Abstract
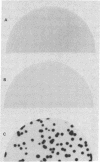
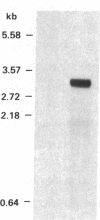
The assimilation of nitrate in plants involves the reduction of nitrate to ammonia in two steps. The first step requires nitrate reductase, a nitrate-inducible enzyme. When seedlings of squash (Cucurbita maxima L.) were treated with nitrate, both nitrate reductase activity and protein were induced in the cotyledons. Poly(A)+ RNA was prepared from cotyledons of nitrate-treated seedlings and was used to construct a λgt11 cDNA library. Using antibodies from mice immunized against purified nitrate reductase from squash, a recombinant λ phage was isolated that encoded part of the nitrate reductase mRNA. The antigens produced by the recombinant phage were used to affinity purify anti-nitrate reductase antibody from ascites fluid of immunized mice. The purified antibody bound to nitrate reductase protein on immunoblots and immunoprecipitated the enzyme from squash protein extracts. The cDNA insert (1.2 kilobases) hybridized to a 3.2-kilobase RNA that was 120-fold more abundant in nitrate-induced cotyledons compared with the uninduced tissue.
Keywords: plant gene expression, λgt11 cDNA cloning
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Oaks A., Huffaker R. C. Effect of light and glucose on the induction of nitrate reductase and on the distribution of nitrate in etiolated barley leaves. Plant Physiol. 1976 Oct;58(4):588–591. doi: 10.1104/pp.58.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Campbell W. H., Remmler J. L. Regulation of Corn Leaf Nitrate Reductase : I. Immunochemical Methods for Analysis of the Enzyme's Protein Component. Plant Physiol. 1986 Feb;80(2):435–441. doi: 10.1104/pp.80.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. H., Smarrelli J. Purification and Kinetics of Higher Plant NADH:Nitrate Reductase. Plant Physiol. 1978 Apr;61(4):611–616. doi: 10.1104/pp.61.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hageman R. H., Flesher D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media. Plant Physiol. 1960 Sep;35(5):700–708. doi: 10.1104/pp.35.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Shen T. C. Nitrate reductase in Agrostemma githago. Comparison of the inductive effects of nitrate and cytokinin. Biochim Biophys Acta. 1972 Nov 24;286(1):118–125. doi: 10.1016/0304-4165(72)90097-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Campbell W. H. Purification of Squash NADH:Nitrate Reductase by Zinc Chelate Affinity Chromatography. Plant Physiol. 1983 Jan;71(1):205–207. doi: 10.1104/pp.71.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Campbell W. H. Quaternary structure and composition of squash NADH:nitrate reductase. J Biol Chem. 1985 Mar 25;260(6):3380–3385. [PubMed] [Google Scholar]
- Remmler J. L., Campbell W. H. Regulation of Corn Leaf Nitrate Reductase : II. Synthesis and Turnover of the Enzyme's Activity and Protein. Plant Physiol. 1986 Feb;80(2):442–447. doi: 10.1104/pp.80.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner D. L., Boyer J. S. Nitrate Reductase Activity in Maize (Zea mays L.) Leaves: II. Regulation by Nitrate Flux at Low Leaf Water Potential. Plant Physiol. 1976 Oct;58(4):505–509. doi: 10.1104/pp.58.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarrelli J., Campbell W. H. Immunological approach to structural comparisons of assimilatory nitrate reductases. Plant Physiol. 1981 Dec;68(6):1226–1230. doi: 10.1104/pp.68.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers D. A., Kuo T. M., Kleinhofs A., Warner R. L., Oaks A. Synthesis and degradation of barley nitrate reductase. Plant Physiol. 1983 Aug;72(4):949–952. doi: 10.1104/pp.72.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke H. R., Filner P. Synthesis and turnover of nitrate reductase induced by nitrate in cultured tobacco cells. J Biol Chem. 1971 Mar 25;246(6):1772–1779. [PubMed] [Google Scholar]