Abstract
Despite improvements in the multi-modality treatment of colorectal liver metastasis (CRLM), survival after resection remains varied. Determining prognosis after surgical resection has historically been predicated on preoperative clinicopathological factors such as primary tumor stage, carcinoembryonic antigen levels, number of liver metastases, presence of extrahepatic disease, as well as other factors. While scoring systems have been developed by combining certain preoperative factors, these have been inconsistent in accurately determining prognosis. There has been increasing interest in the use of biologic and molecular markers to predict prognosis following CRLM. The role of markers such as KRAS, BRAF, p53, human telomerase reverse transcriptase, thymidylate synthase, Ki-67, and hypoxia inducible factor-1α and their correlation with accurately predicting survival after surgical resection have been supported by several studies. Furthermore, other elements such as pathological response to chemotherapy and the presence of circulating tumor cells have shown promise in accurately determining prognosis after resection for colorectal liver metastasis. We herein review past, present, and possible future markers of prognosis among colorectal cancer patients with liver metastasis undergoing resection with curative intent.
Keywords: Colorectal, Metastasis, Prognosis, Risk score, Molecular markers, Outcomes
Core tip: Historically, prognosis after resection has been largely assessed based on preoperative clinicopathologic features. Data validating the prognostic value of patient and tumor specific factors have been mixed, with many recent studies showing these scoring systems to correlate poorly with survival. Rather, there has been an emerging interest in biological or molecular markers of prognosis to more effectively assess patient prognosis after resection of colorectal liver metastasis. In this review, we discuss past, present, and possible future markers of prognosis among colorectal cancer patients with liver metastasis undergoing resection with curative intent.
INTRODUCTION
Colon cancer is the 3rd most common cancer worldwide. It has an estimated incidence of 42.5 per 100000 with over 140000 estimated new cases expected in the United States in 2013 as reported by the Center for Disease Control[1,2]. About 14%-25% of patients with colorectal cancer will have liver metastasis at presentation and up to 60% of patients will develop metastasis at some point after diagnosis[3-6]. Surgical resection remains the only hope for cure. Contemporary series have demonstrated that surgical therapy for colorectal liver metastasis (CRLM) is associated with a low operative mortality of 1% to 2%[7,8]. The reported 5- and 10-year survival does vary, however, ranging from 25% to 74% (median 38%) and 9% to 50% (median 26%), respectively, depending on the era from which the data were reported and the underlying patient population. Historically, prognosis after resection has been largely assessed based on preoperative clinicopathologic features. Data validating the prognostic value of patient and tumor specific factors have been mixed, with many recent studies showing these scoring systems to correlate poorly with survival. Rather, there has been an emerging interest in biological or molecular markers of prognosis to more effectively assess patient prognosis after resection of CRLM. In this review, we discuss past, present, and possible future markers of prognosis among colorectal cancer patients with liver metastasis undergoing resection with curative intent.
CLINICAL MARKERS
Numerous clinical prognostic factors have been identified in an attempt to estimate overall prognosis among patients with CRLM. The most relevant factors have been included in clinicopathological scoring systems, proposed in the late 90s and now widely used by many clinicians[9,10]. The role of each of these factors in determining the prognosis of patients with CRLM is still, however, a matter of some debate. Furthermore, there remains no consensus regarding which of these clinicopathological factors has the “best” prognostic value (Table 1).
Table 1.
Studies of prognostic clinicopathological factors
| Study | Primary stage | CEA level | Size of major metastasis | Number liver metastases | Disease free interval | Extrahepatic disease | Surgical margins |
| Scheele et al[11] | P | NA | P | NP | P | P | P |
| Nordlinger et al[10] | P | P | P | P | P | NA | P |
| Fong et al[9] | P | P | P | P | P | P | P |
| Mann et al[12] | P | P | P | NP | NP | NA | NA |
| Rees et al[15] | P | P | P | P | NA | P | P |
| John et al[21] | NP | P | NP | NP | NA | NP | P |
| Doci et al[27] | P | NP | NP | NP | NP | NA | NA |
| Hughes et al[34] | P | P | P | P | P | NA | NA |
| Gayowski et al[37] | P | NA | NP | P | NP | P | P |
CEA: Carcinoembryonic antigen; P: Prognostic; NP: Non prognostic; NA: Non available.
Primary tumor stage
Advanced primary tumor stage has been considered a negative prognostic factor by multiple investigators. Scheele et al[11] initially proposed a correlation between the primary tumor grade and overall survival (OS) as well as disease free survival (DFS). Primary tumor stage was later incorporated into clinical prognostic scoring systems[9,10]. Specifically, Fong et al[9] proposed the stage of the primary tumor as an adverse prognostic factor, concluding that the nodal status of the primary cancer was highly predictive of outcome[9,12-15]. A subsequent meta-analysis reported an association between primary tumor stage, nodal metastasis, and worse outcomes following resection of CRLM[13-15]. Tranchart et al[16] similarly noted that primary tumor lymph node metastasis was an independent predictor of adverse OS and DFS. Previously Bennett et al[17] analyzed the prognostic value of perihepatic lymph node micrometastases in patients with CRLM. Patients with at least one perihepatic lymph node with metastases had a shorter recurrence free survival.
Preoperative carcinoembryonic antigen level
The role of carcinoembryonic antigen (CEA) as a robust predictor of long-term survival following resection of CRLM remains poorly defined[9,10,12,18-22]. Among many patients, CEA can be an effective marker to monitor for recurrence, as well as to assess response to systemic therapy[18,21]. CEA levels can correlate with the radiological response to preoperative chemotherapy; however, other data have suggested that the absolute change in CEA level with chemotherapy may not correlate with long-term outcome[23]. As a pre-operative prognostic factor, Mann et al[12] reported that CEA levels did correlate with 5-year survival (CEA levels < 200 ng/mL: 48.9% vs > 200 ng/mL: 0.0%). Other studies have similarly noted that preoperative CEA > 200 ng/mL was an independent factor of poor OS and disease specific survival (DSS), respectively[18,21]. In a one study, Park et al[19] looked at both tissue CEA and serum CEA concentration after resection for CRLM and noted that CEA expression was an independent prognostic factor for OS and DFS. Of note, patients with elevations in both tissue CEA expression and serum CEA had a worse OS and DFS compared with patients who had only one CEA category elevated[19]. Despite these data, other studies have noted that CEA level was not a significant predictor of survival or recurrence after hepatic resection for metastatic colorectal cancer[24-27]. The reason for the disparate finding from various studies may be due to the different cut-off values used for CEA, as well as differences in how the statistical models were constructed (e.g., which other competing risk factors were put into the model, how many patients in any given study had a particular factor, etc.).
Number of liver metastases
Several studies have reported that a higher number of CRLM lesions is a poor prognostic factor[28-32]. A recent large meta-analysis examining nearly 10000 patients reported a 5-year survival of only 17.1% for patients with four or more CLMs[28]. Other studies have found no difference in survival based on the number of tumors with 5-year survival ranging from 40%-50% regardless of tumor burden[9,27,29-32]. The reason for these differences may be related to patient selection, differences in surgical approach (resection only, resection plus ablation, etc.), as well as differences in the use of neoadjuvant chemotherapy. For example, in a study by Pawlik et al[33] the 5-year survival among patients with 4 or more CRLM was 50.9%, however many of the patients had been pretreated with neoadjuvant chemotherapy and response to neoadjuvant therapy was strongly associated with survival. As such, the impact of tumor number on prognosis needs to be considered in light of other important clinical and therapeutic information. While the limit of hepatic involvement that precludes a patient from being “operable” is still a matter of debate, the general consensus is that tumor number should not be used as an absolute contraindication to surgery. When all the lesions can be resected with a microscopically negative margin (R0) in the setting of an adequate future liver remnant (FLR), surgery should at least be contemplated. Considering that as the number of tumor metastases increases, a curative resection becomes more technically challenging, the number of liver tumors may impact survival when all tumors are not able to be completely removed.
Size of liver metastases
The size of the largest metastasis is another clinical factor that has long been considered a prognostic factor. Mann et al[12] reported that 5-year survival was 51.6% among patients undergoing surgery for CRLM ≤ 5 cm compared with 27% for those patients with a tumor > 5 cm. In other studies, patients with CRLM measuring > 5 cm were similarly noted to have a worse survival[15]. Specifically, Aldrighetti et al[22] reported that patients with a CRLM lesion measuring > 5 cm had a survival of only 18.8% vs 30% for patients with smaller tumors. In a separate study, Rees et al[15] similarly reported that CRLM diameter > 5 cm was an independent predictor of survival. As such, tumor size > 5 cm has been adopted by several investigators as a predictor of adverse long-term outcome, evidenced by the inclusion of tumor size in multiple clinical scoring systems[9,11,14,34-36]. However, several other studies have been unable to find any differences in recurrence and survival with relation to tumor size[20,24,27,37]. Modern era chemotherapeutic agents are now frequently able to cyto-reduce or downsize metastasis. In this context, it is not clear if tumor size continues to hold important prognostic information. Response to chemotherapy - as evidenced by change in tumor size - may be a more important and relevant prognostic marker than initial CRLM tumor size[38,39].
