Abstract
Background
The notion that cerebellar deficits may underlie clinical symptoms in people with schizophrenia is tested by evaluating 2 forms of cerebellar learning in patients with recent-onset schizophrenia. A potential medication effect is evaluated by including patients with or without antipsychotics.
Methods
We assessed saccadic eye movement adaptation and eyeblink conditioning in men with recent-onset schizophrenia who were taking antipsychotic medication or who were antipsychotic-free and in age-matched controls.
Results
We included 39 men with schizophrenia (10 who were taking clozapine, 16 who were taking haloperidol and 13 who were antipsychotic-free) and 29 controls in our study. All participants showed significant saccadic adaptation. Adaptation strength did not differ between healthy controls and men with schizophrenia. The speed of saccade adaptation, however, was significantly lower in men with schizophrenia. They showed a significantly lower increase in the number of conditioned eyeblink responses. Over all experiments, no consistent effects of medication were observed. These outcomes did not correlate with age, years of education, psychopathology or dose of anti psychotics.
Limitations
As patients were not randomized for treatment, an influence of confounding variables associated with medication status cannot be excluded. Individual patients also varied along the schizophrenia spectrum despite the relative homogeneity with respect to onset of illness and short usage of medication. Finally, the relatively small number of participants may have concealed effects as a result of insufficient statistical power.
Conclusion
We found several cerebellar learning deficits in men with schizophrenia that we cannot attribute to the use of antipsychotics. Although this finding, combined with the fact that deficits are already present in patients with recent-onset schizophrenia, could suggest that cerebellar impairments are a trait deficit in people with schizophrenia. This should be confirmed in longitudinal studies.
Introduction
A dysfunction of the cortico–cerebellar–thalamic–cortical circuit (CCTCC) has been implicated in some of the psychopathological processes occurring in people with schizophrenia.1 Andreasen’s hypothesis of “cognitive dysmetria” suggests a disconnectivity in this circuitry, resulting in language discoordination and thought disorder, key aspects of the cognitive deficits seen in people with schizophrenia.1 Although a functional disconnectivity of the CCTCC does not necessarily imply a cerebellar deficit as the primary cause for such symptoms, converging evidence suggests cerebellar anomalies are at least associated with schizophrenia.2,3
The cerebellum is considered to be mainly involved in motor coordination and motor learning.4,5 It is thought to adjust the output of various brain areas involved in movement by evaluating disparities between intention and action, both online and offline. Cerebellar functions are carried out by separate and specific modules, which all have the same circuit archi tecture.6 Several lines of evidence support an analogous role of the cerebellum in cognitive functions, evaluating and fine-tuning neural processes of “higher” cortical functions.7–11
Based on the uniform architecture of the cerebellum, it is plausible that potential cerebellar deficits in people with schizophrenia similarly affect modules involved in both cognitive and motor processes. Studies have suggested that several “neurological soft signs” (NSS) in people with schizophrenia can be attributed to cerebellar dysfunction.12,13 Although most patients with schizophrenia do not clearly show gross movement abnormalities, subtle cerebellar motor control deficits can be masked or compensated for by various mechanisms and are therefore likely to be missed in routine clinical examination. Numerous studies have shown that cerebellar deficits lead to subtle impairments in short-term motor learning, whereas overall motor performance, which also involves various other adaptation mechanisms, is often left intact.14 Therefore, the most sensitive way to observe subtle deficits in cerebellar function is to assess cerebellar plasticity. In the present study this was assessed in treated and untreated men with recent-onset schizophrenia and matched controls using saccade adaptation and eyeblink conditioning. Animal research has demonstrated extensively that changes in cerebellar output underlie both of these forms of short-term motor learning. Both tasks have previously been studied in various groups of patients.15–18
Saccade adaptation describes the gradual modification of reflexive target-directed saccades by displacing the target in midflight of a saccade.19 It has been shown in nonhuman primates that saccade adaptation is driven by changes in activity of Purkinje cells in the cerebellar vermis.20,21 Abnormalities in other types of saccadic eye movements have been abundantly reported in studies on schizophrenia using a gap-overlap paradigm, antisaccades or memory-guided saccades, 3,22,23 but none of these studies has specifically addressed cerebellar learning. Recently, saccade adaptation was examined in patients with chronic schizophrenia with increased NSS24 who showed reduced saccade adaptation.
Eyeblink conditioning is another form of cerebellar-dependent motor learning. For eyeblink conditioning, the essential neuronal memory trace is stored in the cerebellar interpositus nucleus, and neuronal plasticity also occurs in the cerebellar cortex.25 Eyeblink conditioning has been studied in patients with schizophrenia, yielding inconsistent and even conflicting results26 demonstrating a large variation in eyeblink conditioning capacity in these patients, most likely owing to natural interindividual variation in eyeblink conditioning capacity within groups and to experimental differences and imperfections. To our knowledge, no studies until now have explicitly compared medicated and medication-free patients with schizophrenia. Here we compare eyeblink conditioning among antipsychotic-free patients, patients using haloperidol or clozapine, and controls.
