Abstract
Background
Heart transplantation has become a widely accepted surgical option for end-stage heart failure in Korea since its first success in 1992. We reviewed early postoperative complications and mortality in 239 patients who underwent heart transplantation using bicaval technique in Asan Medical Center.
Methods
Between January 1999 and December 2011, a total of 247 patients aged over 17 received heart transplantation using bicaval technique in Asan Medical Center. After excluding four patients with concomitant kidney transplantation and four with heart-lung transplantation, 239 patients were enrolled in this study. We evaluated their early postoperative complications and mortality. Postoperative complications included primary graft failure, cerebrovascular accident, mediastinal bleeding, renal failure, low cardiac output syndrome requiring intra-aortic balloon pump or extracorporeal membrane oxygenation insertion, pericardial effusion, and inguinal lymphocele. Follow-up was 100% complete with a mean follow-up duration of 58.4±43.6 months.
Results
Early death occurred in three patients (1.3%). The most common complications were pericardial effusion (61.5%) followed by arrhythmia (41.8%) and mediastinal bleeding (8.4%). Among the patients complicated with pericardial effusion, only 13 (5.4%) required window operation. The incidence of other significant complications was less than 5%: stroke (1.3%), low cardiac output syndrome (2.5%), renal failure requiring renal replacement (3.8%), sternal wound infection (2.0%), and inguinal lymphocele (4.6%). Most of complications did not result in the extended length of hospital stay except mediastinal bleeding (p=0.034).
Conclusion
Heart transplantation is a widely accepted option of surgical treatment for end-stage heart failure with good early outcomes and relatively low catastrophic complications.
Keywords: Heart transplantation, Complication, Mortality
INTRODUCTION
Since the world's first successful heart transplantation was performed by Dr. Christiaan Barnard in 1967, it has come to be regarded as a standard treatment for end-stage heart failure. The first heart transplantation was performed successfully in Korea in 1992 [1], and today, this procedure has become a widely accepted surgical option [2,3]. The long-term results of heart transplantation in Korea are comparable with the results reported by the International Society of Heart and Lung Transplantation (ISHLT) [4,5].
From a surgical perspective, there are several operative techniques and their modifications that are currently in use. Among them, the bicaval technique is known to be associated with a decreased incidence of atrial arrhythmias and reduced need for pacemaker implantation because of the preservation of normal atrial geometry and sinus function [6,7]. Since January 1999, we have employed the bicaval technique instead of the standard biatrial technique [4]. Although both these techniques have shown excellent long-term outcomes as previously reported, few studies have evaluated their early postoperative outcomes, including early complications and mortality. Such postoperative outcomes need to be studied further as they are the primary areas of interest for cardiac surgeons.
Therefore, we reviewed the early postoperative complications and mortality in adult recipients who underwent heart transplantations at the Asan Medical Center, using the bicaval technique. We are presenting our experience with early postoperative complications and their management, and the analysis of early mortality.
METHODS
A retrospective chart review was carried out on all patients who underwent orthotopic heart transplantation using the bicaval technique between January 1999 and December 2011. A total of 286 patients underwent orthotopic heart transplantation, and of these, 247 patients were 17 years or older. Among them, patients undergoing multi-organ transplantations were excluded: four patients with heart-lung transplantations and four patients with heart-kidney transplantations. Finally, 239 patients were enrolled in this study, comprising the current study population. Data were extracted from the prospectively registered database of Asan Medical Center, and supplemental information was obtained by reviewing the relevant medical records. The collected variables were basic demographic characteristics, preoperative diagnosis and laboratory data, preoperative need for inotropic support, preoperative intensive care unit (ICU) stay, preoperative mechanical circulatory support (MCS), total length of ICU and hospital stay, and postoperative complications. Postoperative complications included early graft failure, cerebrovascular accident (CVA), bleeding requiring re-exploration, renal failure requiring renal replacement therapy, low cardiac output syndrome requiring MCS, pericardial effusion, and inguinal lymphocele. This study was approved by the institutional ethics committee/review board at the Asan Medical Center (no. S2012-2199-0001), which waived the requirement for informed consent because of the retrospective nature of this study.
