Abstract
Background
Tumor recurrence is the most common cause of treatment failure, even after complete resection of early-stage non-small cell lung cancer (NSCLC). In this study, we investigated the prognosis of patients with early recurrence in order to identify independent risk factors related to early recurrence.
Methods
Between February 1995 and December 2012, 242 patients who underwent surgical resection for stage I NSCLC at Dong-A University Hospital were reviewed. The factors predicting overall survival (OS) and early recurrence were investigated. We also investigated the relationship between the patterns and period of recurrence and clinicopathological factors.
Results
For patients with stage IA and IB NSCLC, the 5-year OS rate was 75.7% and 57.3% (p=0.006), respectively. A multivariate Cox proportional hazards model demonstrated that gender (p=0.004), comorbidity number (p=0.038), resection type (p=0.002), and tumor size (p=0.022) were the statistically significant predictors of OS. Moreover, the multivariate analysis revealed that smoking history (p=0.023) and histologic grade (p=0.012) were the independent predictors of early recurrence. Additionally, only histologic grade (poor differentiation) was found to be significantly associated with a higher frequency of distant metastasis; there was no relationship between the patterns and period of recurrence and clinicopathological factors.
Conclusion
The present study demonstrated that smoking history and histologic grade were independent prognostic factors for early recurrence within two years in patients with early-stage NSCLC. Patients with these predictive factors may be good candidates for adjuvant therapy.
Keywords: Carcinoma, non-small cell, lung; Recurrence; Surgery; Risk factors; Adjuvant therapy
INTRODUCTION
Lung cancer is the leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancer patients. The treatment strategy chosen for patients with NSCLC is generally guided by the tumor-node-metastasis (TNM) classification. Only approximately 25% of the patients are diagnosed with early-stage (stage I) disease, for which surgical resection offers the possibility of a cure. However, even after complete resection of early-stage NSCLC, there is a wide spectrum of outcomes [1,2]. Tumor recurrence is the most common cause of disease failure after surgical resection and the main obstacle for long-term survival. TNM staging indicates the level of disease progression and malignant potential of primary lung cancer [3]. However, even patients with disease at the same stage are split between recurrence and the non-recurrence groups after complete resection. Therefore, the current TNM staging system may have reached the limit of its usefulness [4]. The identification of factors related to recurrence following surgery may help to evaluate the risk of disease recurrence in NSCLC patients. Moreover, identification of risk factors associated with recurrence patterns (local recurrence and distant metastasis) after surgical resection can help stratify patients with higher risks in the administration of potentially harmful adjuvant therapies. Only a few studies have reported the survival, patterns of recurrence, and risk factors analyses predicting recurrence in resected stage I NSCLC [1,3,5]. The purpose of this study is to elucidate the prognosis of patients with early recurrence within two years of surgery and to identify independent risk factors related to early recurrence. We also investigated the relationship between the patterns and period of recurrence and clinicopathological factors.
METHODS
A total of 272 patients (including those of previous bronchial alveolar carcinoma: 48 patients) who underwent surgical resection for stage I NSCLC at Dong-A University Hospital between February 1995 and December 2012 were reviewed. All of these patients underwent lobectomy and systemic lymph node dissection, and had no history of malignant disease. The following exclusion criteria were applied: sublobar resection (n=7); preoperative chemotherapy, radiotherapy, or both (n=15); and death within 30 days of operation (n=8). Of the 272 patients, the remaining 242 patients provided sufficient data to be included in the study. Video-assisted thoracoscopic surgery was performed on 88 of these patients (36%).
Preoperative evaluation included physical examination, chest radiography, chest computed tomography (CT) scan, abdominal CT scan or ultrasonography, bone scintigraphy, blood examination, and brain CT scan or magnetic resonance imaging, and since 2004, positron-emission tomography (PET)-CT scan. The chest CT scan or PET-CT scan and the blood examination (including serum tumor markers) were performed within 6 months after surgery, and every 6 months thereafter. Patients were staged according to the 7th TNM staging proposed by the International Staging Committee of the International Association for the Study of Lung Cancer. Histologic subtypes of lung cancer were determined according to the World Health Organization classification.
Follow-up was carried out through routine office visits or by telephone contact. All of the patients were followed through March 2013. Second primary lung cancer was differentiated from recurrent NSCLC according to the criteria proposed by Martini et al. [6]. Recurrences were diagnosed by physical examination and diagnostic imaging. Histological or cytological confirmation of the recurrence was made when clinically feasible. Local recurrence was defined as disease recurrence in contiguous anatomic sites, including the surgical margin, ipsilateral hemithorax, and mediastinum after surgical resection. Distant metastasis was defined as tumor recurrence in the contralateral lung or outside the hemithorax and mediastinum after surgical resection.