Although effects of psychopharmaceuticals on cerebellar function have received little attention, the cerebellum is known to receive serotonergic and noradrenergic afferents as well as cholinergic, dopaminergic and histaminergic projections.27 Therefore antipsychotics affecting these neuromodulatory systems are also likely to influence cerebellar processing. It has been proposed that various neuromodulators influence different forms of cerebellar learning.27 Based on their receptor profiles, various antipsychotics might differentially influence cerebellar learning. Haloperidol, for example, predominantly binds the dopamine D2 receptor, whereas clozapine binds receptors of various neurotransmitters.28 However, a direct link between antipsychotics, neuronal activities in the cerebellum and behaviour is yet to be established.
The first aim of the present study was to assess the influence of schizophrenia on short-term cerebellar motor learning. The second was to investigate whether antipsychotics differentially affect this type of learning.
Methods
Participants
We recruited men with recent-onset schizophrenia aged between 18 and 35 years and age-matched controls for participation in our study. Among patients, the date of illness onset was defined either as the time of first occurrence of positive symptoms or as the time of occurrence of marked limitations in social or occupational functioning, if these latter problems were to show first. Owing to the methodological problems inherent in matching participants on variables such as education or intelligence in schizophrenia research,29 we opted not to try to match the groups in terms of these variables.
All patients were or had been hospitalized in our department, and schizophrenia was diagnosed according to DSM-IV criteria. Diagnoses were made by clinical consensus among clinicians highly experienced working with patients with psychosis and were confirmed from case notes using OPCRIT criteria.30 Patients with symptoms present for fewer than 6 months at the time of examination were reassessed after 6 months to comply with the DSM-IV criteria. We used the Positive and Negative Symptom Scale (PANSS) to assess symptom severity;31 it was administered the day before or after testing. Exclusion criteria were psychiatric comorbidity; substance dependence; any history of serious head trauma; and neurologic, cardiovascular or respiratory disease.
Among patients, those in the antipsychotic-free group were required to be antipsychotic-naive or to be off antipsychotics for at least 4 weeks before participating in the study. We calculated chlorpromazine (CPZ) equivalents for each patient.
Not all participants were tested in both experiments. Participants first completed the eyeblink conditioning task and then the saccade adaptation task. The time between the experiments was about 45 minutes.
The experimental protocol was approved by the Erasmus MC medical ethics committee, according to the 1994 Declaration of Helsinki. All participants provided written informed consent.
Saccade adaptation task
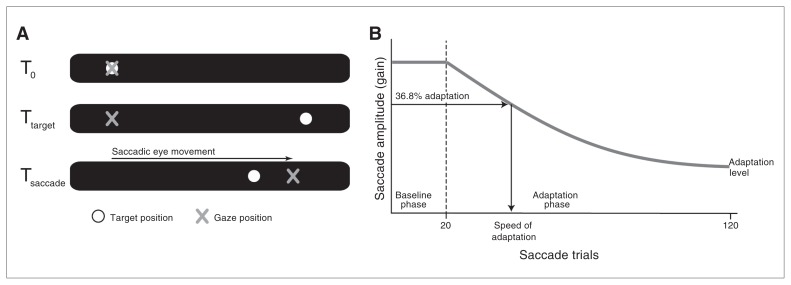
We performed a classic backward saccade adaptation experiment.18 On each trial, a single small, red dot (0.5° of visual angle) was displayed at 10° left of centre on a black screen. After a random interval (1.5–2 s), the dot disappeared and subsequently reappeared 10° right of centre, which evoked a saccade from left to right. In the 20 baseline trials, the dot stayed on the right side of the screen for about 1.5 s, before the next trial started. In the next 100 trials (adaptation phase), the dot on the right stepped 6° (i.e., 30.0% of the target displacement) to the left (backward), about 30 ms after the reflexive rightward saccade was initiated (Fig. 1A). The (random) intertrial interval was between 0.5 and 1.5 s.
Fig. 1.
Saccade adaptation paradigm. (A) At trial onset the participant is looking at the target on the right. After 1.5–2 seconds (at Ttarget) the target jumps to the right, which evokes a saccadic eye movement. In the adaptation phase, the saccade triggers a backward displacement of the target (at Tsaccade). (B) The first 20 trials are baseline trials in which the target is not displaced. During the 100 trials in the adaptation phase, the amplitude of saccadic eye movements gradually decreases. Adaptation strength is calculated by the formula (GainBaseline – GainAdaptation) ÷ 30% GainBaseline, in which GainBaseline is the mean gain in the baseline phase and GainAdaptation is the mean of the last 10 trials of the adaptation phase. Adaptation speed is defined as the number of trials needed to induce 36,8% of the final adaptation at trial 120.
Participants were seated in a darkened room at about a 70 cm distance in front of a 21 inch computer screen covered with a red filter eliminating the monitor’s light reflections. Head movements were restrained using a custom-made biteboard. We recorded binocular eye position using noninvasive infrared video-oculography (EyeLink 2.04, SensoMotoric Instruments) at a sample rate of 250 Hz. Eye position was calibrated with the built-in automatic routine.
For each participant and each trial, saccadic gain was defined as saccadic amplitude divided by target amplitude; a gain of 1 reflects a saccade amplitude of 20°. We calculated basic saccade characteristics (velocity, duration and gain) for the baseline saccades. Adaptation strength was defined as gain difference between the last 10 adaptation trials and baseline, divided by the theoretically expected difference (i.e., 30.0% of average baseline gain; an adaptation strength of 1 corresponds to 100% adaptation). Speed of adaptation was calculated by fitting a decaying natural exponential curve and reflected the number of adaptation trials needed to induce about one-third (1/e = 0.3679) of the final adaptation achieved after 100 trials (Fig. 1B).