1) Operative technique
During the harvest, the heart was procured using cardioplegia with histidine-tryptophan-ketoglutarate solution (Custodiol HTK; Essential Pharmaceuticals, Newtown, PA, USA) injected through the aortic root cannula, with venting through the inferior vena cava (IVC) and the right upper pulmonary vein. A heart allograft is usually flushed with 2 L of Custodiol HTK solution and preserved with the same solution. In the case when the cold ischemic time >120 minutes, 1 L of Custodiol HTK solution was infused again into the heart allograft before anastomosis in some of the cases.
All recipients were prepared in a manner similar to that in the case of other open heart surgeries. Standard median sternotomy was performed, and the preparation for cardiopulmonary bypass (CPB) included aortic and bicaval cannulations. Efforts were made to cannulate the aorta and both the superior and IVC as distally as possible. After the initiation of CPB, recipient cardiectomy was performed so that it could be completed simultaneously with the arrival of the donor heart. The bicaval technique sequenced the left atrial (LA) anastomosis first, usually followed by the IVC, pulmonary artery (PA), aorta, and superior vena cava (SVC) anastomosis. Otherwise, anastomosis was performed in the sequence of LA, PA, aorta, IVC, and SVC. After the completion of anastomosis, 500 mg of solumedrol was administered intravenously, followed by the declamping of the aorta. In situations in which there were concerns regarding the prolonged ischemic time, aortic anastomosis was performed with the subsequent declamping of the aorta before the rest of the anastomosis was completed. After the completion of anastomosis, standard deairing maneuvers were performed, and the reperfusion of the implanted heart was begun. The entire procedure of anastomosis was performed under hypothermia using ice slush. After the patient was weaned off CPB, the usual pericardial, mediastinal, and pleural chest tubes and a pericardial soft drain (Hemovac Evacuators; Zimmer, Warsaw, IN, USA) were placed. Before closing the sternum, we placed a pair of ventricular pacing wires.
2) Postoperative management and immunosuppression
Prior to June 1999, the immunosuppression protocol consisted of induction with cyclosporine, azathioprine, and methylprednisolone [4]. Thereafter, all patients received anti-interleukin-2 receptor monoclonal antibody (basiliximab) preoperatively at the day of surgery, and started again on postoperative day 2. Intraoperatively, all patients received a bolus of methylprednisolone (500 mg), with tapering doses over months. Mycophenolate mofetil (CellCept; Roche Laboratories, Nutley, NJ, USA) was routinely started immediately after surgery, once the patient was extubated. Tacrolimus was usually given on postoperative day 2 once the creatinine level stabilized.
Once the patient was transferred to the ICU after surgery, the patient was given postoperative care in a manner similar to that in the case of other open heart surgery cases. After the patient was cleared off of mediastinal bleeding or neurologic dysfunction, he/she was weaned off mechanical ventilation and was usually extubated within 24 hours of surgery. We tried to maintain adequate vital signs: the target systolic blood pressure and heart rate was 110 to 130 mmHg and 90 to 110 beats/min, respectively. Dopamine was the first choice of inotropes for maintaining adequate blood pressure, and the target heart rate was maintained using dobutamine and/or isoproterenol as appropriate. Further, we tried to maintain the urine output at least 100 mL/hr during the first 24 hours. When the systolic pulmonary blood pressure was greater than 40 mmHg, iloprost (Ventavis; Bayer Schering Pharma, Berlin, Germany) inhalation was administered every 4 to 6 hours.
3) Follow-up
All patients underwent endomyocardial biopsy for the monitoring of acute rejection. Biopsies were taken on a regular basis for a two-year period after surgery, as previously reported [4]. A single expert cardiologist was involved in immediate postoperative care and long-term medical management at our institution. After the patients were discharged from the hospital, clinical follow-ups were performed during outpatient clinic visits. Follow-up was 100% complete with a mean follow-up duration of 58.4±43.6 months.