Synchronous metastases and disease free interval
Approximately 25% of patients have a synchronous presentation of their primary tumor and CRLM at the time of diagnosis[1]. Some authors have found an association between the presence of synchronous metastasis and a worse prognosis[9-11,34,40], while others have not noted that synchronous presentation has an effect on survival[12,20,27]. Similarly, there is no consensus regarding the impact of disease-free interval on outcomes. Some authors have reported that a short disease-free interval did not impact disease-free or OS[12], however other investigators consider disease-free interval a reliable prognostic factor[9,22]. Fong et al[9] concluded that disease-free interval of < 12 mo after resection of the colorectal primary was predictive of adverse outcomes, and included this factor in the clinical risk score. Tan et al[18] similarly noted that a disease-free interval < 12 mo was an independent predictor of disease-specific survival (DSS) at 3 years. The prognostic role of disease-free interval is still controversial. One reason why the impact of disease-free interval may have changed over time is that there is more effective adjuvant treatment for patients with advanced colorectal cancer. More effective chemotherapy may prolong the disease-free interval among these patients and may contribute to why studies conducted in the past might not be comparable to the ones conducted in the era of modern chemotherapy.
Extrahepatic disease
Traditionally extrahepatic disease (EHD) has been considered a contraindication to hepatectomy for CRLM due to the unfavorable prognosis previously noted in multiple studies[10,34,41-43]. While the presence of EHD has clear prognostic implications, the impact of the extent and location of the EHD and its effect on prognosis has been debated. In a study by Elias et al[44], the investigators argued that the total number of metastases was more prognostically important than the site of EHD. While other groups have shown that multiple EHD sites is clearly associated with a worse survival[45,46], the site of EHD also has prognostic importance. Specifically, Pulitanò et al[46] noted that the location of EHD was associated with prognosis, as patients having pulmonary metastasis had the best prognosis and patients with retroperitoneal/aortocaval lymph node metastasis had the worse prognosis. Pulmonary metastasectomy has been demonstrated to prolong survival in selected patients and has a clear benefit in patients with solitary or oligometastatic disease[47-49]. Specifically, 5-year survival after pulmonary resection of colorectal metastasis has been reported to be as high as 48.0%[50]. In contrast, regional lymph node involvement has been correlated with a worse survival, with observed 5-year OS of 25% for pedicular, 0% for celiac, and 0% for para-aortic lymph node involvement[51].
Surgical margin status
Microscopically negative surgical margins (R0) have traditionally been considered an important prognostic factor following resection of CRLM. Most authors have indeed reported that an R1 (microscopically positive) and R2 (macroscopically positive) margin are associated with worse long-term OS[9,15,20,21,52-55]. While there has been some lack of consensus as to what constitutes a “truly” microscopically negative margin[56-59], Pawlik et al[60] demonstrated in a large cohort of patients that margin width > 1 mm was not associated with overall risk or pattern of recurrence. Kokudo et al[30], using a sensitive genetic analysis detecting KRAS and p53 mutations, found micrometastases in the liver parenchyma surrounding CRLM in only 2% of patients, all within 4 mm of the tumor border. Andreou et al[61] did report that it was important to achieve an R0 margin as patients who had an R1 resection were noted to have a worse outcome. Some investigators have argued, however, that it is biology, not millimeters that dictate prognosis following resection[62]. Specifically, these investigators note that margin status is often confounded by the extent of intrahepatic disease. Patients with a larger intrahepatic tumor burden are most at risk for an R1 margin; it is these patients who also have worse overall tumor biology and overall recurrence. To this point, de Haas et al[23] did not find a difference in OS among patients undergoing an R0 vs R1 resection. These data may suggest that, in an era of more effective chemotherapy options, leaving microscopic disease behind may result in increased local failure but not necessarily a worse OS. The impact of margin status on outcomes may therefore be influenced by patient and tumor factors, as well as the utilization of chemotherapy[61]. Regardless of the impact of margin status on prognosis, complete macroscopic and microscopic removal of all lesions with negative resection margins should remain the gold standard in the surgical treatment of CRLM[23].
Operative and post-operative factors
There is no consensus regarding the impact of blood loss, transfusion, or postoperative complications on survival following resection of CRLM[63-66]. The most convincing prognostic factor seems to be the effect of infections and other postoperative complications[40,67]. Specifically, Mavros et al[68] reported that postoperative complications were independently associated with decreased long-term survival after surgery for CRLM with curative intent. The effect of complications on long-term survival may be due to the immune modulating effects of sepsis, impaired immune system and consequent metastatic spread. Moreover, a high rate of complications, longer hospital stays and the delayed wound healing may cause a postponement or avoidance of necessary adjuvant treatments, which in turn may have implications for long-term survival.
Clinical scoring system
One of the first preoperative prognostic scoring systems was described by Nordlinger et al[10] in 1996. In this scoring system, one point was given to each of the following factors: age, size of largest metastasis, CEA level, stage of the primary tumor, disease-free interval, number of liver nodules, and resection margin[10]. Subsequently, Fong et al[9] proposed a “clinical risk score” to predict long-term outcome and recurrence. In a cohort of 1001 patients treated with resection of CRLM, the authors identified 5 criteria as significantly impacting prognosis: nodal status of the primary tumor, disease-free interval, number of hepatic metastases > 1, preoperative CEA level > 200 ng/mL, and size of the largest metastasis > 5 cm[9]. One point was assigned to each factor (Table 2) and the total score was reported to be highly predictive of long-term outcome (Figure 1). This score has been widely utilized; while some groups have validated the scoring system, other investigators have questioned its prognostic accuracy[12,25,26,69-72]. In a separate study, Iwatsuki et al[73] proposed a different prognostic score that included tumor number ≥ 3, tumor size > 8 cm, time to hepatic recurrence ≤ 30 mo as well as the presence of bilobar tumors. The prognostic score, calculated by summing these prognostic factors, was suggested to predict 5-year survival. When comparing the Fong score[9] with other described clinical scoring systems, including the Nordlinger score[10], Iwatsuki score[73], Mayo Clinic scoring system and Basingstoke index, several authors have found that only the Fong and the Iwatsuki scores provide a statistically significant stratification of disease specific survival[9,10,15,70,71,73,74]. In 2008, the Memorial Sloan Kettering Cancer Center (MSKCC) proposed the first nomogram for predicting disease-specific survival for the individual patient[75]. The nomogram appears to better represent characteristics of individual patients, for instance incorporating the true preoperative CEA value rather than applying an arbitrary cutoff value[76].
Table 2.
Survival based on the clinical risk cumulative score (adapted from Fong et al[9])
| Cumulative score | 1 yr | 2 yr | 3 yr | 4 yr | 5 yr | Median, mo |
| 0 | 93 | 79 | 72 | 60 | 60 | 74 |
| 1 | 91 | 76 | 66 | 54 | 44 | 51 |
| 2 | 89 | 73 | 60 | 51 | 40 | 47 |
| 3 | 86 | 67 | 42 | 25 | 20 | 33 |
| 4 | 70 | 45 | 38 | 29 | 25 | 20 |
| 5 | 71 | 45 | 27 | 14 | 14 | 22 |
| Prognostic factor | Score 0 | Score 0 | Score 0 | Score 1 | Score 1 | Score 1 |
| Node-positive primary | negative | negative | negative | positive | positive | positive |
| Disease-free interval | ≥ 12 mo | ≥ 12 mo | ≥ 12 mo | < 12 mo | < 12 mo | < 12 mo |
| Number of liver metastases | 1 | 1 | 1 | > 1 | > 1 | > 1 |
| Size of major liver metastases | ≤ 5 cm | ≤ 5 cm | ≤ 5 cm | > 5 cm | > 5 cm | > 5 cm |
| CEA (ng/mL) | < 200 ng/mL | < 200 ng/mL | < 200 ng/mL | > 200 ng/mL | > 200 ng/mL | > 200 ng/mL |
CEA: Carcinoembryonic antigen.
Figure 1.
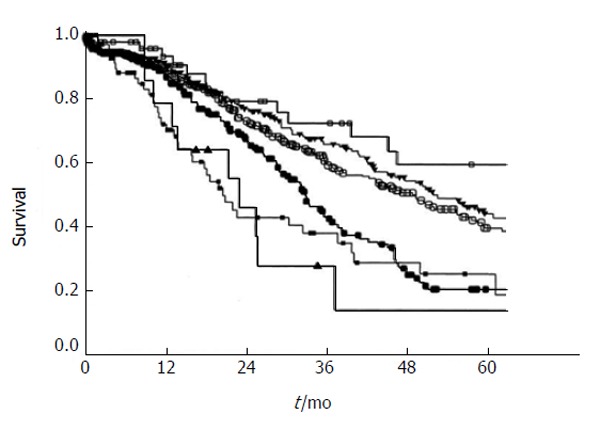
Survival after hepatic resection stratified by the clinical risk score. Open box: score 0 (n = 52); filled triangle: score 1 (n = 262); open circle: score 2 (n = 350); filled circle: score 3 (n = 243); filled box: score 4 (n = 80); open triangle: score 5 (n = 14). P < 0.0001 (from Fong et al[9]). Used with permission.
The ultimate clinical value of these prognostic scoring systems remains debatable. In a study by Nathan et al[77], the authors reported a c-statistic of only 0.5 to 0.6 for many of the scoring systems. The authors postulated that the moderate-to-poor accuracy of the staging systems was related to the inability to account for neoadjuvant treatments, varying R0 resection rates, as well as differences in establishing categorical cutoff values for continuous data fields (e.g., CEA level > 200 ng/mL, and size of the largest metastasis > 5 cm, etc.). Moreover, despite some external validation, these score are based on single-institution cohorts and have not been modified based on newer developments in treatments. Lastly, the variations observed in the OS of patients with similar prognostic scores suggest that other factors may play a role in determining survival after resection of CRLM, most intriguingly patient-specific biological and molecular factors[78].
BIOLOGICAL, PATHOLOGICAL, AND MOLECULAR MARKERS
Recently, attention has turned to the use of biological and molecular markers as a more accurate means to predict long-term outcomes. Patient and tumor specific markers may provide more accurate predictions of survival after hepatic resection for colorectal metastasis (Table 3).