Eyeblink conditioning task
The eyeblink conditioning experiment in humans is described in detail elsewhere.17 Briefly, we recorded eyelid movements with a magnetic distance measurement technique (Shebot system, www.neurasmus.com) measuring the distance between the upper and lower eyelid. Participants wore a headset with integrated movie viewing goggles and headphones to which an air puff nozzle was attached. The headset allowed free head movement and provided sound isolation from the environment. Air puffs (20 ms duration) were delivered onto the cornea of the right eye (lateral scleral conjunctiva), with an intensity evoking a single blink reflex (unconditioned stimulus [US]). Before the experiment, stimulus intensity was slowly increased until a single blink reflex was reliably obtained. We did not observe marked differences in sensitivity among the participants. The sound of the movie (background noise) and 650 Hz tones (conditioned stimulus [CS], 75 dB, 520 ms duration) were presented bilaterally through the headphones. While watching the movie, participants received 80 conditioning trials divided into 10 blocks. Each block contained 1 CS-only trial, 1 US-only trial, and 6 paired US+CS trials. In paired trials the air puff was presented with a delay of 500 ms after onset of the CS, so the 2 stimuli ended simultaneously (delayed eyeblink conditioning). Trials were delivered in random order within the blocks, separated by random intervals of 20–30 s. Blocks were separated by about 1 minute. The head-free recording situation and the movie minimized the interference of attention deficits or anxiety.
For each trial, occurrence of an eyeblink was determined automatically and checked manually (for details, see Smit and colleagues17). For each participant and block, the number of conditioned responses (CR; a blink made in response to the tone) was calculated. The rate of CRs per block was defined as the ratio between the number of CRs and the number of CS-only and paired trials (i.e., 7 per block). We defined a learning index as the difference in number of CRs between the first and the last block.
Statistical analysis
We compared basic saccade characteristics (duration, amplitude and peak velocity) and saccade adaptation parameters (adaptation strength and adaptation speed) between patients with schizophrenia and controls using a t test for independent groups. A correction was made to the t statistic in the case of unequal variances (Levene test, p < 0.05). We analyzed the 3 subgroups of patients with univariate analysis of variance (ANOVA).
We analyzed eyeblink conditioning using a mixed-model (group and blocks) repeated-measures ANOVA. We compared the learning index between controls and patients with schizophrenia using a Student t test and among the patient subgroups using univariate ANOVA. Degrees of freedom of all ANOVAs were Greenhouse–Geisser corrected.
Differences in psychopathology as measured by PANSS, age and level of education were analyzed using univariate ANOVA. We compared differences in CPZ equivalents using a t test for independent groups. Finally we performed a cross-correlation analysis (Spearman ρ), comparing all parameters with age, years of education, PANSS subscores and CPZ values. Error probability was predefined at p < 0.05 (2-tailed), and significance level was adjusted for multiple comparisons using Bonferroni correction where appropriate. We performed our analyses using PASW version 18.0 (SPSS 2010).
Results
Participants
We enrolled 39 men with recent-onset schizophrenia (mean illness duration 21.8 ± 16.2 mo, mean age 23.9 yr) and 30 age-matched controls (mean 24.6 yr, t = 0.72, p = 0.47). Most patients had received psychiatric care only for a short time (median of 1 mo), and 75% of patients had received psychiatric care for fewer than 6 months before inclusion. Participant characteristics are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the participants*
| Group; mean ± SD† | Patient subgroup; mean ± SD† | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Characteristic | Controls n = 30 | Schizophrenia n = 39 | p value | Antipsychotic-free, n = 13 | Clozapine n = 10 | Haloperidol n = 16 | p value |
| Age, yr; mean (range) | 24.57 (18–31) | 23.87 (18–35) | 0.47 | 25.5 (20–35) | 22.2 (18–32) | 22.63 (18–32) | 0.21 |
| Education, yr | 11.8 ± 0.41 | 10.46 ± 0.85 | < 0.001 | 10.5 ± 0.88 | 10.8 ± 0.92 | 10.19 ± 0.75 | 0.19 |
| PANSS | |||||||
| Positive | 14.2 ± 5.6 | — | 17.3* ± 4.6 | 12.5 ± 7.1 | 12.8 ± 4.5 | 0.047 | |
| Negative | 18.3 ± 6.1 | — | 17.9 ± 4.8 | 17.8 ± 7.1 | 19.0 ± 6.7 | 0.86 | |
| General | 31.0 ± 6.9 | — | 33.8 ± 8.0 | 27.8 ± 7.0 | 30.7 ± 5.0 | 0.11 | |
| Total | 63.5 ± 14.9 | — | 69.0 ± 14.9 | 58.1 ± 18.4 | 62.4 ± 11.5 | 0.21 | |
| Duration of illness, mo. | 21.8 ± 16.2 | 21.85 ± 18.8 | 30.6 ± 16.5 | 16.2 ± 11.4 | 0.08 | ||
| Range | 1–60 | — | 1–60 | 6–54 | 1–38 | — | |
| CPZ equivalents | 353 ± 132 | 223 ± 62 | 0.013 | ||||
| DDD | 0.88 ± 0.32 | 0.46 ± 0.12 | 0.002 | ||||
CPZ = chlorpromazine; DDD = defined daily dose; PANSS = Positive and Negative Symptoms Scale; SD = standard deviation.