4) Statistical analysis
Categorical variables were presented as frequencies and percentages, and continuous variables as mean±standard deviation. Differences in baseline characteristics between the patient groups, categorized on the basis of the presence of complications, were compared using the Student t-test or Mann-Whitney U-test for continuous variables and the chi-square test or Fisher's exact test for categorical variables, as appropriate. The survival rates were calculated using the Kaplan-Meier method.
All reported p-values were two-sided, and values of p<0.05 were considered statistically significant. IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) was used for the statistical analysis.
RESULTS
1) Baseline characteristics
Of the 239 patients considered, 171 (71.5%) were male. Further, the mean age at the time of transplantation was 45.1±12.5 years. Thirty-seven patients (15.5%) had undergone previous cardiac operations. Table 1 summarizes the baseline characteristics of all of the patients. The most common diagnosis among recipients was dilated cardiomyopathy (67.8%), followed by ischemic cardiomyopathy (13.4%) and hypertrophic cardiomyopathy (5.4%). Preoperative inotropic and mechanical support was provided to 114 patients (47.7%) and 7 patients (2.9%), respectively. Meanwhile, the mean age of the cardiac donors was 31.9±10.3 years, and 190 of these donors (79.5%) were male.
Table 1.
Baseline and demographic characteristics of patients
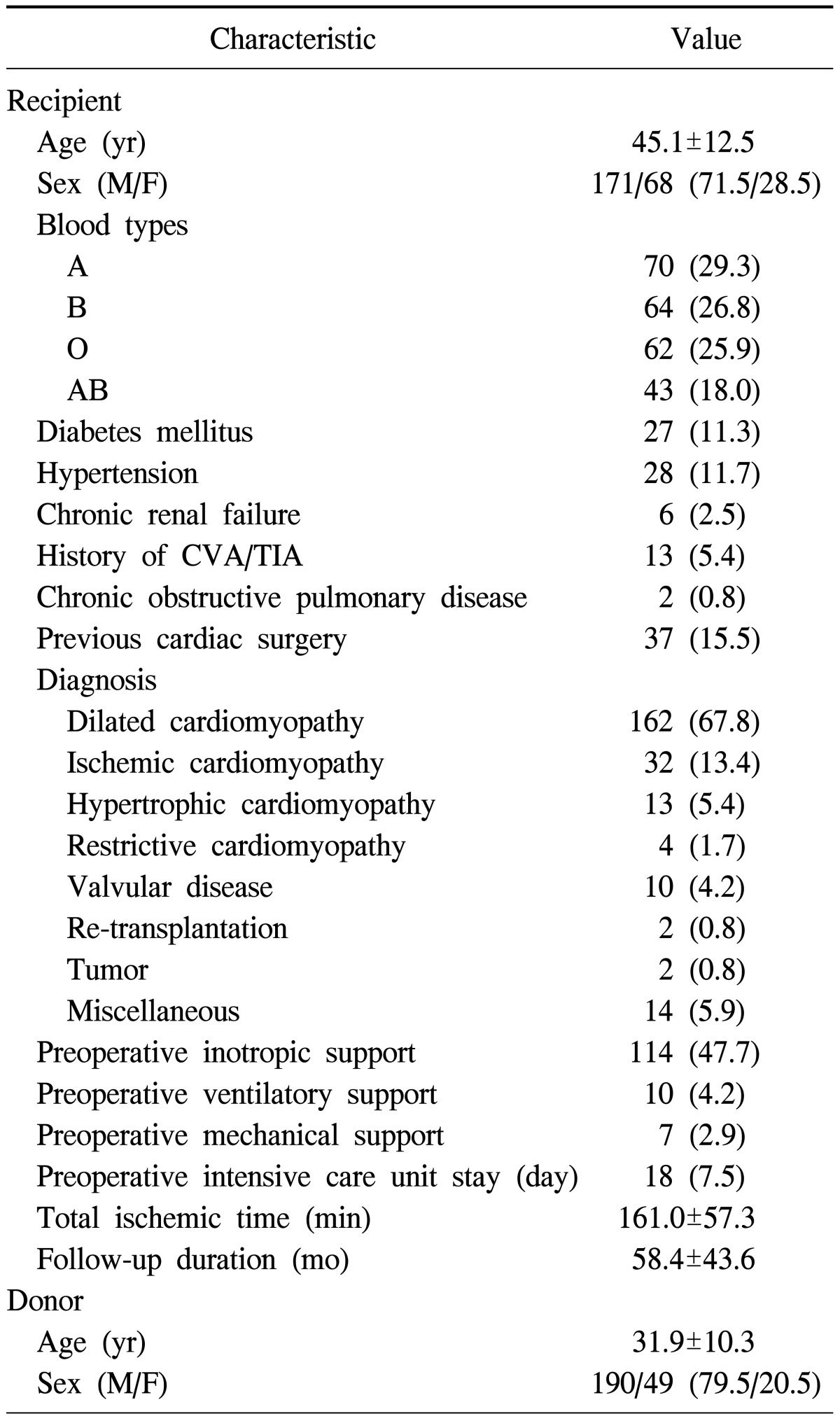
Values are presented as mean±standard deviation or number (%). CVA, cerebrovascular accident; TIA, transient ischemic attack.