Table 3.
Studies of prognostic biomarkers
| Study | No. of patients | Positive case (%) | Biomarker | Correlation with survival |
| Nash et al[110] | 188 | 27 | KRAS | Independent predictor of poor survival (HR = 1.9) |
| Nash et al[110] | 188 | 62 | Ki-67 | Independent predictors of poor survival (HR = 2.6) |
| Teng et al[113] | 292 | 2.1 | BRAF | Independent prognostic biomarker after metastasectomy (HR = 6.245, P < 0.003) |
| Smith et al[26] | 66 | 36 | Ki-67 | Ki-67 correlate with survival (P = 0.04) |
| Smith et al[26] | 66 | 35 | hTERT | Htert correlate with survival (P = 0.0001) |
| Dômont et al[25] | 201 | 43 | hTERT | Independent predictor of poor survival |
| (RR = 2.03, P < 0.0001) | ||||
| Gonen et al[123] | 156 | Not reported | TS | Independent predictor of poor survival |
| (RR = 4.22, P < 0.01) | ||||
| Costa et al[137] | 104 | Not reported | TLI | High TLI independently |
| Predicted decreased DFS | ||||
| (P = 0.035) | ||||
| Nitti et al[128] | 69 | 64 | p53 | Independent predictor of poor survival |
| (RR = 2.53, P = 0.008) | ||||
| Mehta et al[144] | 50 | 30 | FGA | A high FGA is an independent predictor of survival (P = 0.01) |
| Shimomura et al[136] | 64 | 31 | HIF-1α | High HIF-1α is an independent risk factor for recurrence |
hTERT: Human telomerase reverse transcriptase; TS: Thymidylate synthase; TLI: Thymidylate labeling index; DFS: Disease free survival; FGA: Fraction of genome altered; HIF-1α: Hypoxia inducible factor-1α.
Tumor response to preoperative chemotherapy on imaging
Preoperative chemotherapy is increasingly being used, especially among patients with advanced CRLM. Preoperative “conversion” chemotherapy has allowed many previously unresectable patients to be treated and converted/downsized so that surgery becomes possible[79,80]. In some centers, neoadjuvant therapy for patients with resectable disease is also frequently being used[32,79]. The use of preoperative systemic chemotherapy provides the opportunity to assess response. Response to chemotherapy has been shown to improve 5-year survival from 35% to 85% in one study when compared with patients who did not receive chemotherapy[38]. Adam et al[32] reported a 30% increase in 5-year survival among patients who underwent hepatectomy after an objective tumor response vs patients who had tumor progression while receiving neoadjuvant chemotherapy. Similarly, recurrence-free survival (RFS) has been shown to be influenced by tumor response. In a study by Gruenberger et al[39], patients who had a response to chemotherapy had a RFS of 24.7 mo vs only 3 mo for patients with progressive disease.
Most commonly, response to chemotherapy can be assessed by standard cross-sectional imaging using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria. RECIST allows for the assessment of changes in the standard cross-sectional diameter of lesions. Oxaliplatin- and irinotecan-based cytotoxic chemotherapeutic regimens may result in radiographic “shrinkage” of tumors. In contrast, the use of biologic or targeted agents, such as bevacizumab, can sometimes be difficult to assess using RECIST criteria on cross-sectional imaging. For example, in a phase III study examining the addition of bevacizumab to oxaliplatin-based chemotherapy for metastatic colorectal cancer, the investigators noted an improved progression-free survival without affecting RECIST-defined response rates[81]. In a separate study, Chun et al[82] reported that morphological changes in CRLM lesions - rather than RECIST changes - were prognostic with regard to long-term outcomes.
Tumor response to preoperative chemotherapy on pathology
In addition to assessment on preoperative imaging, tumor response can be assessed on pathological examination after extirpation of the tumor. Andreou et al[61] reported on the effect of pathological response to neoadjuvant chemotherapy in achieving negative margins. Patients with a minor pathologic response to preoperative chemotherapy (≥ 50% residual viable tumor cells) had significantly worse OS (5-year OS rate 46% after R0 resection vs 0% after R1 resection). In a study by Adam et al[83], complete pathological response (CPR) was similarly correlated with an increase in overall 5-year survival from 45% to 76%. This finding was subsequently confirmed by correlating pathologic response, considered as mean of the percentage of cancer cells remaining within each tumor, with 5-year overall survival. In a separate study, Blazer et al[84] reported on 305 patients who underwent preoperative irinotecan- or oxaliplatin-based chemotherapy, followed by resection of CRLM. In this group of patients, 9% had a complete response (no residual cancer cells), 36% a major response (1% to 49% residual cancer cells), and 55% a minor response (≥ 50% residual cancer cells). The residual tumor was assessed semiquantitatively, estimating the proportion of residual cancer cells in relation to the tumor area, comprehensive of areas of chemotherapy-related tissue injury, tumor necrosis, fibro-collagenous proliferation, and other reparative changes. Survival was strongly correlated with pathologic response: 5-years survival was 75%, 56%, and 33% for patients with a complete response, major response, or minor response respectively[84].
A semi-quantitative analysis of the proportion of viable cancer cells, however, is limited due to the difficulty in determining the baseline percentage of tumor cell before preoperative chemotherapy. Therefore, it could be that this type of pathological response would have a better prognostic role than a predictive one based on response to chemotherapy[82,84]. Interestingly Tanaka et al[85] found that a complete pathological response in all the metastases is not necessary to obtain a correlation with OS. The authors showed that patients with multiple metastases and complete response in some of those tumors still experienced a higher OS and DFS compared with pathologic non-responders. The “best” OS was, however, noted among those patients in whom all CRLM lesions showed a complete response[85].
Tumor regression grading, as well as tumor thickness at the tumor-normal interface, have been proposed as prognostic histopathological factors[83-90]. Based on the tumor regression scheme proposed for esophageal carcinoma, Rubbia-Brandt et al[90] described a pathological grading system for CRLM[90]. In this schema, tumor regression was characterized by fibrosis overgrowing on tumor cells, decreased necrosis, and the presence or absence of tumor glands at the periphery of liver metastases. Based on this, a tumor regression grade (TRG) score, ranging from 1 to 5, was proposed and subsequently shown to correlate with DFS[91]. Maru et al[89] recently introduced the idea of using tumor thickness measured at the tumor-normal interface as a new prognostic factor for therapy response and survival. Greater tumor thickness predicted shorter recurrence-free survival: 70% for patients with a tumor thickness of < 0.5 mm, 51% for patients with a tumor thickness between 0.5 mm and 5 mm, and 35% for patients with a tumor thickness of ≥ 5 mm[89].
Other factors noted on pathology beyond response to preoperative therapy may impact prognosis. Rudolf Virchow hypothesized in 1863 that the origin of cancer was at sites of chronic inflammation. Today, the causal relationship between inflammation, innate immunity and cancer is widely acknowledged. Nonetheless, many of the molecular and cellular mechanisms mediating this relationship still remain unresolved[92,93]. Okano et al[94] reported that patients with dense tumor infiltrating lymphocytes (TIL) surrounding metastatic liver survived longer than patients with weak TILs after hepatic resection. Canna et al[95] recently examined the relationship between local and systemic inflammatory responses and outcomes in patients undergoing resection of colorectal cancer. A low tumor CD4+ T-lymphocyte infiltrate was associated with an elevated circulating C-reactive protein (CRP) and both were associated with a poor outcome. Furthermore CRP was superior to tumor T-lymphocytic infiltration in predicting cancer specific survival[95]. There is increasing evidence that a host’s inflammatory response to tumor (IRT) is associated with recurrence and lower survival in patients undergoing potentially curative resection for colorectal cancer[96,97]. Similar studies have shown worse OS and DFS in patients with an elevated preoperative CRP > 10 mg/L and neutrophil to lymphocyte ratio (NLR) > 5:1[93,95-100]. NLR > 5:1 has been shown to be an independent predictor of recurrence and worse survival in patients undergoing resection for CRLM[101].
MOLECULAR MARKERS OF PROGNOSIS
KRAS, BRAF
KRAS, along with HRAS and NRAS, belongs to a family of GTPases. When activated, KRAS can induce a cascade of mitogen-activated protein kinases (MAPKs) that transfers signals from the cell membrane via the cytoplasm into the nucleus. The ras gene products activate proteins in the Raf family, which consists of the ARAF, BRAF and RAF-1 members[102]. Mutations of the KRAS gene predicts resistance to epidermal growth factor receptor (EGFR)-targeted monoclonal antibodies, and acquired resistance to anti-EGFR therapies may be due to the late switch in KRAS mutational status[103,104]. The reported prevalence of KRAS mutations in liver metastases varies from 15% to 44%. While several studies have reported no statistically significant association between KRAS mutation and metastatic progression, proliferative index, or survival has been reported[105-109], Nash et al[110] did report a prevalence of 27% KRAS mutation in liver metastasis and noted an independent association between KRAS mutation and worse survival after liver resection (Figure 2). In a separate study, Karagkounis et al[111] reported KRAS and BRAF analysis performed on 202 patients undergoing surgery for CRLM at the Johns Hopkins Hospital. In this study, the authors noted that KRAS mutations were found in approximately one third of patients, while BRAF mutations were found in only 2% of patients undergoing surgery for CRLM. KRAS status was an independent predictor of overall and recurrence-free survival (Figure 3); the low incidence of BRAF mutation limited assessment of its prognostic impact[111]. Other studies, however, have noted BRAF mutation to be an independent prognostic factor of worse survival following resection of CRLM, as well as a poor prognostic factor for colon cancer patients of various stages[102,112-114]. KRAS status may not only predict overall recurrence, but perhaps also the pattern of recurrence. Vauthey et al[115] recently reported that RAS mutation was predictive of early lung recurrence after curative resection of CRLM.