Differences between controls and patients with schizophrenia were assessed using the Student t test. Differences among patient subgroups were assessed using univariate analyses of variance.
Unless otherwise indicated.
Among the patients with schizophrenia, 10 were taking clozapine, 16 were taking haloperidol and 13 were antipsychotic-free. Six of the 13 antipsychotic-free patients were antipsychotic-naive; the other 7 had been off antipsychotics for at least 4 weeks. These 7 patients had been treated previously with either aripiprazol (n = 1), haloperidol (n = 3) or risperidone (n = 3). One antipsychotic-free patient received lorazepam at the time of examination (Table 1). No further medication was given.
Four controls participated only in the saccade adaptation task and 1 participated only in the eyeblink conditioning task. The remaining 25 controls completed both tasks. One patient receiving haloperidol participated only in the eyeblink conditioning task, and 1 patient receiving clozapine participated only in the saccade adaptation task. In all, 26 controls and 38 patients (13 antipsychotic-free, 16 haloperidol, 9 clozapine) participated in the eyeblink conditioning task, and 29 controls and 38 patients (13 antipsychoticfree, 15 haloperidol, 10 clozapine) participated in the sac cade adaptation task.
All participants had normal hearing, normal or corrected-to-normal visual acuity and had no obvious oculomotor disturbances.
Illness-duration was not significantly different among the patient groups (30.6 mo in the clozapine group, 21.9 mo in the medication-free group and 16.2 mo in the haloperidol group, p = 0.08). Patient groups did not differ in years of education and PANSS subscales (all p > 0.15), except for the positive subscale (F2,36 = 3.34, p = 0.047, η2 = 0.16). Medication-free patients had significantly higher PANSS positive values than patients treated with haloperidol (t25.7 = 2.67, p = 0.013) and marginally higher values than patients treated with clozapine (t14.6 = 1.87, p = 0.08). The clozapine group had significantly higher CPZ values than the haloperidol group (353.3 v. 223.1; t11.5 = 2.92, p = 0.013).
Saccade adaptation task
Basic saccade characteristics (peak velocity, duration and gain) did not differ between controls and patients (all p > 0.18) or among the 3 patient subgroups (all p > 0.20; Table 2). Baseline saccade gains did not differ between controls (mean 0.93 ± 0.04) and patients (mean 0.91 ± 0.05, t65 = 1.86, p = 0.07) or among the patient subgroups (Table 2).
Table 2.
Saccade characteristics of controls and patients with schizophrenia*
| Group; mean ± SD | Patient subgroup; mean ± SD | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Characteristic | Controls, n = 29 | Schizophrenia, n = 38† | Antipsychoticfree, n = 13 | Clozapine, n = 10 | Haloperidol, n = 15† |
| Peak velocity, º/s | 634.0 ± 82.5 | 602.1 ± 114.1 | 615.3 ± 93.2 | 643.8 ± 86.9 | 562.0 ± 137.7 |
| Duration, ms | 88.2 ± 17.3 | 91.3 ± 18.0 | 91.9 ± 14.2 | 83.9 ± 7.0 | 96.1 ± 24.3 |
| Pregain | 0.93 ± 0.04 | 0.91 ± 0.05 | 0.93 ± 0.06 | 0.91 ± 0.04 | 0.89 ± 0.05 |
| Postgain | 0.75 ± 0.05 | 0.73 ± 0.06 | 0.73 ± 0.06 | 0.76 ± 0.05 | 0.71 ± 0.06 |
SD = standard deviation.
No differences in basic saccade characteristics (peak velocity, duration, gains) were found among any of the groups. Saccade gains (defined as saccade amplitude/target amplitude) were calculated at baseline (pregain) and after saccade adaptation (postgain) and did not differ significantly between controls and patients with schizophrenia (Student t tests) or among patient subgroups (univariate analyses of variance).
Not significant.
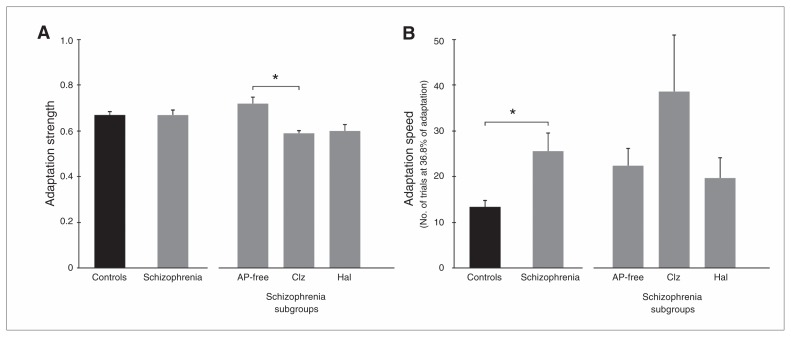
Adaptation strength did not differ between controls (0.67 ± 0.16) and patients (0.67 ± 0.16, t65 = 0.42, p = 0.97; Fig. 2A). The univariate ANOVA revealed significant differences among the patient subgroups (F2,35 = 3.47, p = 0.042, η2 = 0.17). The Tukey post hoc test showed that the clozapine group had reduced adaptation strength compared with antipsychotic-free patients (p = 0.044).