The demographic data collected included age, gender, number of comorbidities, smoking status, tumor location (upper vs. non-upper), tumor laterality (right vs. left), extent of resection (single lobectomy vs. more than bilobectomy), tumor size, pathologic stage (stage IA or IB), histology (adenocarcinoma, squamous, and others: adenosquamous cell carcinoma, large cell carcinoma, adenocarcinoma mixed with large cell carcinoma, squamous cell carcinoma mixed with large cell carcinoma, etc.), tumor differentiation (well/moderate vs. poor), adjuvant therapy, and PET-CT maximum standardized uptake value (maxSUV). The subtypes of adenocarcinoma were categorized by its differentiation degree, that is lepidic type (including bronchial alveolar carcinoma) into well differentiated, acinar and papillary type into moderate, and solid and micropapillary into poor.
All statistical analyses were performed using IBM SPSS ver. 20 (IBM Co., Armonk, NY, USA). To investigate their impact on disease-free status (no recurrence vs. recurrence), patterns of recurrence (local recurrence vs. distant metastasis), period of recurrence (early recurrence vs. late recurrence), and clinicopathological factors were analyzed. To compare between the groups with respect to categorical and continuous variables, the chi-square test or Student t-test was used. Statistically significant differences were accepted at p<0.05. Overall survival (OS) was measured from the date of surgery to the date of death from any cause or date on which the patient was last known to be alive. Disease-free survival (DFS) was measured from the date of resection to the date of the first recurrence or last follow-up showing no recurrence. Survival was estimated using the Kaplan-Meier method, and the significance of comparisons was examined using the log-rank test. Mutivariate analyses were performed by means of a logistic regression analysis and the Cox proportional hazards model. Only variables with p<0.05 after the univariate analysis were considered in the multivariate analysis.
RESULTS
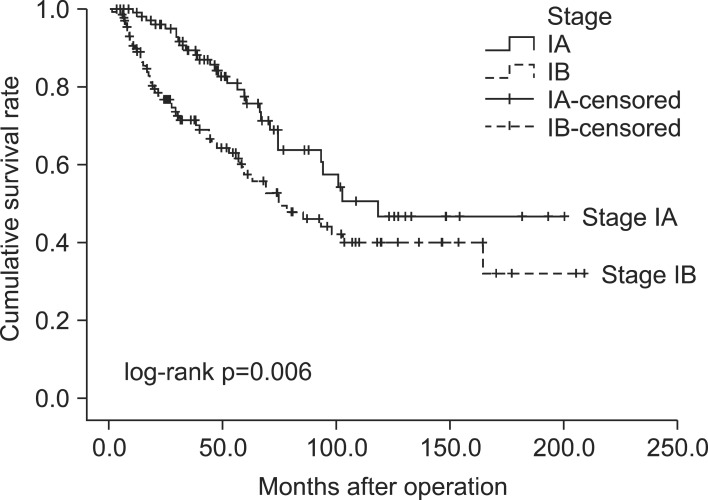
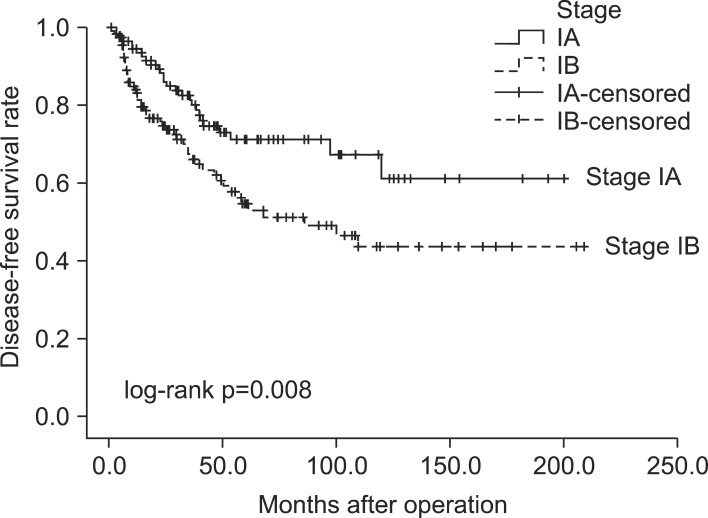
The median follow-up time for all 242 resected early-stage NSCLC patients was 44.3 months (mean, 54.4±44.4 months). The baseline characteristics of these 242 patients are listed in Table 1. Fig. 1 and Fig. 2 show the OS and DFS curve of 242 patients with stage I NSCLC. For patients with stage IA and IB NSCLC, the 5-year OS rates were 75.7% and 57.3% (p=0.006), respectively, whereas the 5-year DFS rates were 71.7% and 54.7% (p=0.008), respectively.
Table 1.