Figure 2.
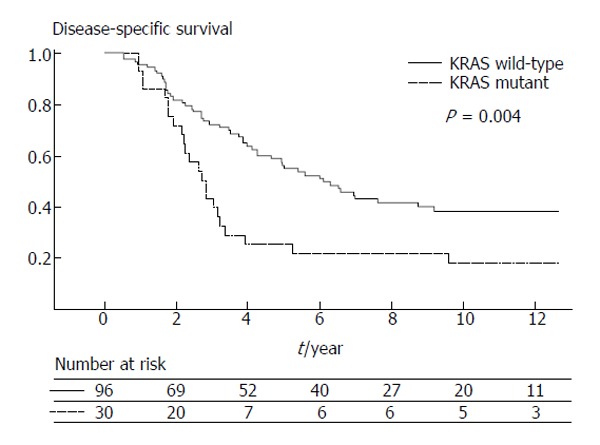
Disease specific survival after liver resection stratified by KRAS mutation (from Nash et al[110]). Used with permission.
Figure 3.
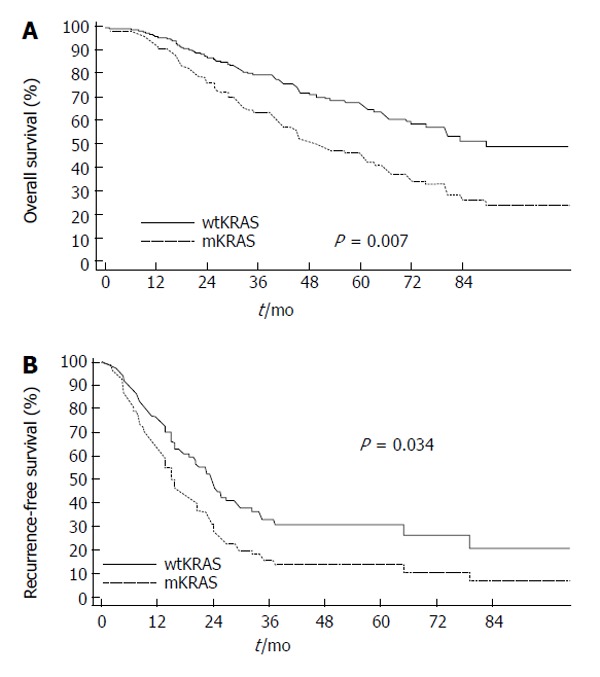
Overall (A) and disease-free (B) survival after hepatic surgery for colorectal liver metastasis depicted by KRAS mutation status (multivariate Cox model) (wtKRAS, wild type KRAS; mKRAS, mutated KRAS) (from Karagkounis et al[111]). Used with permission.
As the MAP kinase signaling pathway is involved in the inflammatory cascade, Huang et al[112] described the role of the activated MAP kinase pathway and CRP in liver metastases. This study demonstrated the significance of both specific C reactive protein (CRP) single nucleotide polymorphisms (SNP) and mutations in KRAS/BRAF in liver metastases with respect prognosis after resection of CRLM[116]. CRP SNP rs7553007 and KRAS mutations were found to be independent prognostic factors for CRC patients with synchronous liver metastasis[112].
hTERT
Telomerase is a ribonucleoprotein enzyme responsible for the replication of telomeres, preventing cell senescence and death. Telomerase has two core functional components: the catalytic subunit of hTERT (with telomere-specific reverse transcriptase activity) and a telomerase RNA template. hTERT is the rate-limiting component of telomerase complex and its expression correlates with telomerase activity[117]. Despite the growing evidence that hTERT is predictive of response to neoadjuvant chemoradiation among patients with rectal cancer, the prognostic role of hTERT among patients with resected CRLM has not been well studied[114,115]. Fong et al[9] and Smith et al[26] did compare the prognostic value of the Fong clinical scoring system vs markers of cell proliferation, such as hTERT. In this study, the authors noted that hTERT correlated better with survival than predictions based on the clinical risk score[9,26]. The independent prognostic value of hTERT has subsequently been validated as predictor of worse overall survival among patients with surgically resected CRLM[25,118,119].
Thymidylate synthase
Thymidylate synthase (TS), the target enzyme of fluorouracil (FU)-based chemotherapy, is commonly reported to correlate with response to systemic therapy and survival[120-123]. A few small studies have suggested that TS gene overexpression might be associated with poor prognosis in patients undergoing resection of CRLM[120-122]. A separate study by Gonen et al[123] confirmed that TS was an independent poor prognostic factor for OS and progression-free survival in a multivariate analysis using data from a large cohort of patients with resected CRLM. Interestingly, this same group also analyzed tumor mRNA and confirmed that tumor TS expression was associated with lower RFS and disease specific survival[123]. Other authors have also noted that TS seems to correlate with the clinic risk score in patients who undergo resection for CRLM[124].
p53
p53 is a tumor suppressor gene with a central role in controlling the cell cycle and apoptosis through regulation of Bax activity. p53 mutation correlates with the development of CRLM, as well as increased metastatic burden[125]. While such findings have raised interest in the potential of p53 as a predictive biomarker, data on the prognostic role of p53 in patients with resected CRLM has yielded mixed and inconclusive results[126]. Bellucco et al[127] showed a lower median survival among patients with p53-positive tumors with synchronous unresectable CRLM treated by hepatic artery infusional chemotherapy. These data were later confirmed in patients undergoing curative hepatic resection for CRLM, as p53 protein status was the single best predictor of survival (median survival: p53 wild type, 93 mo vs p53 mutated 27 mo)[128]. Similarly, 3- and 5-year survival were better among patients with p53 wild type CRLM[128]. Tanaka et al[129] also showed that mutated p53 remained an independent prognostic factor for worse survival after hepatectomy based on a multivariate analysis. In contrast, Yang et al[130] reported a separate study in which patients with p53 mutated CRLM actually had a better long-term survival after liver resection compared with patients who had wild type p53 tumors. Thus, the results of p53 on prognosis are conflicting and the actual role of p53 in defining long-term outcome remains to be determined.
Ki-67
Ki-67 is a proliferation marker, present in the nucleus during cellular proliferation. Due to its correlation cellular proliferation, Ki-67 has been identified as a possible predictive factor of outcome after liver resection of CRLM. Weber et al[29] conducted a large single-institutional study showing that Ki-67 labeling index was a reliable prognostic factor of survival among patients with resected CRLM. The prognostic impact of Ki-67 was subsequently confirmed on a meta-analysis that identified Ki-67 overexpression as a strong predictor of survival[131]. In a comparison between the Fong clinical scoring system[9] and the expression of Ki-67 as prognostic factors, Smith et al[26] concluded that both Ki-67 correlated better with survival than the clinical score.
Hypoxia inducible factor-1α
Hypoxia inducible factor-1α (HIF-1α) is a transcription factor involved in crucial aspects of cancer biology, including angiogenesis, cell survival, glucose metabolism and invasion[132]. Recent studies have shown that inflammation induces HIF-1α activity[132-135]. Moreover, constitutive activation of Ras-MAPK pathway and the PI3K-AKT pathway, or loss of function of tumor suppressor protein, as p53, regulate HIF-1α activity. Recently Shimomura et al[136] evaluated the clinical significance of HIF-1α expression in CRLM. The authors concluded that overexpression of HIF-1α is an independent risk factor for cancer recurrence after curative resection for CRLM[136]. With new confirmatory studies, HIF-1α may prove to be an important prognostic factor for survival after CRLM resection.
Miscellaneous Markers (p21, H-thymidine labeling index and markers of angiogenesis)
Studies have been unable to correlate prognosis between p21, a cyclin-dependent-kinase inhibitor and a key effector of p53 anti-proliferative activity, and OS in patients with CRLM[126]. Similarly, only one study has analyzed the relation between 3H-thymidine labeling index (TLI) and clinical outcome[137]. In this study, the authors did find that TLI correlated relapse at 4 years following surgery[137]. There is insufficient data to evaluate the prognostic value of markers of angiogenesis and thrombospondin-7.
Circulating tumor cells and circulating tumor DNA
Circulating tumor cells (CTC) and disseminated tumor cells (DTC) may serve as prognostic factors for tumor relapse after potentially curative resection of CRLM. Some investigators have suggested that intraoperative manipulation of the tumor may increase CTC and DTC, spreading malignant cells and causing an increase in intrahepatic or extrahepatic tumor recurrences[138]. The data on this hypothesis are scarce and there is no consensus regarding the matter. While most studies analyzing CTC and DTC have largely focused on their prognostic value in the setting of primary colorectal cancer, a few studies have examined their role in the setting of CRLM. Vogelaar et al[139] demonstrated that patients free of DTC in their bone marrow assessed by RT-PCR had a significantly better DFS and OS after resection of CRLM. A recent meta-analysis investigated the association between outcomes in patients with resected CRLM and tumor cells in the blood or bone marrow[140]. Specifically, in a cohort of 1329 patients (16 studies), the authors reported strong evidence suggesting that CTC correlated with worse OS and DFS. Of note, patients with detectable CTC had a 2 fold risk of progression or recurrence and a 2.5 fold increased risk of death compared with patients who had no CTC detected[140].
