp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 108.Kopp C, Rudolph U, Low K, et al. Modulation of rhythmic brain activity by diazepam: GABA(A) receptor subtype and state specificity. Proc Natl Acad Sci U S A. 2004;101:3674–9. doi: 10.1073/pnas.0306975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Lier H, Drinkenburg WHIM, van Eeten YJW, et al. Effects of diazepam and zolpidem on EEG beta frequencies are behaviorspecific in rats. Neuropharmacology. 2004;47:163–74. doi: 10.1016/j.neuropharm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 110.Association AP. Diagnostic and Statistical Manual of Mental Disorders. ed 4. Washington (DC): American Psychiatric Press; 1994. [Google Scholar]
- 111.Golombek DA, Martini M, Cardinali DP. Melatonin as an anxiolytic in rats: time dependence and interaction with the central GABAergic system. Eur J Pharmacol. 1993;237:231–6. doi: 10.1016/0014-2999(93)90273-k. [DOI] [PubMed] [Google Scholar]
- 112.Golus P, King MG. The effects of melatonin on open field behavior. Pharmacol Biochem Behav. 1981;15:883–5. doi: 10.1016/0091-3057(81)90048-4. [DOI] [PubMed] [Google Scholar]
- 113.Papp M, Litwa E, Gruca P, et al. Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav Pharmacol. 2006;17:9–18. doi: 10.1097/01.fbp.0000181601.72535.9d. [DOI] [PubMed] [Google Scholar]
- 114.Garfinkel D, Zisapel N, Wainstein J, et al. Facilitation of benzodiazepine discontinuation by melatonin: a new clinical approach. Arch Intern Med. 1999;159:2456–60. doi: 10.1001/archinte.159.20.2456. [DOI] [PubMed] [Google Scholar]
- 115.Nava F, Carta G. Melatonin reduces anxiety induced by lipopolysaccharide in the rat. Neurosci Lett. 2001;307:57–60. doi: 10.1016/s0304-3940(01)01930-9. [DOI] [PubMed] [Google Scholar]
- 116.Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- 117.Larson J, Jessen RE, Uz T, et al. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett. 2006;393:23–6. doi: 10.1016/j.neulet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 118.Nandi A, Beard JR, Galea S. Epidemiologic heterogeneity of common mood and anxiety disorders over the lifecourse in the general population: a systematic review. BMC Psychiatry. 2009;9:31. doi: 10.1186/1471-244X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Green S. Benzodiazepines, putative anxiolytics and animal models of anxiety. Trends Neurosci. 1991;14:101–4. doi: 10.1016/0166-2236(91)90070-b. [DOI] [PubMed] [Google Scholar]
- 120.Rosenstein RE, Cardinali DP. Melatonin increases in vivo GABA accumulation in rat hypothalamus, cerebellum, cerebral cortex and pineal gland. Brain Res. 1986;398:403–6. doi: 10.1016/0006-8993(86)91505-2. [DOI] [PubMed] [Google Scholar]
- 121.Lemoine P, Garfinkel D, Laudon M, et al. Prolonged-release melatonin for insomnia — an open-label long-term study of efficacy, safety, and withdrawal. Ther Clin Risk Manag. 2011;7:301–11. doi: 10.2147/TCRM.S23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 123.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 124.Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 125.Gerrow K, Triller A. Synaptic stability and plasticity in a floating world. Curr Opin Neurobiol. 2010;20:631–9. doi: 10.1016/j.conb.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 126.Gaiarsa J-L, Caillard O, Ben-Ari Y. Long-term plasticity at GABA-ergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–70. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- 127.Abraham WC, Logan B, Greenwood JM, et al. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–34. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cooke SF, Bliss TVP. Plasticity in the human central nervous system. Brain. 2006;129:1659–73. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- 129.Bob P, Fedor-Freybergh P. Melatonin, consciousness, and traumatic stress. J Pineal Res. 2008;44:341–7. doi: 10.1111/j.1600-079X.2007.00540.x. [DOI] [PubMed] [Google Scholar]