Characteristics of 242 patients with resected early stage non-small cell lung cancer
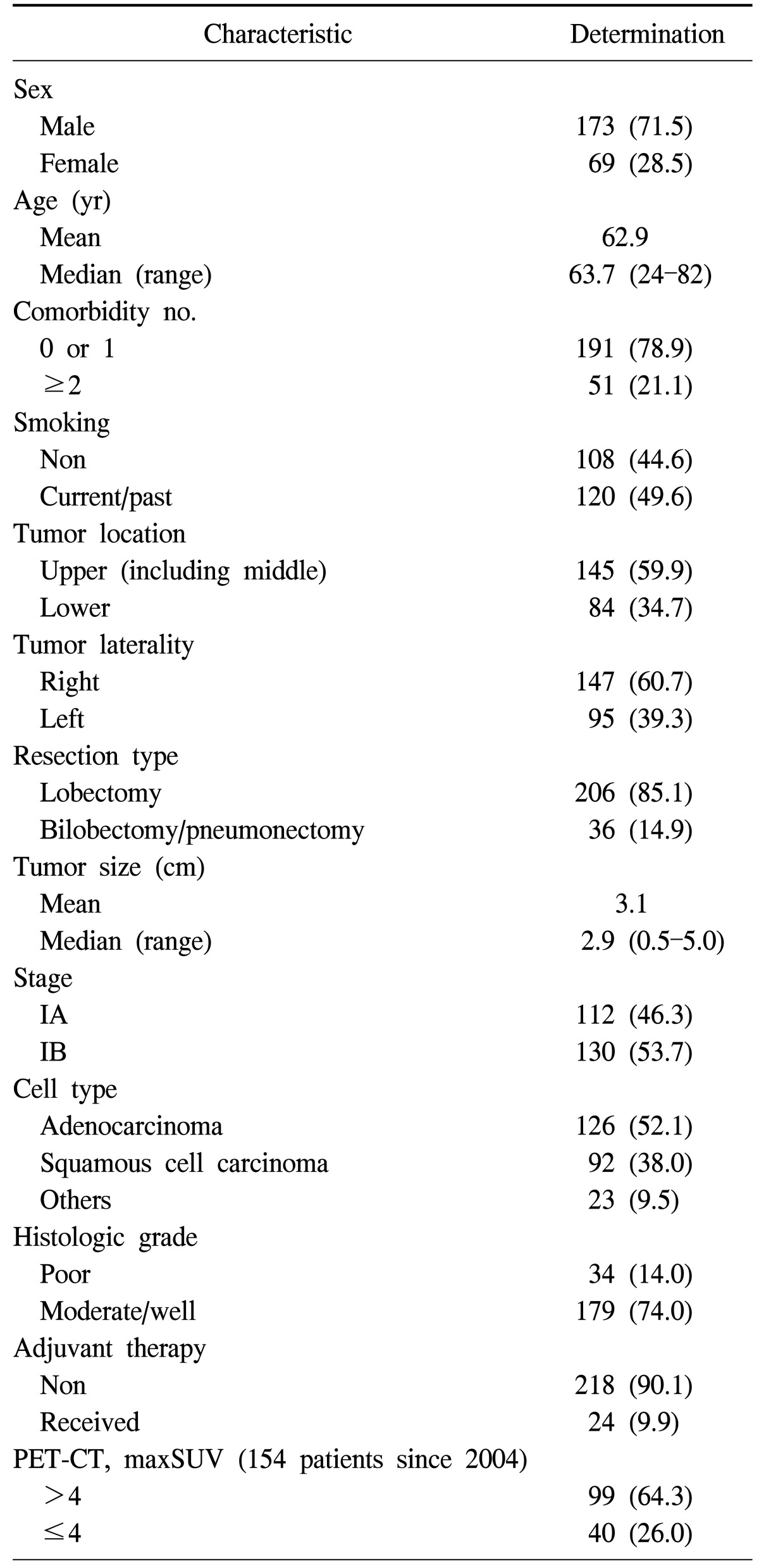
Values are presented as number (%).
PET-CT, positron emission tomography-computed tomography; maxSUV, maximum standardized uptake value.
Fig. 1.
Overall survival curve for stage IA and IB.
Fig. 2.
Disease-free survival curve for stage IA and IB.
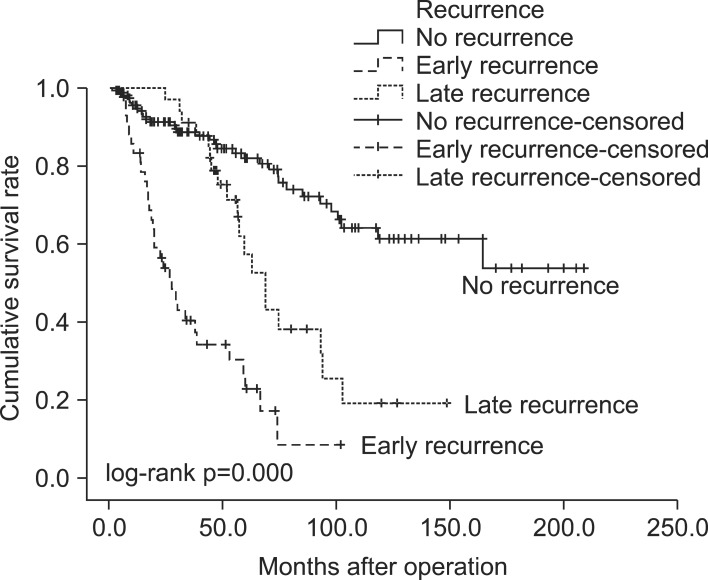
The patients were classified into three groups according to the interval between operation and recurrence: 165 patients (68.2%) had no recurrence (NR), 43 patients (17.8%) had recurrence within two years (early recurrence, ER), and 34 patients (14.0%) had recurrence more than two years after surgery (late recurrence, LR). The distribution of time to recurrence is shown in Table 2. Patients with recurrence had a significantly worse survival (p=0.000). The 5-year survival rate for patients in the ER group was 22.8%, while this was 57.3% in the LR group and 82.1% in the NR group (Fig. 3). Table 3 shows the results of the univariate and multivariate analyses of the risk factors for overall recurrence in patients after surgical resection. The results of the univariate analysis showed that the following six factors were significantly associated with increased recurrence rates: gender, smoking status, tumor size, pathologic stage, histologic grade, and PET-CT maxSUV. There was no statistical difference in terms of age, number of comorbidities, tumor location, tumor laterality, resection type, histology, and adjuvant therapy. In the multivariate analysis, the independent predictor of recurrence was the histologic grade (p=0.017).