Another developing field of cancer research is the detection of tumor-derived circulating mutant DNA. Circulating tumor DNA (ctDNA) represents a small part of the circulating DNA, making detection challenging. Recently Diehl et al[141] proposed a multistep approach to quantify ctDNA in patients with metastatic colorectal cancer undergoing surgery and receiving chemotherapy. Using this beaming technique, the authors were able to detect ctDNA in a subset of patients following resection of CRLM. Among those patients with detectable ctDNA following resection of CRLM, recurrence was universal (Figure 4). In addition, the investigators noted a significant difference in DFS among patients with and without detectable ctDNA. Although preliminary in nature, these results suggest that ctDNA might be a promising prognostic factor of outcome following resection of CRLM.
Figure 4.
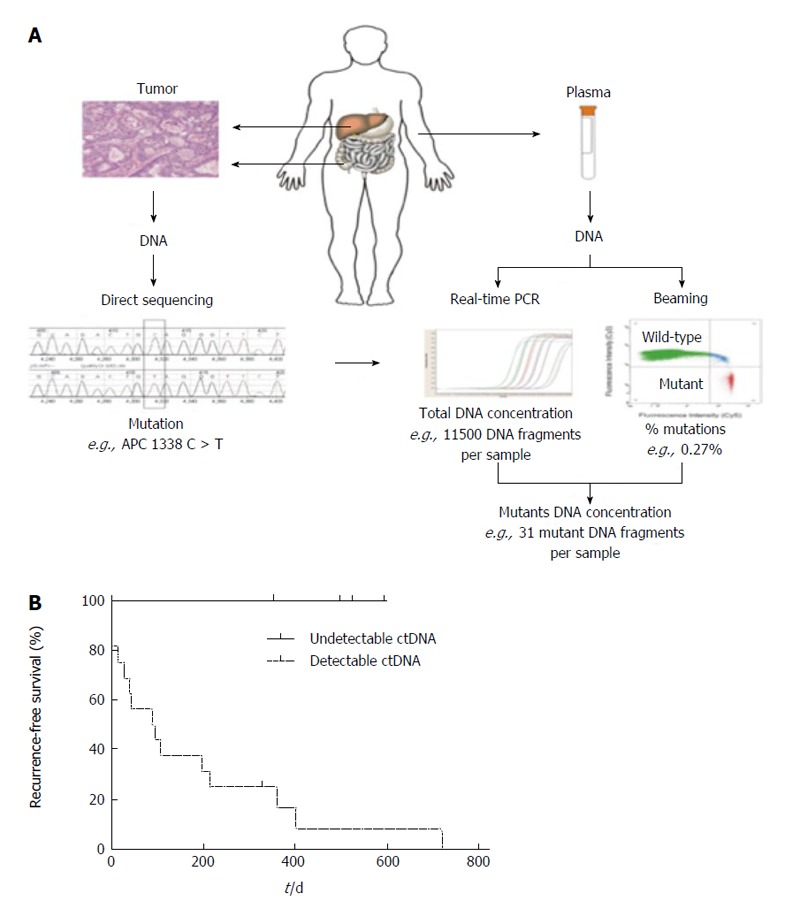
Patients with detectable ctDNA following resection of colorectal liver metastasis, recurrence was universal. A: Depiction of process by which ctDNA is detected and amplified from the specimen and plasma of patients; B: Representative flow cytometric data of ctDNA of one subject who underwent resection of colorectal liver metastasis. Note that the notable difference in recurrence-free survival in subjects with detectable vs undetectable ctDNA (from Diehl et al[141]). Used with permission. PCR: Polymerase chain reaction.
Genetic integrity
The development and progression of colorectal cancer is a multistep process leading to the accumulation of genomic alterations that occur over the lifetime of a tumor[142] (Figure 5). The loss of genomic integrity, in terms of gross chromosomal aberrations and abnormalities of nuclear DNA content (aneuploidy), has been examined in relation to long-term outcome. In the early 1990’s, Cady et al[143] found that aneuploidy was an independent prognostic factor, negatively impacting DFS. More recently, Metha et al[144] used an array-based comparative genomic hybridization to investigate the association of DNA copy number alterations with survival in patients with CRLM resected with curative intent. The total fraction of genome altered (FGA) in the metastases was noted to be an independent predictor of survival in patients with resected hepatic colorectal cancer metastases. In addition, the authors described a direct proportionality between level of FGA and probability of survival[144]. Although genetic instability seems to be correlated with tumor aggressiveness in primary colorectal cancer, it is not clear yet if it has a prognostic value in following resection of CRLM[126].
Figure 5.
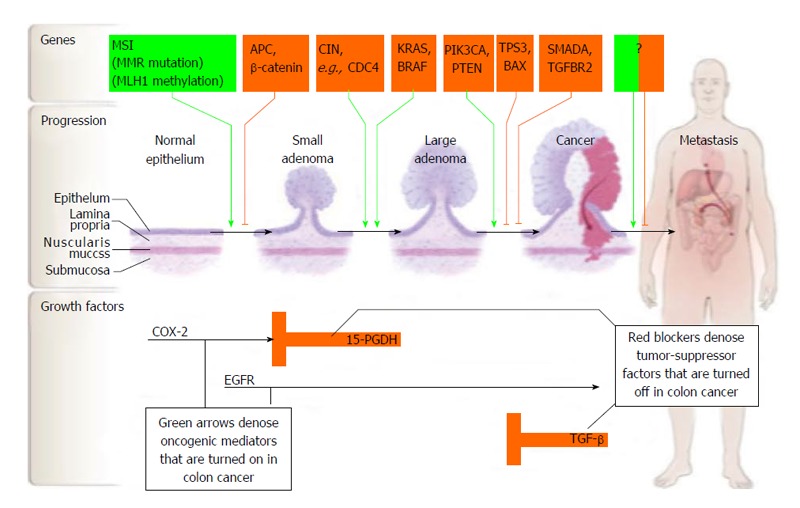
Carcinogenesis of colorectal cancer (from Markowitz et al[145]). Used with permission. EGFR: Epidermal growth factor receptor; TGF: Transforming growth factor; COX-2: Cyclooxygenase-2.
CONCLUSION
Survival following resection of CRLM varies and is dependent on clinical, tumor, and molecular factors. Accurate predictors of prognosis are important for patients, as well as providers. While some preoperative clincopathologic factors are associated with outcome, the emergence of biologic and molecular markers may allow for a more individualized approach to prognosis. Factors such as KRAS, BRAF, TS, hTERT, Ki-67 can help predict long-term prognosis following CRLM. In addition, more recent data on CTC and ctDNA holds for a more sensitive and powerful metric of prognosis near the time of surgery for CRLM. With more accurate markers of prognosis in the future, a greater emphasis on patient-specific treatments and prognostic information will hopefully continue to emerge.
Footnotes
P- Reviewers: Franko J, Siriwardena AK S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Sawyers CL, Abate-Shen C, Anderson KC, Barker A, Baselga J, Berger NA, Foti M, Jemal A, Lawrence TS, Li CI, et al. AACR Cancer Progress Report 2013. Clin Cancer Res. 2013;19:S4–98. doi: 10.1158/1078-0432.CCR-13-2107. [DOI] [PubMed] [Google Scholar]
- 3.Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465–474. doi: 10.1002/bjs.5278. [DOI] [PubMed] [Google Scholar]
- 4.Mayo SC, Heckman JE, Shore AD, Nathan H, Parikh AA, Bridges JF, Anders RA, Anaya DA, Becker NS, Pawlik TM. Shifting trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery. 2011;150:204–216. doi: 10.1016/j.surg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andres A, Toso C, Adam R, Barroso E, Hubert C, Capussotti L, Gerstel E, Roth A, Majno PE, Mentha G. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases: a LiverMetSurvey-based study. Ann Surg. 2012;256:772–778; discussion 778-779. doi: 10.1097/SLA.0b013e3182734423. [DOI] [PubMed] [Google Scholar]
- 6.Cardona K, Mastrodomenico P, D’Amico F, Shia J, Gönen M, Weiser MR, Paty PB, Kingham TP, Allen PJ, De Matteo RP, et al. Detailed pathologic characteristics of the primary colorectal tumor independently predict outcome after hepatectomy for metastases. Ann Surg Oncol. 2013;20:148–154. doi: 10.1245/s10434-012-2540-y. [DOI] [PubMed] [Google Scholar]
- 7.House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D’Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752, 752-755. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 8.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318; discussion 318-321. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 11.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 12.Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–1172. doi: 10.1001/archsurg.139.11.1168. [DOI] [PubMed] [Google Scholar]
- 13.Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, Kosuge T, Yamamoto J, Imamura H. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno H, Mochizuki H, Hatsuse K, Hase K, Yamamoto T. Indicators for treatment strategies of colorectal liver metastases. Ann Surg. 2000;231:59–66. doi: 10.1097/00000658-200001000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 16.Tranchart H, Chirica M, Faron M, Balladur P, Lefevre LB, Svrcek M, de Gramont A, Tiret E, Paye F. Prognostic impact of positive surgical margins after resection of colorectal cancer liver metastases: reappraisal in the era of modern chemotherapy. World J Surg. 2013;37:2647–2654. doi: 10.1007/s00268-013-2186-3. [DOI] [PubMed] [Google Scholar]
- 17.Bennett JJ, Schmidt CR, Klimstra DS, Grobmyer SR, Ishill NM, D’Angelica M, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Perihepatic lymph node micrometastases impact outcome after partial hepatectomy for colorectal metastases. Ann Surg Oncol. 2008;15:1130–1136. doi: 10.1245/s10434-007-9802-0. [DOI] [PubMed] [Google Scholar]
- 18.Tan MC, Butte JM, Gonen M, Kemeny N, Fong Y, Allen PJ, Kingham TP, Dematteo RP, Jarnagin WR, D’Angelica MI. Prognostic significance of early recurrence: a conditional survival analysis in patients with resected colorectal liver metastasis. HPB (Oxford) 2013;15:803–813. doi: 10.1111/hpb.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park JW, Chang HJ, Kim BC, Yeo HY, Kim DY. Clinical validity of tissue carcinoembryonic antigen expression as ancillary to serum carcinoembryonic antigen concentration in patients curatively resected for colorectal cancer. Colorectal Dis. 2013;15:e503–e511. doi: 10.1111/codi.12304. [DOI] [PubMed] [Google Scholar]
- 20.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, Lillemoe KD, Yeo CJ, Cameron JL. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John SK, Robinson SM, Rehman S, Harrison B, Vallance A, French JJ, Jaques BC, Charnley RM, Manas DM, White SA. Prognostic Factors and Survival after Resection of Colorectal Liver Metastasis in the Era of Preoperative Chemotherapy: An 11-Year Single-Centre Study. Dig Surg. 2013;30:293–301. doi: 10.1159/000354310. [DOI] [PubMed] [Google Scholar]
- 22.Aldrighetti L, Castoldi R, Di Palo S, Arru M, Stella M, Orsenigo E, Gavazzi F, Ferla G, Di Carlo V, Staudacher C. [Prognostic factors for long-term outcome of hepatic resection for colorectal liver metastases] Chir Ital. 2005;57:555–570. [PubMed] [Google Scholar]
- 23.de Haas RJ, Wicherts DA, Flores E, Ducreux M, Lévi F, Paule B, Azoulay D, Castaing D, Lemoine A, Adam R. Tumor marker evolution: comparison with imaging for assessment of response to chemotherapy in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:1010–1023. doi: 10.1245/s10434-009-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fortner JG, Silva JS, Cox EB, Golbey RB, Gallowitz H, Maclean BJ. Multivariate analysis of a personal series of 247 patients with liver metastases from colorectal cancer. II. Treatment by intrahepatic chemotherapy. Ann Surg. 1984;199:317–324. doi: 10.1097/00000658-198403000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dômont J, Pawlik TM, Boige V, Rose M, Weber JC, Hoff PM, Brown TD, Zorzi D, Morat L, Pignon JP, et al. Catalytic subunit of human telomerase reverse transcriptase is an independent predictor of survival in patients undergoing curative resection of hepatic colorectal metastases: a multicenter analysis. J Clin Oncol. 2005;23:3086–3093. doi: 10.1200/JCO.2005.06.944. [DOI] [PubMed] [Google Scholar]