2) Early postoperative outcomes
There were three (1.3%) early deaths. Table 2 summarizes the early postoperative mortality and complications. The mean period of ICU stay was 6.7±3.1 days. Postoperative bleeding leading to exploration occurred in 20 patients (8.4%), among whom three experienced delayed bleeding at postoperative day 6, 7, and 20, respectively.
Table 2.
Early postoperative mortality and complications
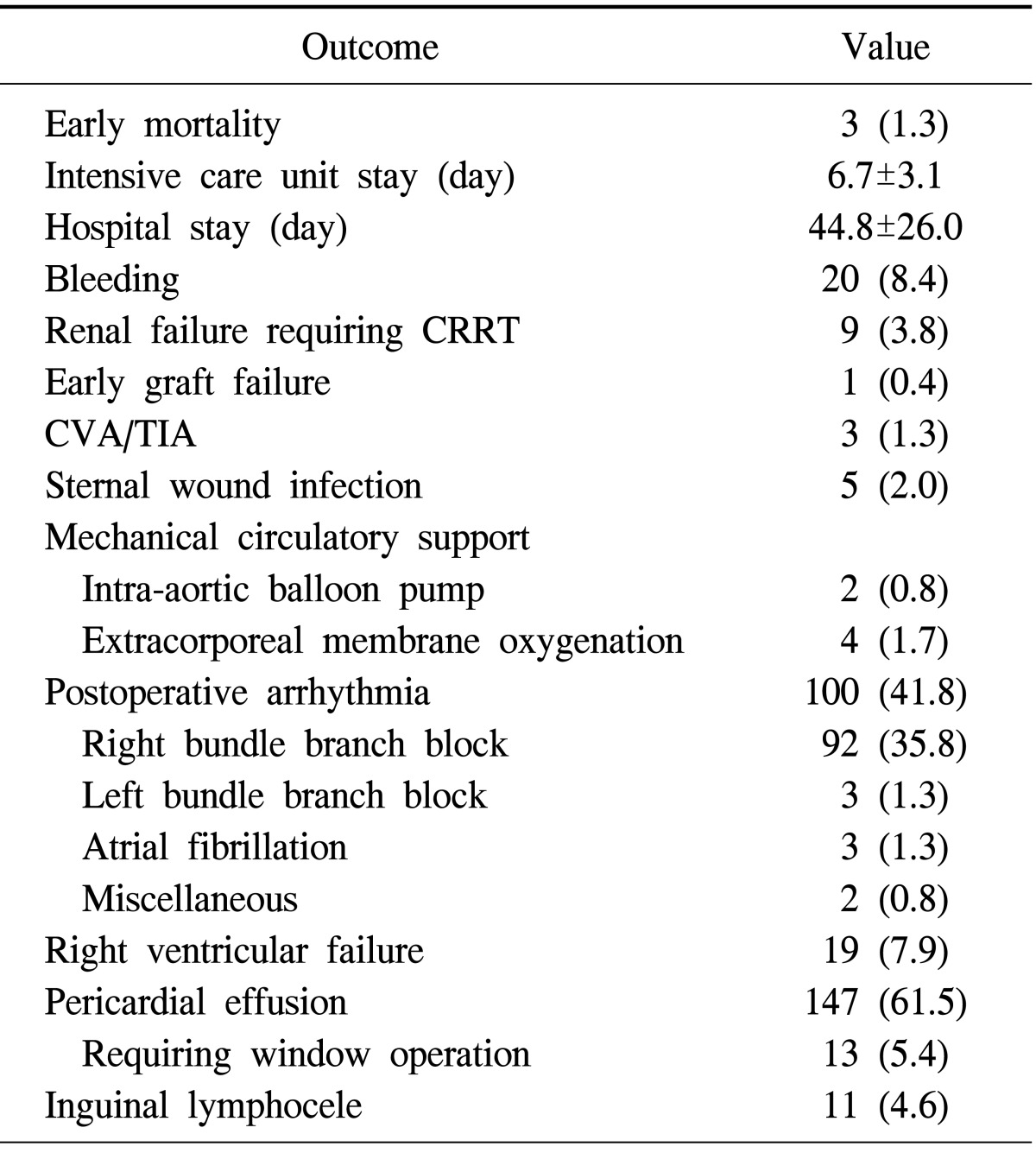
Values are presented as number (%) or mean±standard deviation. CRRT, continuous renal replacement therapy; CVA, cerebrovascular accident; TIA, transient ischemic attack.