Table 2.
Group according to the interval between surgery and recurrence.

Values are presented as number (%).
Fig. 3.
Overall survival for early-stage non-small cell lung cancer according to the interval from surgical resection to recurrence.
Table 3.
Univariate and multivariate analyses of risk factors for overall recurrent patients after surgical resection
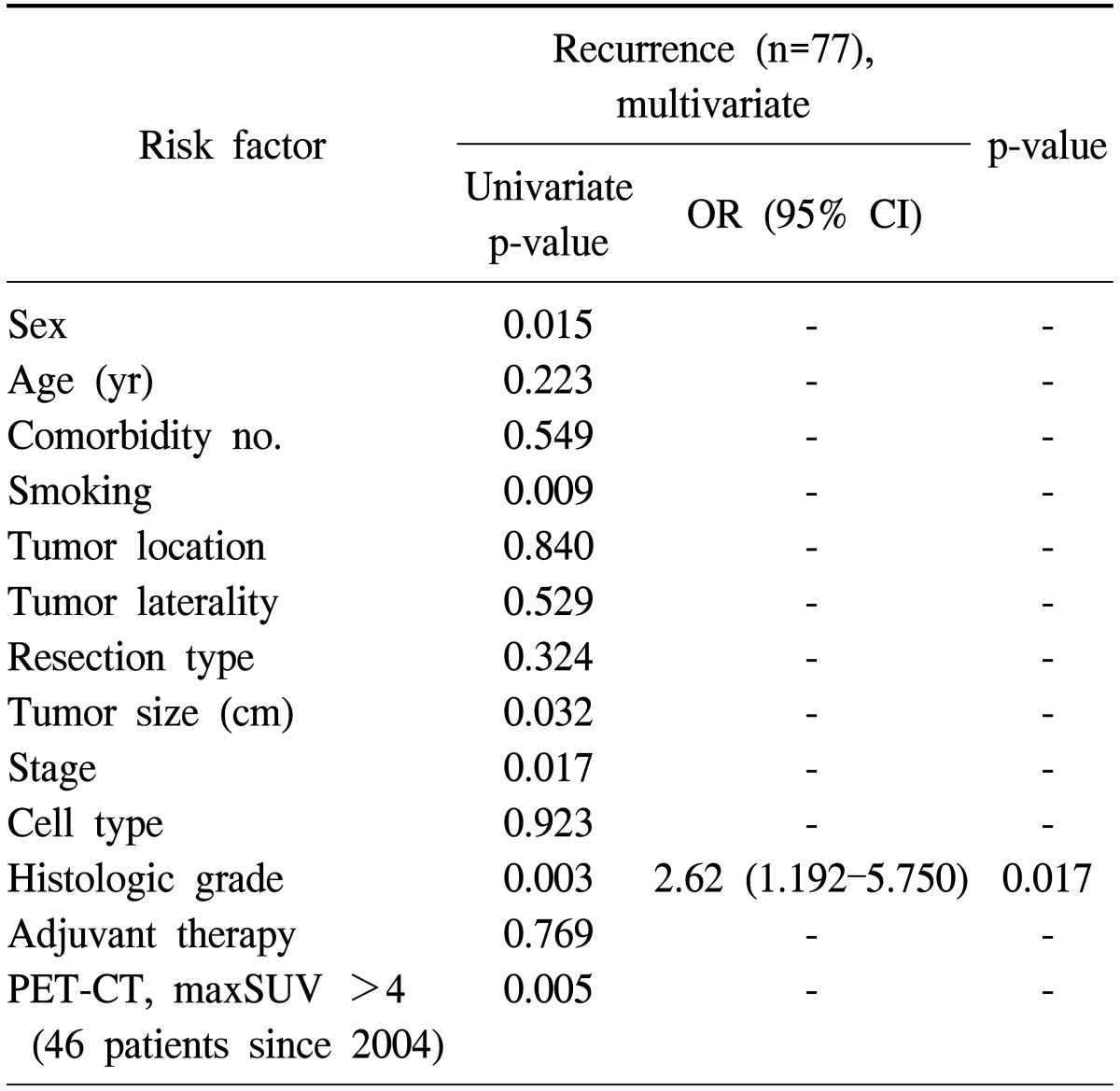
OR, odds ratio; CI, confidence interval; PET-CT, positron emission tomography-computed tomography; maxSUV, maximum standardized uptake value.