- 26.Smith DL, Soria JC, Morat L, Yang Q, Sabatier L, Liu DD, Nemr RA, Rashid A, Vauthey JN. Human telomerase reverse transcriptase (hTERT) and Ki-67 are better predictors of survival than established clinical indicators in patients undergoing curative hepatic resection for colorectal metastases. Ann Surg Oncol. 2004;11:45–51. doi: 10.1007/BF02524345. [DOI] [PubMed] [Google Scholar]
- 27.Doci R, Gennari L, Bignami P, Montalto F, Morabito A, Bozzetti F. One hundred patients with hepatic metastases from colorectal cancer treated by resection: analysis of prognostic determinants. Br J Surg. 1991;78:797–801. doi: 10.1002/bjs.1800780711. [DOI] [PubMed] [Google Scholar]
- 28.Smith MD, McCall JL. Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg. 2009;96:1101–1113. doi: 10.1002/bjs.6735. [DOI] [PubMed] [Google Scholar]
- 29.Weber JC, Nakano H, Bachellier P, Oussoultzoglou E, Inoue K, Shimura H, Wolf P, Chenard-Neu MP, Jaeck D. Is a proliferation index of cancer cells a reliable prognostic factor after hepatectomy in patients with colorectal liver metastases. Am J Surg. 2001;182:81–88. doi: 10.1016/s0002-9610(01)00656-0. [DOI] [PubMed] [Google Scholar]
- 30.Kokudo N, Imamura H, Sugawara Y, Sakamoto Y, Yamamoto J, Seki M, Makuuchi M. Surgery for multiple hepatic colorectal metastases. J Hepatobiliary Pancreat Surg. 2004;11:84–91. doi: 10.1007/s00534-002-0754-2. [DOI] [PubMed] [Google Scholar]
- 31.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231:743–751. doi: 10.1097/00000658-200005000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases. Ann Surg. 2004;240:1052–1061; discussion 1061-1064. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawlik TM, Abdalla EK, Ellis LM, Vauthey JN, Curley SA. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg. 2006;10:240–248. doi: 10.1016/j.gassur.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 34.Hughes KS, Rosenstein RB, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D. Resection of the liver for colorectal carcinoma metastases. A multi-institutional study of long-term survivors. Dis Colon Rectum. 1988;31:1–4. doi: 10.1007/bf02552560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 36.Hamady ZZ, Malik HZ, Finch R, Adair R, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Hepatic resection for colorectal metastasis: impact of tumour size. Ann Surg Oncol. 2006;13:1493–1499. doi: 10.1245/s10434-006-9105-x. [DOI] [PubMed] [Google Scholar]
- 37.Gayowski TJ, Iwatsuki S, Madariaga JR, Selby R, Todo S, Irish W, Starzl TE. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–710; discussion 710-711. [PMC free article] [PubMed] [Google Scholar]
- 38.Allen PJ, Kemeny N, Jarnagin W, DeMatteo R, Blumgart L, Fong Y. Importance of response to neoadjuvant chemotherapy in patients undergoing resection of synchronous colorectal liver metastases. J Gastrointest Surg. 2003;7:109–115; discussion 116-117. doi: 10.1016/S1091-255X(02)00121-X. [DOI] [PubMed] [Google Scholar]
- 39.Gruenberger B, Scheithauer W, Punzengruber R, Zielinski C, Tamandl D, Gruenberger T. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. doi: 10.1186/1471-2407-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 41.Foster JH. Survival after liver resection for secondary tumors. Am J Surg. 1978;135:389–394. doi: 10.1016/0002-9610(78)90072-7. [DOI] [PubMed] [Google Scholar]
- 42.Adson MA. The resection of hepatic metastases. Another view. Arch Surg. 1989;124:1023–1024. doi: 10.1001/archsurg.1989.01410090029005. [DOI] [PubMed] [Google Scholar]
- 43.Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 44.Elias D, Liberale G, Vernerey D, Pocard M, Ducreux M, Boige V, Malka D, Pignon JP, Lasser P. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol. 2005;12:900–909. doi: 10.1245/ASO.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 45.Carpizo DR, Are C, Jarnagin W, Dematteo R, Fong Y, Gönen M, Blumgart L, D’Angelica M. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol. 2009;16:2138–2146. doi: 10.1245/s10434-009-0521-6. [DOI] [PubMed] [Google Scholar]
- 46.Pulitanò C, Bodingbauer M, Aldrighetti L, de Jong MC, Castillo F, Schulick RD, Parks RW, Choti MA, Wigmore SJ, Gruenberger T, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol. 2011;18:1380–1388. doi: 10.1245/s10434-010-1459-4. [DOI] [PubMed] [Google Scholar]
- 47.Inoue M, Kotake Y, Nakagawa K, Fujiwara K, Fukuhara K, Yasumitsu T. Surgery for pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2000;70:380–383. doi: 10.1016/s0003-4975(00)01417-x. [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto T, Tsubota N, Iwanaga K, Yuki T, Matsuoka H, Yoshimura M. Pulmonary resection for metastases from colorectal cancer. Chest. 2001;119:1069–1072. doi: 10.1378/chest.119.4.1069. [DOI] [PubMed] [Google Scholar]
- 49.Mansel JK, Zinsmeister AR, Pairolero PC, Jett JR. Pulmonary resection of metastatic colorectal adenocarcinoma. A ten year experience. Chest. 1986;89:109–112. doi: 10.1378/chest.89.1.109. [DOI] [PubMed] [Google Scholar]
- 50.Inoue M, Ohta M, Iuchi K, Matsumura A, Ideguchi K, Yasumitsu T, Nakagawa K, Fukuhara K, Maeda H, Takeda S, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg. 2004;78:238–244. doi: 10.1016/j.athoracsur.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, Bismuth H, Castaing D. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement. J Clin Oncol. 2008;26:3672–3680. doi: 10.1200/JCO.2007.15.7297. [DOI] [PubMed] [Google Scholar]
- 52.Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am. 2003;12:165–192, xi. doi: 10.1016/s1055-3207(02)00091-1. [DOI] [PubMed] [Google Scholar]
- 53.Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, Codina-Barreras A, Mojal S. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007;18:1190–1195. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 54.Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, Blumgart LH, D’Angelica M. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elias D, Lasser P, Rougier P, Ducreux M, Bognel C, Roche A. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg. 1995;180:213–219. [PubMed] [Google Scholar]
- 56.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 57.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, Lemoine A, Bismuth H. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, Abascal A, Majno P, Engerran L. Repeat hepatectomy for colorectal liver metastases. Ann Surg. 1997;225:51–60; discussion 60-62. doi: 10.1097/00000658-199701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722, discussion 722-724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andreou A, Aloia TA, Brouquet A, Dickson PV, Zimmitti G, Maru DM, Kopetz S, Loyer EM, Curley SA, Abdalla EK, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg. 2013;257:1079–1088. doi: 10.1097/SLA.0b013e318283a4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pawlik TM, Vauthey JN. Surgical margins during hepatic surgery for colorectal liver metastases: complete resection not millimeters defines outcome. Ann Surg Oncol. 2008;15:677–679. doi: 10.1245/s10434-007-9703-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869; discussion 869-870. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giuliante F, Ardito F, Pulitanò C, Vellone M, Giovannini I, Aldrighetti L, Ferla G, Nuzzo G. Does hepatic pedicle clamping affect disease-free survival following liver resection for colorectal metastases. Ann Surg. 2010;252:1020–1026. doi: 10.1097/SLA.0b013e3181f66918. [DOI] [PubMed] [Google Scholar]
- 65.Zorzi D, Mullen JT, Abdalla EK, Pawlik TM, Andres A, Muratore A, Curley SA, Mentha G, Capussotti L, Vauthey JN. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10:86–94. doi: 10.1016/j.gassur.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 66.Ferrero A, Russolillo N, Viganò L, Lo Tesoriere R, Muratore A, Capussotti L. Does Pringle maneuver affect survival in patients with colorectal liver metastases. World J Surg. 2010;34:2418–2425. doi: 10.1007/s00268-010-0682-2. [DOI] [PubMed] [Google Scholar]
- 67.Farid SG, Aldouri A, Morris-Stiff G, Khan AZ, Toogood GJ, Lodge JP, Prasad KR. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. Ann Surg. 2010;251:91–100. doi: 10.1097/SLA.0b013e3181bfda3c. [DOI] [PubMed] [Google Scholar]
- 68.Mavros MN, de Jong M, Dogeas E, Hyder O, Pawlik TM. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg. 2013;100:711–718. doi: 10.1002/bjs.9060. [DOI] [PubMed] [Google Scholar]