Renal replacement therapy due to renal failure was required in 9 patients (3.8%), two of whom were already diagnosed with chronic renal failure preoperatively. One of them died of septic shock (patient 1 in Table 3) during continuous renal replacement therapy (CRRT) support in the ICU. Five patients recovered renal function, but the remaining three patients required conventional hemodialysis after transfer to the general ward. The median duration of CRRT among survivors was 4.5 days (interquartile range, 4 to 8.75 days).
Table 3.
Clinical characteristics and early mortality of patients receiving heart transplantation
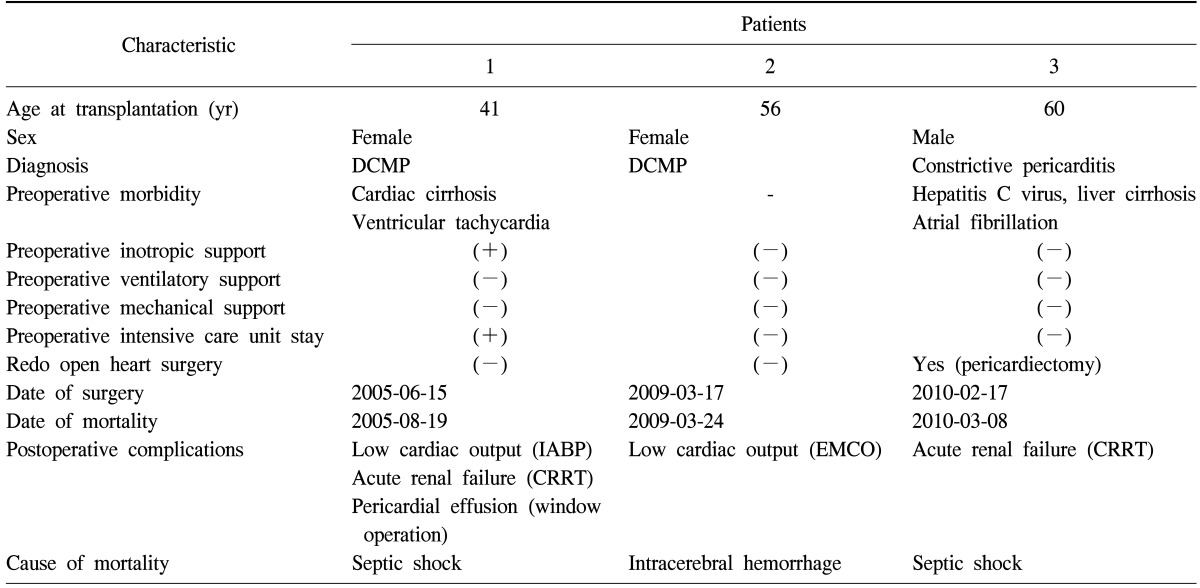
DCMP, dilated cardiomyopathy; IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; CRRT, continuous renal replacement therapy.