The patterns of recurrence included local recurrence in 26 patients (33.8%) and distant metastasis in 51 patients (66.2%). Table 4 shows a comparison of clinicopathological factors according to the pattern of recurrence (local recurrence only or distant metastasis). Only histologic grade (poor differentiation) was found to be significantly associated with a higher frequency of distant metastasis.
Table 4.
Relationship between pattern of recurrence and clinicopathologic variables
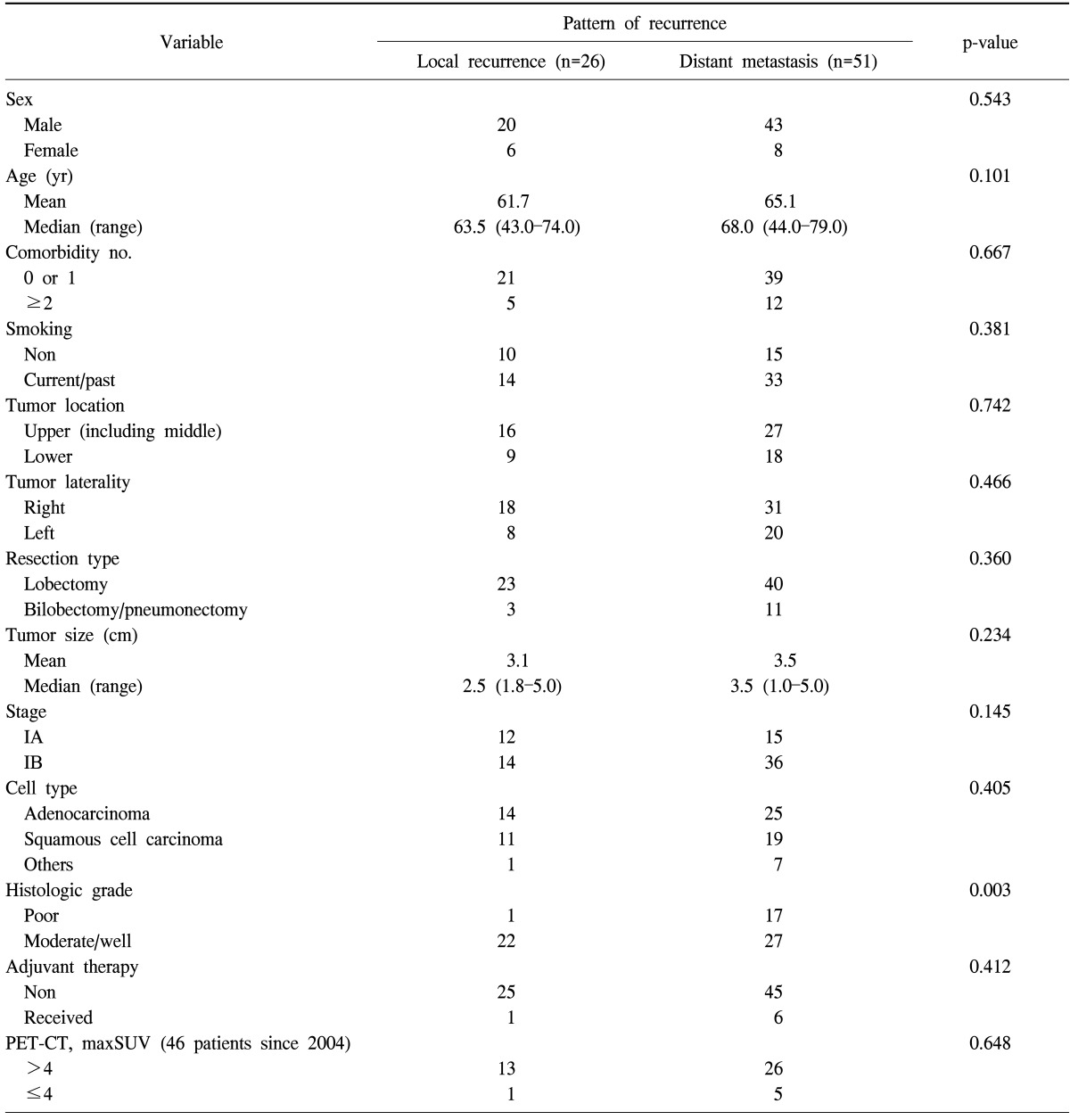
PET-CT, positron emission tomography-computed tomography; maxSUV, maximum standardized uptake value.