Fig. 2.
Saccade adaptation. (A) Adaptation strength did not differ between 38 patients with schizophrenia and 29 controls. The 10 patients taking clozapine (Clz) showed a lower adaptation strength than the 13 antipsychotic (AP)–free patients. (B) Adaptation speed (expressed as the number of trials needed to reach 36.8% of adaptation) was reduced in patients with schizophrenia compared with controls. The 3 patient groups did not differ from each other. Error bars represent standard errors of the mean. Hal = haloperidol (n = 15). *p < 0.05.
The controls adapted significantly faster than patients (13.4 ± 8.3 v. 25.6 ± 24.1 trials, respectively, t65 = 2.80, p = 0.007; Fig. 2B). The univariate ANOVA revealed no significant differences among the patient subgroups (F2,35 = 1.95, p = 0.16, η2 = 0.10).
Adaptation strength and speed did not correlate (ρ = 0.20, p = 0.11). Furthermore, no significant correlations were found between adaptation strength and speed or between age, years of education, PANSS (sub)scores and CPZ equivalents after Bonferroni correction for multiple comparisons (all ρ < 0.32).
Eyeblink conditioning task
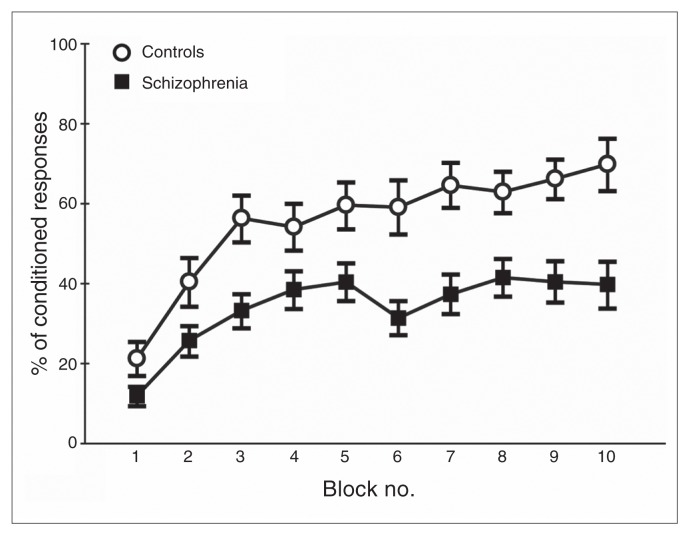
Over the conditioning blocks, patients and controls showed a gradual increase in the number of CRs (Fig. 3). In a preliminary analysis, a mixed-model repeated-measures ANOVA with all blocks as a within-subjects factor and group (patient v. control) as a between-subjects factor revealed significant main effects of block (F6.5,394 = 23.01, p < 0.001, η2 = 0.27) and group (F1,61 = 11.34, p < 0.001, η2 = 0.16), and a trend toward significant interaction (F6.5,394 = 1.972, p = 0.06, η2 = 0.31). In addition, the ANOVA with all blocks as a within-subjects factor and patient subgroup as a between-subjects factor showed a significant main effect of block (F5.8,204 = 8.27, p < 0.001, η2 = 0.19), but no effect of group (F2,35 = 1.74, p = 0.19, η2 = 0.09) or interaction (F11.7,204 = 0.34, p = 0.87, η2 = 0.03).
Fig. 3.
Eyeblink conditioning. The increase in the percentage of conditioned eyeblink responses was significantly less in the 38 patients with schizophrenia (filled squares) than in the 26 controls (open circles). Error bars represent standard errors of the mean.
The learning index between controls (0.48 ± 0.23) and patients (0.26 ± 0.22) differed significantly (t61 = 3.67, p < 0.001). A univariate ANOVA (F2,35 = 0.83, p = 0.92, η2 = 0.01) among the patient subgroups showed no differences in learning index (0.29 ± 0.30 in the haloperidol group, 0.25 ± 0.31 in the clozapine group and 0.31 ± 0.28 in the medication-free group).
No significant correlations were found between learning index and age, years of education, PANSS (sub)scores and CPZ equivalents after Bonferroni correction for multiple comparisons (all ρ < 0.27).
The learning index obtained in the eyeblink conditioning task mildly correlated with the adaption strength (ρ = 0.27, p = 0.039) but not with adaption speed (ρ = −0.21, p = 0.11) obtained in the saccade adaptation task when we considered all participants together. When we considered controls and patients separately, we observed a significant correlation between eyeblink conditioning learning index and adaptation strength in the control group (ρ = 0.43, p = 0.035), but not in the patient group (ρ = 0.20, p = 0.23). The correlations between eyeblink conditioning learning index and adaptation speed were not significant (ρ = −0.15, p = 0.47 in the control group and ρ = −0.16, p = 0.36 in patients with schizophrenia).
Discussion
In the present study we used 2 experiments to investigate the cerebellar learning capacity of patients with schizophrenia. Compared with a group of controls, both medicated and medication-free patients showed deficits in saccade adaptation and eyeblink conditioning. We found no abnormalities in baseline saccade or eyeblink behaviour that could account for cerebellar learning deficits.