- 130.El-Sherif Y, Tesoriero J, Hogan MV, et al. Melatonin regulates neuronal plasticity in the hippocampus. J Neurosci Res. 2003;72:454–60. doi: 10.1002/jnr.10605. [DOI] [PubMed] [Google Scholar]
- 131.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 132.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–50. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 134.Laudon M, Nir I, Zisapel N. Melatonin receptors in discrete brain areas of the male rat. Impact of aging on density and on circadian rhythmicity. Neuroendocrinology. 1988;48:577–83. doi: 10.1159/000125066. [DOI] [PubMed] [Google Scholar]
- 135.Nonno R, Lucini V, Stankov B, et al. 2-[125I]iodomelatonin binding sites in the bovine hippocampus are not sensitive to guanine nucleotides. Neurosci Lett. 1995;194:113–6. doi: 10.1016/0304-3940(95)11742-f. [DOI] [PubMed] [Google Scholar]
- 136.Wang LM, Suthana NA, Chaudhury D, et al. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–7. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Takahashi Y, Okada T. Involvement of the nitric oxide cascade in melatonin-induced inhibition of long-term potentiation at hippocampal CA1 synapses. Neurosci Res. 2011;69:1–7. doi: 10.1016/j.neures.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 138.El-Sherif Y, Witt-Enderby P, Li PK, et al. The actions of a charged melatonin receptor ligand, TMEPI, and an irreversible MT2 receptor agonist, BMNEP, on mouse hippocampal evoked potentials in vitro. Life Sci. 2004;75:3147–56. doi: 10.1016/j.lfs.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 139.Di Carlo M, Giacomazza D, San Biagio PL. Alzheimer’s disease: biological aspects, therapeutic perspectives and diagnostic tools. J Phys Condens Matter. 2012;24:244102. doi: 10.1088/0953-8984/24/24/244102. [DOI] [PubMed] [Google Scholar]
- 140.Savaskan E, Ayoub MA, Ravid R, et al. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J Pineal Res. 2005;38:10–6. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 141.Savaskan E, Jockers R, Ayoub M, et al. The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer’s disease. Curr Alzheimer Res. 2007;4:47–51. doi: 10.2174/156720507779939823. [DOI] [PubMed] [Google Scholar]
- 142.Brunner P, Sozer-Topcular N, Jockers R, et al. Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer’s disease. Eur J Histochem. 2006;50:311–6. [PubMed] [Google Scholar]
- 143.Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 144.Paul CM, Magda G, Abel S. Spatial memory: theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res. 2009;203:151–64. doi: 10.1016/j.bbr.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 145.Bunney WE, Bunney BG. Molecular clock genes in man and lower animals: possible implications for circadian abnormalities in depression. Neuropsychopharmacology. 2000;22:335–45. doi: 10.1016/S0893-133X(99)00145-1. [DOI] [PubMed] [Google Scholar]
- 146.Overstreet DH, Pucilowski O, Retton MC, et al. Effects of melatonin receptor ligands on swim test immobility. Neuroreport. 1998;9:249–53. doi: 10.1097/00001756-199801260-00014. [DOI] [PubMed] [Google Scholar]
- 147.Micale V, Arezzi A, Rampello L, et al. Melatonin affects the immobility time of rats in the forced swim test: the role of serotonin neurotransmission. Eur Neuropsychopharmacol. 2006;16:538–45. doi: 10.1016/j.euroneuro.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 148.Binfaré RW, Mantovani M, Budni J, et al. Involvement of dopamine receptors in the antidepressant-like effect of melatonin in the tail suspension test. Eur J Pharmacol. 2010;638:78–83. doi: 10.1016/j.ejphar.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 149.Kopp C, Vogel E, Rettori MC, et al. The effects of melatonin on the behavioural disturbances induced by chronic mild stress in C3H/He mice. Behav Pharmacol. 1999;10:73–83. doi: 10.1097/00008877-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 150.Sumaya IC, Masana MI, Dubocovich ML. The antidepressant-like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J Pineal Res. 2005;39:170–7. doi: 10.1111/j.1600-079X.