Table 5 shows the results of the univariate and multivariate analyses of risk factors between early recurrence patients and no recurrence patients after surgical resection. The results of the univariate analysis showed that the following six factors were significantly associated with early recurrence: gender, smoking status, tumor size, pathologic stage, histologic grade, and PET-CT maxSUV. The multivariate analysis revealed that smoking status (p=0.023) and histologic grade (p=0.012) were the independent predictors of early recurrence.
Table 5.
Univariate and multivariate analyses of risk factors between early recurrent patients and no recurrent patients
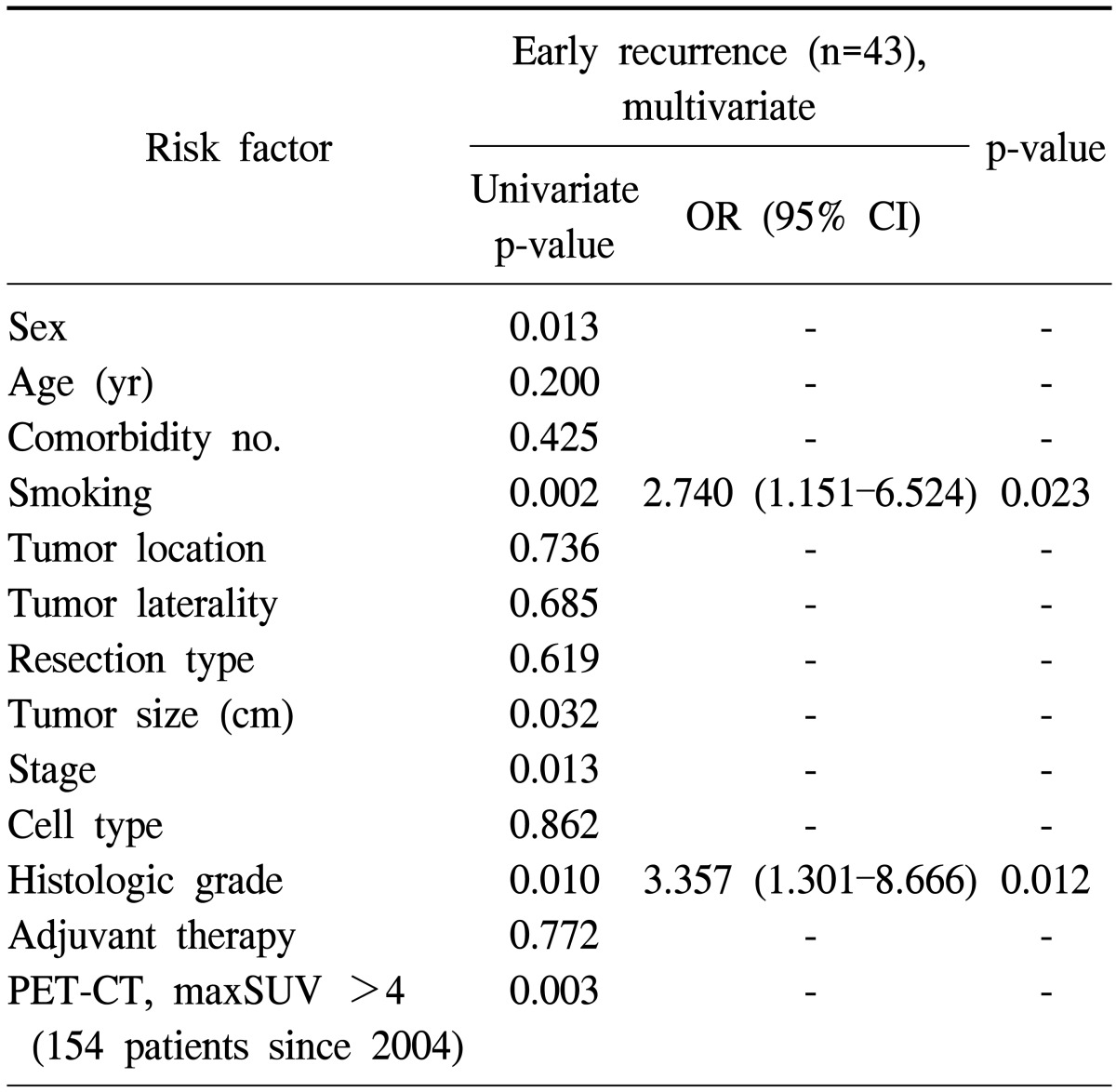
OR, odds ratio; CI, confidence interval; PET-CT, positron emission tomography-computed tomography; maxSUV, maximum standardized uptake value.
Similar analyses were performed to assess the predictors of worse OS in patients with early-stage NSCLC. The univariate analysis indicated that gender, age, number of comorbidities, resection type, tumor size, pathologic stage, adjuvant therapy, and PET-CT maxSUV were the predictors of worse OS. A multivariate Cox proportional hazards model demonstrated that gender (p=0.004), number of comorbidities (p=0.038), resection type (p=0.002), and tumor size (p=0.022) were the statistically significant predictors that carried an increased risk of mortality in patients with early-stage NSCLC (Table 6).