Saccade adaptation has only been studied recently in people with chronic schizophrenia.24 Eyeblink conditioning has been investigated in people with schizophrenia with inconsistent results, most likely owing to large interindividual variation and methodological issues and differences.26 In the present study both paradigms were examined in a homogeneous patient group with respect to sex, duration of illness and actual treatment. Thus, confounding effects of long-term use of medication are unlikely.
In contrast with our findings, Picard and colleagues24 reported impaired saccade adaptation strength, but they observed no differences in adaptation speed in patients with schizophrenia. These differences might have resulted from differences in study population, design and analysis. Their study included 12 patients with schizophrenia with a mean duration of illness of 10.5 years, and all patients were using medication in considerable dosage (mean 645 CPZ equivalents). Their calculation of adaptation strength was not based on individual but on mean baseline gain, and adaptation speed was calculated using a repeated-measures ANOVA. We calculated a gradient reflecting the number of adaptation trials needed to induce about one-third (1/e = 0.3679) of the final adaptation.
In our study, adaptation strength did not differ significantly between patients with schizophrenia and controls, revealing no illness-specific deficit in patients. Differences in adaptation strength were found only among patient subgroups, which might suggest an effect of antipsychotics on cerebellar learning. Such effects may increase with higher doses, which might explain the difference between our findings on adaptation strength and those of Picard and colleagues.24
We investigated a potential effect of antipsychotics on cerebellar learning by directly comparing 2 groups of medicated (haloperidol, clozapine) and medication-free patients with schizophrenia. Importantly, patients received no further neurotropic medication, except for 7 patients who used low-dose benzodiazepines. All patient groups, including the medication-free patients, showed similar learning impairments: a reduced number of CRs in the eyeblink conditioning paradigm and a reduced saccade adaptation speed. This indicates that the use of antipsychotics cannot account for all of the observed learning deficits. Haloperidol and clozapine do not appear to induce additional deficits in eyeblink conditioning or saccade adaptation speed. However, in comparison to medication-free patients, patients using clozapine reached lower saccade-adaptation strength. We find cerebellar motor learning deficits in patients with recent-onset schizophrenia either in the presence or absence of anti psychotic medication.
Previous studies on eyeblink conditioning in patients with schizophrenia have reported both normal and abnormal behaviour.26 Our results are in agreement with those of Bolbecker and colleagues,32 who included 13 medication-free patients showing significantly fewer CRs and earlier CR response latencies than controls. The authors also observed no effect of medication in eyeblink conditioning. Several earlier studies of eyeblink conditioning in medication-free patients with schizophrenia reported no deficits or even an increase in CR rate.33,34 Importantly, studies including only medicated patients35,36 all reported eyeblink conditioning deficits. Lubow26 stated that poorer conditioning has only been found with medicated patients, whereas nonmedicated patients either showed better conditioning than controls or no difference. However, this should not be taken to mean that there is no difference between medicated and medication-free patients with schizophrenia, as none of these studies directly compared a substantial group of medication-free patients with patients using antipsychotics. In both studies comparing medicated and medication-free patients,37,38 the number of medication-free patients was too small to permit a reliable statistical evaluation.
We did not observe differences in eyeblink conditioning between medicated and medication-free patients, possibly as a consequence of relatively low-dose antipsychotics. The relative homogeneity of our study population might explain the fact that we found an eyeblink conditioning deficit in medication-free patients, whereas most previous studies did not. The heterogeneity of symptoms classified under “schizophrenia” can induce variation in study populations. This might especially be the case for eyeblink conditioning data, known to show a great interindividual variation in general. Here interindividual variation is allegedly reduced by including only men with recent-onset schizophrenia. Possible effects of long-term use of antipsychotics was thereby avoided.
Methodological differences and imperfections of previous studies have led to a rather inconsistent picture of eyeblink conditioning in patients with schizophrenia. In agreement with the only other study directly comparing medicated and medication-free patients,32 our data indicate that patients with schizophrenia show impaired eyeblink conditioning, irrespective of medication status.
Although we did not perform a study with a naturalistic design, we aimed to investigate potential effects of antipsychotics on cerebellar learning. Based on their receptor profiles, haloperidol and clozapine might affect cerebellar processing and/or learning in different ways. Haloperidol has a high affinity for dopamine D2 receptors, and a medium affinity for norepinephrine α1 and 5-HT2A receptors,28 all of which have been suggested to be involved in cerebellar function and/or plasticity.27 We did not find any significant differences in saccade adaptation or eyeblink conditioning between medication-free patients with schizophrenia and those taking haloperidol, suggesting that cerebellar functioning is impervious to low therapeutic doses of haloperidol. Patients taking clozapine did not show a significant difference in eyeblink conditioning or in saccade adaptation speed in comparison to medication-free patients, but they did show a significantly lower saccade adaptation strength. We could not find a correlation between clozapine dose and saccade adaptation strength. Clozapine has a high affinity to the dopamine D4 receptor and many other receptor types (e.g., 5-HT2A, muscarinic M1 and histamine H1028), all of which have been connected to cerebellar function and/or plasticity.27
Apart from the possibility of clozapine interfering with cerebellar plasticity directly, a second explanation for the additional deficit in patients taking clozapine could be that these patients belong to a subgroup with more severe deficits who are treated with clozapine when other antipsychotics are insufficiently effective.