CVA leading to death or permanent sequelae occurred in three patients (1.3%). One of them developed intracerebral hemorrhage during extracorporeal membrane oxygenation (ECMO) support and died on postoperative day 7 (patient 2 in Table 3). Two patients were found to develop cerebral infarction on postoperative day 2 and are currently being followed up by neurologists in Asan Medical Center.
Sternal wound infection occurred in five patients (2.0%), and all of these patients underwent sternal wound revisions. Among them, one patient had delayed wound infection one month after surgery. There was no case of recurrence after sternal wound revision.
Four patients (1.7%) and two patients (0.8%) required ECMO and intra-aortic balloon pump (IABP) support, respectively, due to low cardiac output or primary graft failure postoperatively. Of the four patients who were on postoperative ECMO support, one patient died of intracerebral hemorrhage as described previously (patient 2 in Table 3). The other three were successfully weaned off ECMO support, and one of them underwent re-transplantation. The mean period of ECMO support was 3.75 days. Of the two patients who received IABP insertion, one underwent IABP insertion intraoperatively and was successfully weaned off IABP on postoperative day 2. The other received IABP insertion on postoperative day 2, which was removed successfully after three days.
Postoperative arrhythmia documented on electrocardiogram (ECG) was observed in 100 patients (41.8%): right bundle branch block (RBBB) in 92 patients (35.8%), left bundle branch block (LBBB) in 3 patients (1.3%), and atrial fibrillation (AF) in 3 patients (1.3%). Patients whose ECG revealed RBBB and AF did not have hemodynamic instability or its related complications during hospital stay. Only one patient who showed LBBB needed temporary pacemaker (TPM) implantation on postoperative day 1. The TPM was discontinued on postoperative day 2, and the patient was discharged without significant rhythm-related morbidity.
Right ventricular (RV) failure manifested by decreased contractility or RV chamber dilatation on echocardiography was observed in 19 patients (7.9%), none of whom showed hemodynamic instability requiring MCS. All of these patients had gradual improvement of RV function according to the serial follow-up echocardiography.
Echocardiography also revealed pericardial effusion of varying amounts in 147 patients (61.5%), but most of them did not have hemodynamic significance. Among these patients, 13 (5.4%) underwent pericardiostomy or window operation.
Inguinal lymphocele occurred in 11 patients (4.6%), five of whom underwent surgical treatment by us or vascular surgeons in Asan Medical Center. The others showed improvement of symptoms by conservative management. Notably, eight patients received cannulation via the femoral artery or the femoral vein during heart transplantation, implying that inguinal lymphocele is attributable to femoral cannulation.
Table 3 summarizes the clinical characteristics and the cause of death in the three patients with early mortality. In contrast to the gender predilection toward male among the recipients, two out of the three patients were female. Two of the patients had preoperative liver dysfunction with varied etiology. Neither of them received preoperative ventilatory or mechanical support requiring ICU care. The most common cause of death was sepsis, which occurred in two patients. Throughout the study, there were 26 late deaths (10.8%), and the 1-year, 5-year, and 10-year survival rates were 95.1%, 89.1%, and 76.3%, respectively.
We compared the length of hospital and ICU stay between the groups categorized on the basis of the presence of complications. In general, there was no difference in the length of hospital stay according to each complication, except mediastinal bleeding (p=0.034). The presence of renal failure requiring renal replacement therapy (p=0.003), ECMO insertion (p=0.038), and arrhythmia (p=0.011) resulted in the prolongation of ICU stay, while the other complications did not affect the duration of ICU stay.
DISCUSSION
This study represents the experience with early postoperative complications of adult heart transplantation accumulated at the Asan Medical Center over a 13-year period, during which significant advancement in organ procurement and operative techniques, refinement in perioperative care, and changes in immunosuppressive protocols were achieved. As previously reported, the long-term results of our institution were comparable to those reported by the ISHLT [5]. Despite this achievement in long-term outcomes, few studies have evaluated the early postoperative outcomes and complications after heart transplantation from the surgical perspective. This encouraged us to systematically collect and review our experience, focusing on the less-studied aspects.