- 69.Mala T, Bøhler G, Mathisen Ø, Bergan A, Søreide O. Hepatic resection for colorectal metastases: can preoperative scoring predict patient outcome. World J Surg. 2002;26:1348–1353. doi: 10.1007/s00268-002-6231-x. [DOI] [PubMed] [Google Scholar]
- 70.Arru M, Aldrighetti L, Castoldi R, Di Palo S, Orsenigo E, Stella M, Pulitanò C, Gavazzi F, Ferla G, Di Carlo V, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 71.Merkel S, Bialecki D, Meyer T, Müller V, Papadopoulos T, Hohenberger W. Comparison of clinical risk scores predicting prognosis after resection of colorectal liver metastases. J Surg Oncol. 2009;100:349–357. doi: 10.1002/jso.21346. [DOI] [PubMed] [Google Scholar]
- 72.Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, Müller SA, Schemmer P, Büchler MW, Weitz J. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279–3288. doi: 10.1245/s10434-009-0654-7. [DOI] [PubMed] [Google Scholar]
- 73.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, Geller DA, Gayowski TJ, Fung JJ, Starzl TE. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, Nagorney DM. Hepatic resection for colorectal metastases: value for risk scoring systems. Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kattan MW, Gönen M, Jarnagin WR, DeMatteo R, D’Angelica M, Weiser M, Blumgart LH, Fong Y. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:282–287. doi: 10.1097/SLA.0b013e31815ed67b. [DOI] [PubMed] [Google Scholar]
- 76.Feroci F, Fong Y. Use of clinical score to stage and predict outcome of hepatic resection of metastatic colorectal cancer. J Surg Oncol. 2010;102:914–921. doi: 10.1002/jso.21715. [DOI] [PubMed] [Google Scholar]
- 77.Nathan H, de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Gigot JF, Schulick RD, Choti MA, Aldrighetti L, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–764, 764-676. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 78.Ayez N, Lalmahomed ZS, van der Pool AE, Vergouwe Y, van Montfort K, de Jonge J, Eggermont AM, Ijzermans JN, Verhoef C. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:2757–2763. doi: 10.1245/s10434-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tanaka K, Adam R, Shimada H, Azoulay D, Lévi F, Bismuth H. Role of neoadjuvant chemotherapy in the treatment of multiple colorectal metastases to the liver. Br J Surg. 2003;90:963–969. doi: 10.1002/bjs.4160. [DOI] [PubMed] [Google Scholar]
- 80.Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, Majno P, Engerran L. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520; discussion 520-522. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 82.Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, Curley SA, Abdalla EK, Kaur H, Charnsangavej C, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adam R, Wicherts DA, de Haas RJ, Aloia T, Lévi F, Paule B, Guettier C, Kunstlinger F, Delvart V, Azoulay D, et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: myth or reality. J Clin Oncol. 2008;26:1635–1641. doi: 10.1200/JCO.2007.13.7471. [DOI] [PubMed] [Google Scholar]
- 84.Blazer DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, Fogelman D, Eng C, Chang DZ, Wang H, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka K, Takakura H, Takeda K, Matsuo K, Nagano Y, Endo I. Importance of complete pathologic response to prehepatectomy chemotherapy in treating colorectal cancer metastases. Ann Surg. 2009;250:935–942. doi: 10.1097/sla.0b013e3181b0c6e4. [DOI] [PubMed] [Google Scholar]
- 86.Brouquet A, Zimmitti G, Kopetz S, Stift J, Julié C, Lemaistre AI, Agarwal A, Patel V, Benoist S, Nordlinger B, et al. Multicenter validation study of pathologic response and tumor thickness at the tumor-normal liver interface as independent predictors of disease-free survival after preoperative chemotherapy and surgery for colorectal liver metastases. Cancer. 2013;119:2778–2788. doi: 10.1002/cncr.28097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Klinger M, Tamandl D, Eipeldauer S, Hacker S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B, Gruenberger T. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17:2059–2065. doi: 10.1245/s10434-010-0972-9. [DOI] [PubMed] [Google Scholar]
- 88.Knijn N, de Ridder JA, Punt CJ, de Wilt JH, Nagtegaal ID. Histopathological evaluation of resected colorectal cancer liver metastases: what should be done. Histopathology. 2013;63:149–156. doi: 10.1111/his.12124. [DOI] [PubMed] [Google Scholar]
- 89.Maru DM, Kopetz S, Boonsirikamchai P, Agarwal A, Chun YS, Wang H, Abdalla EK, Kaur H, Charnsangavej C, Vauthey JN, et al. Tumor thickness at the tumor-normal interface: a novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am J Surg Pathol. 2010;34:1287–1294. doi: 10.1097/PAS.0b013e3181eb2f7b. [DOI] [PubMed] [Google Scholar]
- 90.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, Sartoretti P, Dousset B, Majno PE, Soubrane O, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18:299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 91.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 92.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okano K, Maeba T, Moroguchi A, Ishimura K, Karasawa Y, Izuishi K, Goda F, Usuki H, Wakabayashi H, Maeta H. Lymphocytic infiltration surrounding liver metastases from colorectal cancer. J Surg Oncol. 2003;82:28–33. doi: 10.1002/jso.10188. [DOI] [PubMed] [Google Scholar]
- 95.Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, McArdle CS. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McMillan DC, Wotherspoon HA, Fearon KC, Sturgeon C, Cooke TG, McArdle CS. A prospective study of tumor recurrence and the acute-phase response after apparently curative colorectal cancer surgery. Am J Surg. 1995;170:319–322. doi: 10.1016/s0002-9610(99)80296-7. [DOI] [PubMed] [Google Scholar]
- 97.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90:215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 98.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, Virtamo J, Taylor PR, Albanes D, Sinha R. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483–2487. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 99.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 100.Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, Lodge JP, Toogood GJ. Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246:806–814. doi: 10.1097/SLA.0b013e318142d964. [DOI] [PubMed] [Google Scholar]
- 101.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 102.Fransén K, Klintenäs M, Osterström A, Dimberg J, Monstein HJ, Söderkvist P. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis. 2004;25:527–533. doi: 10.1093/carcin/bgh049. [DOI] [PubMed] [Google Scholar]
- 103.Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, Papadimitriou CA, Murray S. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–972. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 104.Bouchahda M, Adam R, Giacchetti S, Castaing D, Brezault-Bonnet C, Hauteville D, Innominato PF, Focan C, Machover D, Lévi F. Rescue chemotherapy using multidrug chronomodulated hepatic arterial infusion for patients with heavily pretreated metastatic colorectal cancer. Cancer. 2009;115:4990–4999. doi: 10.1002/cncr.24549. [DOI] [PubMed] [Google Scholar]
- 105.Kastrinakis WV, Ramchurren N, Maggard M, Steele G, Summerhayes IC. K-ras status does not predict successful hepatic resection of colorectal cancer metastasis. Arch Surg. 1995;130:9–14. doi: 10.1001/archsurg.1995.01430010011001. [DOI] [PubMed] [Google Scholar]
- 106.Kato M, Ito Y, Kobayashi S, Isono K. Detection of DCC and Ki-ras gene alterations in colorectal carcinoma tissue as prognostic markers for liver metastatic recurrence. Cancer. 1996;77:1729–1735. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1729::AID-CNCR47>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 107.Petrowsky H, Sturm I, Graubitz O, Kooby DA, Staib-Sebler E, Gog C, Köhne CH, Hillebrand T, Daniel PT, Fong Y, et al. Relevance of Ki-67 antigen expression and K-ras mutation in colorectal liver metastases. Eur J Surg Oncol. 2001;27:80–87. doi: 10.1053/ejso.2000.1029. [DOI] [PubMed] [Google Scholar]
- 108.Russo A, Migliavacca M, Bazan V, Maturi N, Morello V, Dardanoni G, Modica G, Bazan P, Albanese I, La Farina M, et al. Prognostic significance of proliferative activity, DNA-ploidy, p53 and Ki-ras point mutations in colorectal liver metastases. Cell Prolif. 1998;31:139–153. doi: 10.1046/j.1365-2184.1998.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schimanski CC, Linnemann U, Berger MR. Sensitive detection of K-ras mutations augments diagnosis of colorectal cancer metastases in the liver. Cancer Res. 1999;59:5169–5175. [PubMed] [Google Scholar]
- 110.Nash GM, Gimbel M, Shia J, Nathanson DR, Ndubuisi MI, Zeng ZS, Kemeny N, Paty PB. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:572–578. doi: 10.1245/s10434-009-0605-3. [DOI] [PubMed] [Google Scholar]