A third explanation might be that clozapine is more sedative than haloperidol, which might lower vigilance, resulting in reduced saccade adaptation strength. However, both eyeblink conditioning and saccade adaptation are processes requiring no conscious awareness. Lowered vigilance as a leading cause for cerebellar motor learning deficits in our patients is unlikely, since no correlation was found between learning deficits and CPZ equivalents. The only previous study that specifically focused on a differential effect of antipsychotic medication on eyeblink conditioning39 found no difference in eyeblink conditioning between patients using either haloperidol or olanzapine, an antipsychotic with a structural resemblance to clozapine. Likewise, we found no difference in eyeblink conditioning capacities between patients taking clozapine or haloperidol.
Although we did find a correlation between adaptation strength and eyeblink conditioning in healthy controls, this correlation was absent in our population of patients with schizophrenia. Although both saccade adaptation and eyeblink conditioning specifically depend on cerebellar plasticity, they differ in several aspects, such as form of learning, learned behavioural change, underlying neural circuitry and temporal characteristics of the learning process. These differences may account for the absence of a correlation between individual learning capacities in both paradigms. Another interesting finding was that in the eyeblink conditioning experiment the patients’ percentage of correct CRs did not continue to increase until it attained the control level. In contrast, in the saccade adaptation experiment patients eventually did appear to attain the same saccade adaptation strength as the controls, though at a significantly lower speed. Although patients taking clozapine did not manage to reach the same saccade adaptation strength in 100 trials, they may have eventually reached a similar level of adaptation in a prolonged experiment. It has been demonstrated that, although quick saccade adaptation depends on intact cerebellar cortical function, monkeys remain able to slowly change saccade amplitude after cerebellar cortical structures involved in saccade adaptation are lesioned.40 It is plausible that cerebellar deficits present in patients with schizophrenia might also be compensated for by other slower neural adaptation mechanisms.
To our knowledge, the present study is the first assessing saccade adaptation in patients with recent-onset schizophrenia. Investigating saccade adaptation has several benefits over other cerebellar motor learning tests. It specifically addresses cerebellar function; is quick, reliable and noninvasive; and shows less variance in outcome measures than eyeblink conditioning. This relatively small outcome variance could be valuable when searching for subtle deficits or medication effects. In our experiments, this is illustrated by the fact that we found an additional effect of clozapine in saccade adaptation, but not in eyeblink conditioning.
Based on theories of cerebellar structure and function, it is plausible that deficits in cerebellar learning are not limited to motor behaviour, but also extend to “higher” cortical pro cesses thought to be modulated by the cerebellum.7–11 Although our findings imply a cerebellar deficit in patients with schizophrenia, its functional relevance remains up for debate. Recently it has been reported that eyeblink conditioning deficits in patients with schizophrenia can be reduced by administration of secretin, an endogenous neuropeptide involved in modulating cerebellar synaptic transmission.41 It would be interesting to investigate whether secretin also reduces other cognitive symptoms of schizophrenia, such as time perception, working memory, verbal memory and sequence learning, that have been linked to cerebellar dysfunction.
Limitations
There are some limitations to our study. Patients were not randomized for treatment. Thus we cannot fully exclude that confounding variables associated with medication status may have influenced our results. Furthermore, we did not collect intelligence data in our participants. In previous studies no correlation between intelligence and eyeblink conditioning was found in patients with schizophrenia.32,36,39 In addition, we did not find any correlation between years of education and any of our test results. Moreover, in both eyeblink conditioning and saccade adaptation, reflexive movements are adapted by subconscious processes, which require little or no “higher” cognitive input. In addition, to our knowledge, no correlation between saccade adaptation capacity and intelligence has been reported. Although our patient group was relatively homogeneous with respect to onset of illness and short use of medication, patients varied along the schizophrenia spectrum. The relatively small number of participants per subgroup may have concealed effects as a result of insufficient statistical power.
Conclusion
Cerebellar learning deficits in patients with schizophrenia cannot be attributed to the use of antipsychotics. The fact that the deficits are already present in patients with recent-onset schizophrenia and are not influenced by medication could suggest that cerebellar impairments are a trait deficit in these patients. However, this has to be confirmed in longitudinal studies.
Acknowledgements
We thank Dr. N. van Beveren for help with inclusion of patients and Dr. S.A. Kushner for critical reading of the manuscript. We also thank the anonymous reviewers for their critical appraisal of our work. This work was supported by a European Commission (C7: FP7-ITN) grant to C.I. De Zeeuw and M.A. Frens, a Prinses Beatrix Fonds grant to C.I. De Zeeuw and J.N. van der Geest, a TC2N (Interreg) grant to M.A. Frens and J.N. van der Geest, and a ZonMW/Neuras grant to M.A. Frens.
Footnotes
Competing interests: None declared.
Contributors: M. Coesmans, C.H. Röder, C.I. De Zeeuw, M.A. Frens and J.N. van der Geest designed the study. M. Coesmans, A.E. Smit and S.K.E. Koekkoek acquired the data, which M. Coesmans, C.H. Röder, A.E. Smit, S.K.E. Koekkoek, M.A. Frens and J.N. van der Geest analyzed. M. Coesmans, C.H. Röder and J.N. van der Geest wrote the article, which all authors reviewed and approved for publication.