This study demonstrated that the early mortality was excellent [8], compared with that reported by other centers [9-11]. Due to the low incidence of early mortality, we were not able to identify the statistically significant risk factors for mortality. However, notably, two patients had preoperative hepatic dysfunction, which is compatible with previous studies suggesting an association between poor clinical outcomes and preoperative hepatic dysfunction [12]. The leading cause of early mortality was septic shock, followed by intracerebral hemorrhage, owing to anticoagulation during ECMO support. In contrast to the 30-day mortality reported by ISHLT, in which primary graft failure is the leading cause of death [5], graft failure was not listed as a cause of death in our study.
Other postoperative complications, including mediastinal bleeding, renal failure, early graft failure, CVA, and sternal wound infection reported in our study, could be compared favorably with those reported in other studies across the world [13-18]. These complications could be adequately managed with either a surgical or medical modality. The presence of complications did not make a difference in the length of ICU and hospital stay in a majority of cases; patients with renal failure, arrhythmia, and ECMO insertion had a longer ICU stay, and patients with mediastinal bleeding had a longer hospital stay.
Our study recognized that the role of ECMO support has emerged as a preoperative bridge-to-transplantation as well as a postoperative bridge-to-recovery. Out of seven patients who received preoperative ECMO support and were bridged to transplantation, no one had postoperative mortality, and all of them had successful recovery after transplantation. Only one case of early mortality was reported in the postoperative ECMO group (patient 2 in Table 3); the remaining three patients were successfully bridged to recovery. The successful outcome of using ECMO as a preoperative bridge-to-transplantation and postoperative bridge-to-recovery indicates that ECMO support can be a viable option for managing preoperative end-stage heart failure patients and improving survival, particularly when compared with the general outcomes of ECMO support after major cardiac surgery. In addition, given the situation in Korea where ventricular assist device implantation is not generalized for patients with a failing heart [19] and ECMO support may be the only alternative option, the number of patients on the waiting list who may need preoperative ECMO support is expected to increase over time.
Our study showed that ECG-documented arrhythmias and pericardial effusion revealed by echocardiography were frequently observed after heart transplantation. RBBB was the most commonly documented arrhythmia, but none of the patients with RBBB showed hemodynamic instability that required pacemaker insertion. It appears that RBBB after heart transplantation does not have clinically significant implications; however, this needs to be further verified. Similarly, most of the pericardial effusion did not have hemodynamic significance, and only 8.8% of pericardial effusion required a pericardiostomy or window operation. This result suggests that pericardial effusion without tamponade can be observed and needs serial follow-up. Whether the remnant pericardial effusion may cause long-term problems requires further investigation.
This study has several limitations. First, it is a retrospective and non-randomized study with observational data. Therefore, the results might be affected by unmeasured confounders. Second, this study incorporates the surgical outcomes of a thirteen-year period, performed by various surgeons. In spite of the use of the same technique, the difference in surgical outcomes among operating surgeons was not adequately reflected.