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 151.Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–31. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- 152.Fava M, Targum SD, Nierenberg AA, et al. An exploratory study of combination buspirone and melatonin SR in major depressive disorder (MDD): a possible role for neurogenesis in drug discovery. J Psychiatr Res. 2012;46:1553–63. doi: 10.1016/j.jpsychires.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 153.de Bodinat C, Guardiola-Lemaitre B, Mocaër E, et al. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9:628–42. doi: 10.1038/nrd3140. [DOI] [PubMed] [Google Scholar]
- 154.Bourin M, Mocaer E, Porsolt R. Antidepressant-like activity of S 20098 (agomelatine) in the forced swimming test in rodents: involvement of melatonin and serotonin receptors. J Psychiatry Neurosci. 2004;29:126–33. [PMC free article] [PubMed] [Google Scholar]
- 155.Galecka E, Szemraj J, Florkowski A, et al. Single nucleotide polymorphisms and mRNA expression for melatonin MT(2) receptor in depression. Psychiatry Res. 2011;189:472–4. doi: 10.1016/j.psychres.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 156.Ambriz-Tututi M, Rocha-Gonzalez HI, Cruz SL, et al. Melatonin: a hormone that modulates pain. Life Sci. 2009;84:489–98. doi: 10.1016/j.lfs.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 157.Wilhelmsen M, Amirian I, Reiter RJ, et al. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res. 2011;51:270–7. doi: 10.1111/j.1600-079X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- 158.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 159.Ambriz-Tututi M, Rocha-Gonzalez H, Cruz S, et al. Melatonin: a hormone that modulates pain. Life Sci. 2009;84:489–98. doi: 10.1016/j.lfs.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 160.Pang SF, Wan Q, Brown G. Melatonin receptors in the spinal cord. Biol Signals. 1997;6:272–83. [PubMed] [Google Scholar]
- 161.Yoon MH, Park HC, Kim WM, et al. Evaluation for the interaction between intrathecal melatonin and clonidine or neostigmine on formalin-induced nociception. Life Sci. 2008;83:845–50. doi: 10.1016/j.lfs.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 162.Arreola-Espino R, Urquiza-Marin H, Ambriz-Tututi M, et al. Melatonin reduces formalin-induced nociception and tactile allodynia in diabetic rats. Eur J Pharmacol. 2007;577:203–10. doi: 10.1016/j.ejphar.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 163.Yu CX, Zhu CB, Xu SF, et al. Selective MT(2) melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci Lett. 2000;282:161–4. doi: 10.1016/s0304-3940(00)00883-1. [DOI] [PubMed] [Google Scholar]
- 164.Ambriz-Tututi M, Granados-Soto V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain. 2007;132:273–80. doi: 10.1016/j.pain.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 165.Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41:82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Prokopenko I, Langenberg C, Florez JC, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dietrich K, Birkmeier S, Schleinitz D, et al. Association and evolutionary studies of the melatonin receptor 1B gene (MTNR1B) in the self-contained population of Sorbs from Germany. Diabet Med. 2011;28:1373–80. doi: 10.1111/j.1464-5491.2011.03374.x. [DOI] [PubMed] [Google Scholar]
- 168.Song JY, Wang HJ, Ma J, et al. Association of the rs10830963 polymorphism in MTNR1B with fasting glucose levels in Chinese children and adolescents. Obes Facts. 2011;4:197–203. doi: 10.1159/000329306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vlassi M, Gazouli M, Paltoglou G, et al. The rs10830963 variant of melatonin receptor MTNR1B is associated with increased risk for gestational diabetes mellitus in a Greek population. Hormones. 2012;11:70–6. doi: 10.1007/BF03401539. [DOI] [PubMed] [Google Scholar]