Table 6.
Risk factors for overall survival of patients with resected early stage non-small cell lung cancer
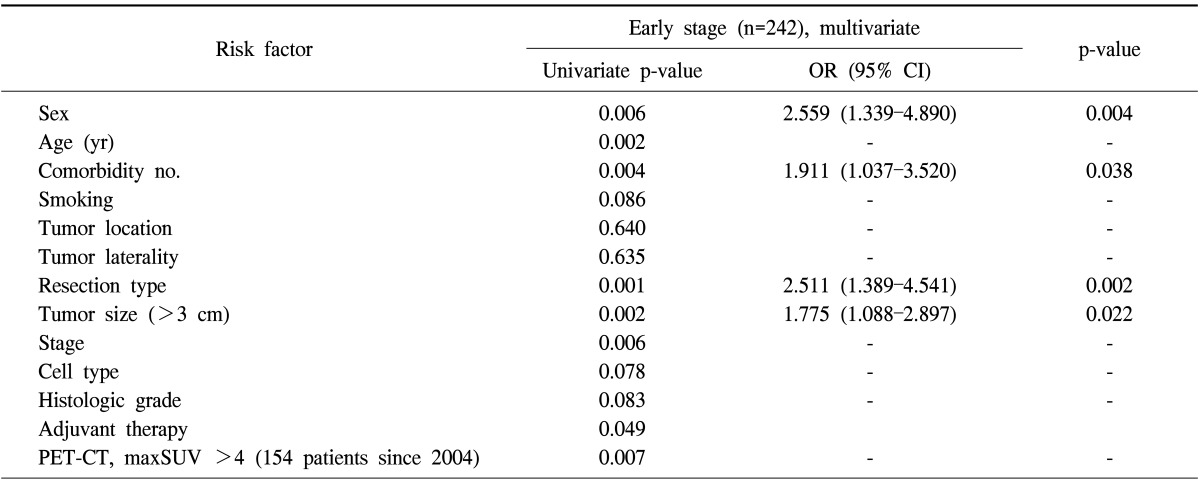
OR, odds ratio; CI, confidence interval; PET-CT, positron emission tomography-computed tomography; maxSUV, maximum standardized uptake value.
DISCUSSION
Lung cancer is the most fatal malignancy in most countries throughout the world. NSCLC represents more than 80% of all newly diagnosed cases of lung cancer. Anatomic surgical resection remains the best curative option for early-stage disease, but unfortunately, only 15% to 25% of patients have early-stage disease that can benefit from radical resection at diagnosis.
Long-term survival in NSCLC following surgical resection is stage-related. However, even when the surgical treatment of stage I lung cancer patients is considered successful, it is still a challenge to control this early-stage disease, mostly because of recurrence. Tumor recurrence is the most common cause of treatment failure after surgical resection [6-10]. The purpose of this study is to identify the factors related to recurrence following surgery and help stratify patients with higher risks into the administration of potentially harmful adjuvant therapies. There are two methods for the identification of factors related to recurrence following surgery. One is the classical determination using clinical variables as in our study. The other is based on molecular biological methods.
We set out to identify the clinicopathological factors associated with recurrence including early recurrence in early-stage NSCLC. Our study revealed that even in the case of curative resection, which is the most effective therapy for early-stage disease, the overall incidence of recurrence was 31.8% (pathologic stage IA, 24.1%; pathologic stage IB, 38.5%). The predictors of recurrence in resected stage I NSCLC according to the 6th edition of the TNM classification (TNM 6) have been described in literature [5-7]. However, only few studies evaluated the risk factors for recurrence in resected stage I NSCLC according to the 7th edition of the TNM classification (TNM 7) [11]. Maeda and colleagues reported that histologic differentiation, vascular invasion, and visceral pleural invasion were significant risk factors for recurrence in stage I NSCLC. In our study, the factor predicting recurrence was a poorly differentiated histology (p=0.017).
A majority of recurrences are distant and occur within the first 2 years of surgery [5-7]. Our study also showed similar results. The sites of the first failure were distant metastases to the brain, bone, liver, or contralateral lung in most of the patients reported by Martini et al. [12] and by Pairolero et al. [13]. In both studies, the brain was the most common site. In contrast, the highest initial failure site in our study was the lung (19/51, 37.3%). To date, most large studies have shown that the patterns of recurrence may differ by histology with more local recurrence seen in patients with squamous cell carcinoma and more distant metastases seen in patients with adenocarcinoma [12-14]. In our study, however, the pattern of recurrence did not differ by histology.
The recurrence was classified into three groups according to the timing of recurrence: none, early, and late recurrence. Early recurrence was seen in 17.8% (43/242) of all patients (pathologic stage IA patients, 11.6%; pathologic stage IB patients, 23.1%). Martini et al. [6] demonstrated that early recurrence (≤1 year) was observed in 27% of the patients with recurrence in pathological stage I NSCLC. The factors influencing lower incidence of recurrence were the extent of lung resection and lymph node dissection. In our study, the univariate analysis for early recurrence identified 6 significant risk factors (male, smoking status, tumor size, advanced pathologic stage, poorly-differentiated carcinoma, and PET-CT maxSUV), while the multivariate analysis showed that smoking status and poorly differentiated carcinoma were independent risk factors for early recurrence.