In conclusion, heart transplantation is a life-saving procedure for patients with end-stage heart failure with good early outcomes and relatively low catastrophic complications. Most of the early postoperative complications could be adequately managed with surgical or medical treatment and did not result in an extended hospital stay. We hope that this study can be used as a reference and can provide guidance for postoperative management after heart transplantation.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Song MG, Seo DM, Lee JW, et al. Cardiac transplantation: 1 case report. Korean J Thorac Cardiovasc Surg. 1993;26:224–227. [Google Scholar]
- 2.Park CB, Song H, Song MG, et al. Early results of heart transplantation: a review of 20 patients. Korean J Thorac Cardiovasc Surg. 1997;30:164–171. [Google Scholar]
- 3.Park KY, Park CH, Kim WS, et al. Heart transplantation: the Sejong General Hospital experience. Korean J Thorac Cardiovasc Surg. 1996;29:606–613. [Google Scholar]
- 4.Jung SH, Kim JJ, Choo SJ, Yun TJ, Chung CH, Lee JW. Long-term mortality in adult orthotopic heart transplant recipients. J Korean Med Sci. 2011;26:599–603. doi: 10.3346/jkms.2011.26.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Davies RR, Russo MJ, Morgan JA, Sorabella RA, Naka Y, Chen JM. Standard versus bicaval techniques for orthotopic heart transplantation: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2010;140:700–708. 708.e1–708.e2. doi: 10.1016/j.jtcvs.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Schnoor M, Schafer T, Luhmann D, Sievers HH. Bicaval versus standard technique in orthotopic heart transplantation: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2007;134:1322–1331. doi: 10.1016/j.jtcvs.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 8.Luckraz H, Goddard M, Charman SC, Wallwork J, Parameshwar J, Large SR. Early mortality after cardiac transplantation: should we do better? J Heart Lung Transplant. 2005;24:401–405. doi: 10.1016/j.healun.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Bocchi EA, Fiorelli A First Guideline Group for Heart Transplantation of the Brazilian Society of Cardiology. The Brazilian experience with heart transplantation: a multicenter report. J Heart Lung Transplant. 2001;20:637–645. doi: 10.1016/s1053-2498(00)00235-7. [DOI] [PubMed] [Google Scholar]
- 10.Dellgren G, Geiran O, Lemstrom K, et al. Three decades of heart transplantation in Scandinavia: long-term follow-up. Eur J Heart Fail. 2013;15:308–315. doi: 10.1093/eurjhf/hfs160. [DOI] [PubMed] [Google Scholar]
- 11.Favaloro R, Peradejordi M, Bertolotti A, et al. Results of heart transplantation: 16 years' experience in a center in Argentina. Transplant Proc. 2010;42:321–323. doi: 10.1016/j.transproceed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Chokshi A, Cheema FH, Schaefle KJ, et al. Hepatic dysfunction and survival after orthotopic heart transplantation: application of the MELD scoring system for outcome prediction. J Heart Lung Transplant. 2012;31:591–600. doi: 10.1016/j.healun.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrier M, Perrault LP, Pellerin M, et al. Sternal wound infection after heart transplantation: incidence and results with aggressive surgical treatment. Ann Thorac Surg. 2001;72:719–723. doi: 10.1016/s0003-4975(01)02824-7. [DOI] [PubMed] [Google Scholar]
- 14.Filsoufi F, Rahmanian PB, Castillo JG, Pinney S, Broumand SR, Adams DH. Incidence, treatment strategies and outcome of deep sternal wound infection after orthotopic heart transplantation. J Heart Lung Transplant. 2007;26:1084–1090. doi: 10.1016/j.healun.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 15.Gude E, Andreassen AK, Arora S, et al. Acute renal failure early after heart transplantation: risk factors and clinical consequences. Clin Transplant. 2010;24:E207–E213. doi: 10.1111/j.1399-0012.2010.01225.x. [DOI] [PubMed] [Google Scholar]
- 16.Kittleson MM, Patel JK, Moriguchi JD, et al. Heart transplant recipients supported with extracorporeal membrane oxygenation: outcomes from a single-center experience. J Heart Lung Transplant. 2011;30:1250–1256. doi: 10.1016/j.healun.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Sénéchal M, LePrince P, Tezenas du Montcel S, et al. Bacterial mediastinitis after heart transplantation: clinical presentation, risk factors and treatment. J Heart Lung Transplant. 2004;23:165–170. doi: 10.1016/S1053-2498(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 18.Kavarana MN, Sinha P, Naka Y, Oz MC, Edwards NM. Mechanical support for the failing cardiac allograft: a single-center experience. J Heart Lung Transplant. 2003;22:542–547. doi: 10.1016/s1053-2498(02)00654-x. [DOI] [PubMed] [Google Scholar]
- 19.Park SJ, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Outcomes of extracorporeal life support for low cardiac output syndrome after major cardiac surgery. J Thorac Cardiovasc Surg. 2012 Dec 5; doi: 10.1016/j.jtcvs.2012.11.006. [Epub]. http://dx.doi.org/10.1016/j.jtcvs.2012.11.006. [DOI] [PubMed] [Google Scholar]