References
- 1.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–8. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konarski JZ, McIntyre RS, Grupp LA, et al. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30:178–86. [PMC free article] [PubMed] [Google Scholar]
- 3.Picard H, Amado I, Mouchet-Mages S, et al. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34:155–72. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohyama T, Nores WL, Murphy M, et al. What the cerebellum computes. Trends Neurosci. 2003;26:222–7. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 5.Raymond JL, Lisberger SG, Mauk MD. The cerebellum: A neuronal learning machine? Science. 1996;272:1126–31. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- 6.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–13. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 8.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 9.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–22. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 10.Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–2. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- 11.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 12.Kinney DK, Yurgelun-Todd DA, Woods BT. Neurologic signs of cerebellar and cortical sensory dysfunction in schizophrenics and their relatives. Schizophr Res. 1999;35:99–104. doi: 10.1016/s0920-9964(98)00121-2. [DOI] [PubMed] [Google Scholar]
- 13.Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–53. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 14.D’Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 2009;32:30–40. doi: 10.1016/j.tins.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Coesmans M, Smitt PA, Linden DJ, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol. 2003;53:325–36. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]
- 16.Krab LC, de Goede-Bolder A, Aarsen FK, et al. Motor learning in children with neurofibromatosis type I. Cerebellum. 2011;10:14–21. doi: 10.1007/s12311-010-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smit AE, van der Geest JN, Vellema M, et al. Savings and extinction of conditioned eyeblink responses in fragile X syndrome. Genes Brain Behav. 2008;7:770–7. doi: 10.1111/j.1601-183X.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Geest JN, Haselen GC, Frens MA. Saccade adaptation in Williams-Beuren Syndrome. Invest Ophthalmol Vis Sci. 2006;47:1464–8. doi: 10.1167/iovs.05-0617. [DOI] [PubMed] [Google Scholar]
- 19.Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol. 2004;72:27–53. doi: 10.1016/j.pneurobio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Catz N, Dicke PW, Thier P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci U S A. 2008;105:7309–14. doi: 10.1073/pnas.0706032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30:3715–27. doi: 10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia. 2001;39:742–56. doi: 10.1016/s0028-3932(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 23.Krebs MO, Bourdel MC, Cherif ZR, et al. Deficit of inhibition motor control in untreated patients with schizophrenia: further support from visually guided saccade paradigms. Psychiatry Res. 2010;179:279–84. doi: 10.1016/j.psychres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Picard H, Le SA, Amado I, et al. Impaired saccadic adaptation in schizophrenic patients with high neurological soft sign scores. Psychiatry Res. 2012;199:18–8. doi: 10.1016/j.psychres.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 25.Thompson RF, Steinmetz JE. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–55. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Lubow RE. Classical eyeblink conditioning and schizophrenia: a short review. Behav Brain Res. 2009;202:1–4. doi: 10.1016/j.bbr.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Schweighofer N, Doya K, Kuroda S. Cerebellar aminergic neuromodulation: towards a functional understanding. Brain Res Brain Res Rev. 2004;44:103–16. doi: 10.1016/j.brainresrev.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Gardner DM, Baldessarini RJ, Waraich P. Modern antipsychotic drugs: a critical overview. CMAJ. 2005;172:1703–11. doi: 10.1503/cmaj.1041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meehl PE. Nuisance variables and the ex post fact design. In: Radner M, Winokur S, editors. Theories & Methods of Physics and Psychology. 4th vol ed. Minneapolis (MN): University of Minnesota; 1970. pp. 373–402. [Google Scholar]
- 30.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48:764–70. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 31.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 32.Bolbecker AR, Mehta CS, Edwards CR, et al. Eye-blink conditioning deficits indicate temporal processing abnormalities in schizophrenia. Schizophr Res. 2009;111:182–91. doi: 10.1016/j.schres.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry. 2000;48:204–9. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- 34.Spain B. Eyelid conditioning and arousal in schizophrenic and normal subjects. J Abnorm Psychol. 1966;71:260–6. doi: 10.1037/h0023596. [DOI] [PubMed] [Google Scholar]
- 35.Brown SM, Kieffaber PD, Carroll CA, et al. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn. 2005;58:94–108. doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Hofer E, Doby D, Anderer P, et al. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51:127–36. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 37.Marenco S, Weinberger DR, Schreurs BG. Single-cue delay and trace classical conditioning in schizophrenia. Biol Psychiatry. 2003;53:390–402. doi: 10.1016/s0006-3223(02)01506-8. [DOI] [PubMed] [Google Scholar]
- 38.Edwards CR, Newman S, Bismark A, et al. Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Res. 2008;162:185–94. doi: 10.1016/j.pscychresns.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens A, Schwarz J, Schwarz B, et al. Implicit and explicit learning in schizophrenics treated with olanzapine and with classic neuroleptics. Psychopharmacology (Berl) 2002;160:299–306. doi: 10.1007/s00213-001-0974-1. [DOI] [PubMed] [Google Scholar]
- 40.Barash S, Melikyan A, Sivakov A, et al. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999;19:10931–9. doi: 10.1523/JNEUROSCI.19-24-10931.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolbecker AR, Hetrick WP, Johannesen JK, et al. Secretin effects on cerebellar-dependent motor learning in schizophrenia. Am J Psychiatry. 2009;166:460–6. doi: 10.1176/appi.ajp.2008.08040597. [DOI] [PubMed] [Google Scholar]