Some recent clinical studies have shown that postoperative chemotherapy is effective in the management of patients after a complete resection of stage II and IIIA NSCLC. The Lung Adjuvant Cisplatin Evaluation (LACE) pooled data of the five largest randomized trials completed since 2004 and reanalyzed this collectively [15]. This study concluded that postoperative cisplatin-based chemotherapy significantly improves survival in patients with NSCLC. The LACE trial demonstrated a clear survival benefit of adjuvant platinum-based chemotherapy-at least 5% for OS and 6% for DFS. However, the role of adjuvant chemotherapy in patients with resected stage I NSCLC is controversial because of the lack of a clear survival benefit from randomized trials. On the basis of our study, patients who are at risk for early recurrence may be considered for and may benefit from adjuvant chemotherapy.
However, there are several limitations of our study. Our data were analyzed retrospectively. For more conclusive results, a prospective randomized controlled study is required in the future. Moreover, as ours is a retrospective, single-institution study, patient selection bias with respect to the diagnosis of cancer recurrence might be inevitable compared with a multi-institutional prospective study. In this study, the overall follow-up observation period was short. Therefore, a relatively long follow-up observation period should be considered for long-term results.
In conclusion, the present study focused on the identification of factors related to early recurrence within two years of surgery in early-stage NSCLC patients. The identified risk factors were smoking history and poor histologic differentiation. Therefore, in cases with these risk factors for early recurrence, adjuvant therapy may be used for improving survival.
ACKNOWLEDGMENTS
This work was supported by the Dong-A University research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Dominioni L, Imperatori A, Rovera F, Ochetti A, Torrigiotti G, Paolucci M. Stage I nonsmall cell lung carcinoma: analysis of survival and implications for screening. Cancer. 2000;89(11 Suppl):2334–2344. doi: 10.1002/1097-0142(20001201)89:11+<2334::aid-cncr4>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Goodgame B, Stinchcombe TE, Simon G, Wozniak A, Govindan R. Recent advances in lung cancer: summary of presentations from the 45th annual meeting of the American Society of Clinical Oncology (2009) J Thorac Oncol. 2009;4:1293–1300. doi: 10.1097/JTO.0b013e3181b7ef95. [DOI] [PubMed] [Google Scholar]
- 3.Brundage MD, Davies D, Mackillop WJ. Prognostic factors in non-small cell lung cancer: a decade of progress. Chest. 2002;122:1037–1057. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- 4.Pollack JR. A perspective on DNA microarrays in pathology research and practice. Am J Pathol. 2007;171:375–385. doi: 10.2353/ajpath.2007.070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones DR, Daniel TM, Denlinger CE, Rundall BK, Smolkin ME, Wick MR. Stage IB nonsmall cell lung cancers: are they all the same? Ann Thorac Surg. 2006;81:1958–1962. doi: 10.1016/j.athoracsur.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 6.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 7.Harpole DH, Jr, Herndon JE, 2nd, Young WG, Jr, Wolfe WG, Sabiston DC., Jr Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer. 1995;76:787–796. doi: 10.1002/1097-0142(19950901)76:5<787::aid-cncr2820760512>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa T, Okumura N, Ohata K, Igai H, Matsuoka T, Kameyama K. Postrecurrence survival in patients with stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;34:499–504. doi: 10.1016/j.ejcts.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Sugimura H, Nichols FC, Yang P, et al. Survival after recurrent nonsmall-cell lung cancer after complete pulmonary resection. Ann Thorac Surg. 2007;83:409–417. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 10.Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax. 2009;64:192–196. doi: 10.1136/thx.2007.094912. [DOI] [PubMed] [Google Scholar]
- 11.Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K. Poor prognostic factors in patients with stage IB non-small cell lung cancer according to the seventh edition TNM classification. Chest. 2011;139:855–861. doi: 10.1378/chest.10-1535. [DOI] [PubMed] [Google Scholar]
- 12.Martini N, Flehinger BJ, Zaman MB, Beattie EJ., Jr Prospective study of 445 lung carcinomas with mediastinal lymph node metastases. J Thorac Cardiovasc Surg. 1980;80:390–399. [PubMed] [Google Scholar]
- 13.Pairolero PC, Williams DE, Bergstralh EJ, Piehler JM, Bernatz PE, Payne WS. Postsurgical stage I bronchogenic carcinoma: morbid implications of recurrent disease. Ann Thorac Surg. 1984;38:331–338. doi: 10.1016/s0003-4975(10)62281-3. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Rubinstein L. Cancer recurrence after resection: T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1990;49:242–246. doi: 10.1016/0003-4975(90)90145-v. [DOI] [PubMed] [Google Scholar]
- 15.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]