- 111.Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA, Donehower RC, Hirose K, Ahuja N, Pawlik TM, Choti MA. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119:4137–4144. doi: 10.1002/cncr.28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang CJ, Teng HW, Chien CC, Lin JK, Yang SH. Prognostic significance of C-reactive protein polymorphism and KRAS/BRAF in synchronous liver metastasis from colorectal cancer. PLoS One. 2013;8:e65117. doi: 10.1371/journal.pone.0065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Teng HW, Huang YC, Lin JK, Chen WS, Lin TC, Jiang JK, Yen CC, Li AF, Wang HW, Chang SC, et al. BRAF mutation is a prognostic biomarker for colorectal liver metastasectomy. J Surg Oncol. 2012;106:123–129. doi: 10.1002/jso.23063. [DOI] [PubMed] [Google Scholar]
- 114.Popovici V, Budinska E, Tejpar S, Weinrich S, Estrella H, Hodgson G, Van Cutsem E, Xie T, Bosman FT, Roth AD, et al. Identification of a poor-prognosis BRAF-mutant-like population of patients with colon cancer. J Clin Oncol. 2012;30:1288–1295. doi: 10.1200/JCO.2011.39.5814. [DOI] [PubMed] [Google Scholar]
- 115.Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, Curley SA, Aloia TA, Maru DM. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258:619–626; discussion 626-627. doi: 10.1097/SLA.0b013e3182a5025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Charpin C, Martin M, Balandraud N, Roudier J, Auger I. Autoantibodies to BRAF, a new family of autoantibodies associated with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R194. doi: 10.1186/ar3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- 118.Bertorelle R, Briarava M, Rampazzo E, Biasini L, Agostini M, Maretto I, Lonardi S, Friso ML, Mescoli C, Zagonel V, et al. Telomerase is an independent prognostic marker of overall survival in patients with colorectal cancer. Br J Cancer. 2013;108:278–284. doi: 10.1038/bjc.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pucciarelli S, Rampazzo E, Briarava M, Maretto I, Agostini M, Digito M, Keppel S, Friso ML, Lonardi S, De Paoli A, et al. Telomere-specific reverse transcriptase (hTERT) and cell-free RNA in plasma as predictors of pathologic tumor response in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2012;19:3089–3096. doi: 10.1245/s10434-012-2272-z. [DOI] [PubMed] [Google Scholar]
- 120.Saw RP, Koorey D, Painter D, Gallagher PJ, Solomon MJ. p53, DCC and thymidylate synthase as predictors of survival after resection of hepatic metastases from colorectal cancer. Br J Surg. 2002;89:1409–1415. doi: 10.1046/j.1365-2168.2002.02222.x. [DOI] [PubMed] [Google Scholar]
- 121.Ju J, Pedersen-Lane J, Maley F, Chu E. Regulation of p53 expression by thymidylate synthase. Proc Natl Acad Sci USA. 1999;96:3769–3774. doi: 10.1073/pnas.96.7.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bathe OF, Franceschi D, Livingstone AS, Moffat FL, Tian E, Ardalan B. Increased thymidylate synthase gene expression in liver metastases from colorectal carcinoma: implications for chemotherapeutic options and survival. Cancer J Sci Am. 1999;5:34–40. [PubMed] [Google Scholar]
- 123.Gonen M, Hummer A, Zervoudakis A, Sullivan D, Fong Y, Banerjee D, Klimstra D, Cordon-Cardo C, Bertino J, Kemeny N. Thymidylate synthase expression in hepatic tumors is a predictor of survival and progression in patients with resectable metastatic colorectal cancer. J Clin Oncol. 2003;21:406–412. doi: 10.1200/JCO.2003.06.060. [DOI] [PubMed] [Google Scholar]
- 124.Maithel SK, Gönen M, Ito H, Dematteo RP, Allen PJ, Fong Y, Blumgart LH, Jarnagin WR, D’Angelica MI. Improving the clinical risk score: an analysis of molecular biomarkers in the era of modern chemotherapy for resectable hepatic colorectal cancer metastases. Surgery. 2012;151:162–170. doi: 10.1016/j.surg.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 125.Tullo A, D’Erchia AM, Honda K, Mitry RR, Kelly MD, Habib NA, Saccone C, Sbisà E. Characterization of p53 mutations in colorectal liver metastases and correlation with clinical parameters. Clin Cancer Res. 1999;5:3523–3528. [PubMed] [Google Scholar]
- 126.Neal CP, Garcea G, Doucas H, Manson MM, Sutton CD, Dennison AR, Berry DP. Molecular prognostic markers in resectable colorectal liver metastases: a systematic review. Eur J Cancer. 2006;42:1728–1743. doi: 10.1016/j.ejca.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 127.Belluco C, Guillem JG, Kemeny N, Huang Y, Klimstra D, Berger MF, Cohen AM. p53 nuclear protein overexpression in colorectal cancer: a dominant predictor of survival in patients with advanced hepatic metastases. J Clin Oncol. 1996;14:2696–2701. doi: 10.1200/JCO.1996.14.10.2696. [DOI] [PubMed] [Google Scholar]
- 128.Nitti D, Belluco C, Montesco MC, Bertorelle R, Da Pian PP, Fassina A, Ninfo V, Chieco-Bianchi L, Lise M. Nuclear p53 protein expression in resected hepatic metastases from colorectal cancer: an independent prognostic factor of survival. Eur J Cancer. 1998;34:851–855. doi: 10.1016/s0959-8049(97)10165-4. [DOI] [PubMed] [Google Scholar]
- 129.Tanaka K, Shimada H, Miura M, Fujii Y, Yamaguchi S, Endo I, Sekido H, Togo S, Ike H. Metastatic tumor doubling time: most important prehepatectomy predictor of survival and nonrecurrence of hepatic colorectal cancer metastasis. World J Surg. 2004;28:263–270. doi: 10.1007/s00268-003-7088-3. [DOI] [PubMed] [Google Scholar]
- 130.Yang Y, Forslund A, Remotti H, Lönnroth C, Andersson M, Brevinge H, Svanberg E, Lindnér P, Hafström L, Naredi P, et al. P53 mutations in primary tumors and subsequent liver metastases are related to survival in patients with colorectal carcinoma who undergo liver resection. Cancer. 2001;91:727–736. [PubMed] [Google Scholar]
- 131.Ivanecz A, Kavalar R, Palfy M, Pivec V, Sremec M, Horvat M, Potrč S. Can we improve the clinical risk score The prognostic value of p53, Ki-67 and thymidylate synthase in patients undergoing radical resection of colorectal liver metastases. HPB (Oxford) 2013:Epub ahead of print. doi: 10.1111/hpb.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 133.Frede S, Freitag P, Otto T, Heilmaier C, Fandrey J. The proinflammatory cytokine interleukin 1beta and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 2005;65:4690–4697. doi: 10.1158/0008-5472.CAN-04-3877. [DOI] [PubMed] [Google Scholar]
- 134.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005;4:256–258. [PubMed] [Google Scholar]
- 135.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 136.Shimomura M, Hinoi T, Kuroda S, Adachi T, Kawaguchi Y, Sasada T, Takakura Y, Egi H, Okajima M, Tashiro H, et al. Overexpression of Hypoxia Inducible Factor-1 Alpha is an Independent Risk Factor for Recurrence After Curative Resection of Colorectal Liver Metastases. Ann Surg Oncol. 2013:Epub ahead of print. doi: 10.1245/s10434-013-2945-2. [DOI] [PubMed] [Google Scholar]
- 137.Costa A, Doci R, Mochen C, Bignami P, Faranda A, Gennari L, Silvestrini R. Cell proliferation-related markers in colorectal liver metastases: correlation with patient prognosis. J Clin Oncol. 1997;15:2008–2014. doi: 10.1200/JCO.1997.15.5.2008. [DOI] [PubMed] [Google Scholar]
- 138.Koch M, Kienle P, Hinz U, Antolovic D, Schmidt J, Herfarth C, von Knebel Doeberitz M, Weitz J. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg. 2005;241:199–205. doi: 10.1097/01.sla.0000151795.15068.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vogelaar FJ, Mesker WE, Rijken AM, van Pelt GW, van Leeuwen AM, Tanke HJ, Tollenaar RA, Liefers GJ. Clinical impact of different detection methods for disseminated tumor cells in bone marrow of patients undergoing surgical resection of colorectal liver metastases: a prospective follow-up study. BMC Cancer. 2010;10:153. doi: 10.1186/1471-2407-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Groot Koerkamp B, Rahbari NN, Büchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann Surg Oncol. 2013;20:2156–2165. doi: 10.1245/s10434-013-2907-8. [DOI] [PubMed] [Google Scholar]
- 141.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 143.Cady B, Stone MD, McDermott WV, Jenkins RL, Bothe A, Lavin PT, Lovett EJ, Steele GD. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg. 1992;127:561–568; discussion 568-569. doi: 10.1001/archsurg.1992.01420050085011. [DOI] [PubMed] [Google Scholar]
- 144.Mehta KR, Nakao K, Zuraek MB, Ruan DT, Bergsland EK, Venook AP, Moore DH, Tokuyasu TA, Jain AN, Warren RS, et al. Fractional genomic alteration detected by array-based comparative genomic hybridization independently predicts survival after hepatic resection for metastatic colorectal cancer. Clin Cancer Res. 2005;11:1791–1797. doi: 10.1158/1078-0432.CCR-04-1418. [DOI] [PubMed] [Google Scholar]
- 145.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–2460. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]