- 170.Holzapfel C, Siegrist M, Rank M, et al. Association of a MTNR1B gene variant with fasting glucose and HOMA-B in children and adolescents with high BMI-SDS. Eur J Endocrinol. 2011;164:205–12. doi: 10.1530/EJE-10-0588. [DOI] [PubMed] [Google Scholar]
- 171.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 172.Chambers JC, Zhang W, Zabaneh D, et al. Common genetic variation near melatonin receptor MTNR1B contributes to raised plasma glucose and increased risk of type 2 diabetes among Indian Asians and European Caucasians. Diabetes. 2009;58:2703–8. doi: 10.2337/db08-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bonnefond A, Clement N, Fawcett K, et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet. 2012;44:297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Staiger H, Machicao F, Schafer SA, et al. Polymorphisms within the novel type 2 diabetes risk locus MTNR1B determine beta-cell function. PLoS ONE. 2008;3:e3962. doi: 10.1371/journal.pone.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 176.Adi N, Mash DC, Ali Y, et al. Melatonin MT1 and MT2 receptor expression in Parkinson’s disease. Med Sci Monit. 2010;16:BR61–7. [PubMed] [Google Scholar]
- 177.Qiu XS, Tang NL, Yeung HY, et al. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine. 2007;32:1748–53. doi: 10.1097/BRS.0b013e3180b9f0ff. [DOI] [PubMed] [Google Scholar]
- 178.Ha E, Choe BK, Jung KH, et al. Positive relationship between melatonin receptor type 1B polymorphism and rheumatoid factor in rheumatoid arthritis patients in the Korean population. J Pineal Res. 2005;39:201–5. doi: 10.1111/j.1600-079X.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 179.Drazen DL, Nelson RJ. Melatonin receptor subtype MT2 (Mel 1b) and not mt1 (Mel 1a) is associated with melatonin-induced enhancement of cell-mediated and humoral immunity. Neuroendocrinology. 2001;74:178–84. doi: 10.1159/000054684. [DOI] [PubMed] [Google Scholar]
- 180.Ekmekcioglu C, Thalhammer T, Humpeler S, et al. The melatonin receptor subtype MT2 is present in the human cardiovascular system. J Pineal Res. 2003;35:40–4. doi: 10.1034/j.1600-079x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 181.Lee CH, Yoo KY, Choi JH, et al. Melatonin’s protective action against ischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. J Neurosci Res. 2010;88:2630–40. doi: 10.1002/jnr.22430. [DOI] [PubMed] [Google Scholar]
- 182.Carney RM, Blumenthal JA, Stein PK, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–8. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 183.Eaton WW. Epidemiologic evidence on the comorbidity of depression and diabetes. J Psychosom Res. 2002;53:903–6. doi: 10.1016/s0022-3999(02)00302-1. [DOI] [PubMed] [Google Scholar]
- 184.Taylor DJ, Mallory LJ, Lichstein KL, et al. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 185.Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 186.Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–8. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 187.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 189.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–61. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 190.Olsson L, Pettersen E, Ahlbom A, et al. No effect by the common gene variant rs10830963 of the melatonin receptor 1B on the association between sleep disturbances and type 2 diabetes: results from the Nord-Trondelag Health Study. Diabetologia. 2011;54:1375–8. doi: 10.1007/s00125-011-2106-8. [DOI] [PubMed] [Google Scholar]
- 191.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stadel JM, Wilson S, Bergsma DJ. Orphan G protein-coupled receptors: a neglected opportunity for pioneer drug discovery. Trends Pharmacol Sci. 1997;18:430–7. doi: 10.1016/s0165-6147(97)01117-6. [DOI] [PubMed] [Google Scholar]
- 193.Paxinos G, Watson C. Stereotaxic coordinates. San Diego (CA): Academic Press; 2006. The rat brain in stereotaxic coordinates. [Google Scholar]