Abstract
Collagen crosslinking is a major post-translational modification of collagen which has important roles in determining the biomechanical competence of bone. Crosslinks can be divided into enzymatic lysil oxidase-mediated and non-enzymatic glycation-induced (advanced glycation end products, AGE) molecules. In addition, collagen in bone can also undergo spontaneous isomerization and racemization of the aspartic acid residues with the C-telopeptide (CTX), leading to the formation of two isomers namely α (newly formed collagen) and β (matured isomerized collagen) CTX. Several in vitro and ex vivo studies, relating the bone content of these crosslinks with bone strength, have shown that they contributed to the mechanical competence of trabecular and cortical bone—mainly on the post-yield properties—in part independent of the bone mineral content. In addition, AGEs such as pentosidine have been reported to alter the formation and propagation of microdamage by making the bone more brittle. The bone content of AGEs and isomerization can also be modified by antiresorptive and anabolic therapies. They may thus explain part of the antifracture efficacy of these treatments. The main challenge consists in the transposition of these in vitro/ex vivo studies to clinical applications for the development of a non-invasive biomarker, as none of currently identified collagen crosslinks (both enzymatic and nonenzymatic) is bone specific. Nevertheless, serum or urine levels of pentosidine and the ratio of α/β CTX have been reported to predict fracture risk in postmenopausal women, in men and in patients with type 2 diabetes.
Introduction
Although clinical assessment of bone mineral density (BMD) by dual X-ray absorptiometry, which largely reflects the mineral phase of bone tissue, is the current gold standard for the diagnosis of osteoporosis, ∼50% of postmenopausal women with incident fracture have BMD levels above the WHO criteria for osteoporosis,1,2 indicating that factors that are not reflected in BMD measurement contribute to bone fracture resistance. Indeed, bone fragility also depends on the morphology and architecture of bone, as well as on the material properties of bone matrix that cannot be readily assessed. In osteoporosis, low BMD levels and the microarchitectural alterations of bone tissue are both related to abnormalities of bone turnover. Consequently, it has been suggested that bone fracture resistance in postmenopausal osteoporosis may be reflected in part, independent of BMD levels, by measuring bone turnover using specific serum and urinary markers of bone formation and resorption, although this approach was not successful in idiopathic osteoporosis and patients with hypoparathyroidism. In addition to turnover, changes in the matrix composition, especially modification of the collagen network, may also contribute to bone fragility. The involvement of these modifications in bone strength has recently been a subject of intensive research and will be discussed in this article.
Post-translational modifications of bone collagen
Type I collagen accounts for 90% of the organic matrix, and with aging and disease may be subjected to a series of enzymatic and non-enzymatic intra- and extracellular post-translational modifications that induce conformational changes in collagen molecules and may influence bone strength. The post-translational modifications of bone non-collagenous proteins, which is beyond the scope of this paper has been reviewed by Quin et al.3
Enzymatic crosslinks formation: the pivotal role of lysyl oxidase.
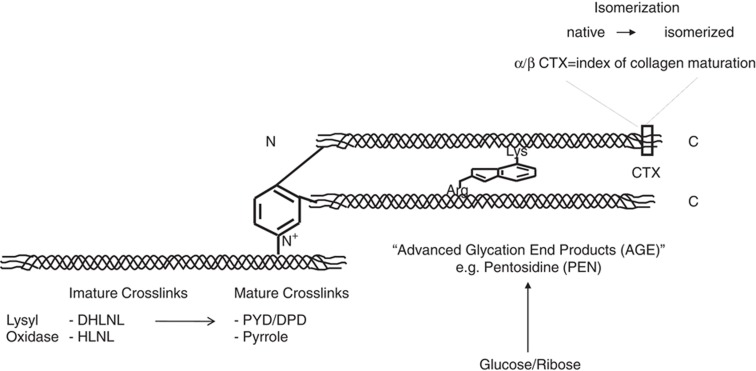
Enzymatic modifications include the formation of pyridinium and pyrrolic molecules within the N- and C-terminal telopeptides (CTX). They are initiated by the oxidation of lysine and hydroxylysine residues that are catalyzed by the enzyme lysyl oxidase (LOX) (Figure 1). The first chemical entities that are formed under the activity of LOX are the divalent immature molecules dihydroxylysinornorleucine (DHLNL) and hydroxylysinonorleucine (HLNL), which then with maturation are transformed to trivalent crosslinks pyrdinoline (PYD), deoxypyridinoline (DPD) and pyrrolic analogues (Figure 1).
Figure 1. Pathways of enzymatic and non-enzymatic crosslink formation in bone collagen.
deH-DHLNL, dehydro-dihydroxylysinornorleucine; deH-HLNL, dehydro-hydroxylysinonorleucine; deH-LNL, dehydro-lysinonorleucine, HLKNL, hydroxylysino-5-k-ketonorleucine; LKNL, lysine-5-ketonorleucine.
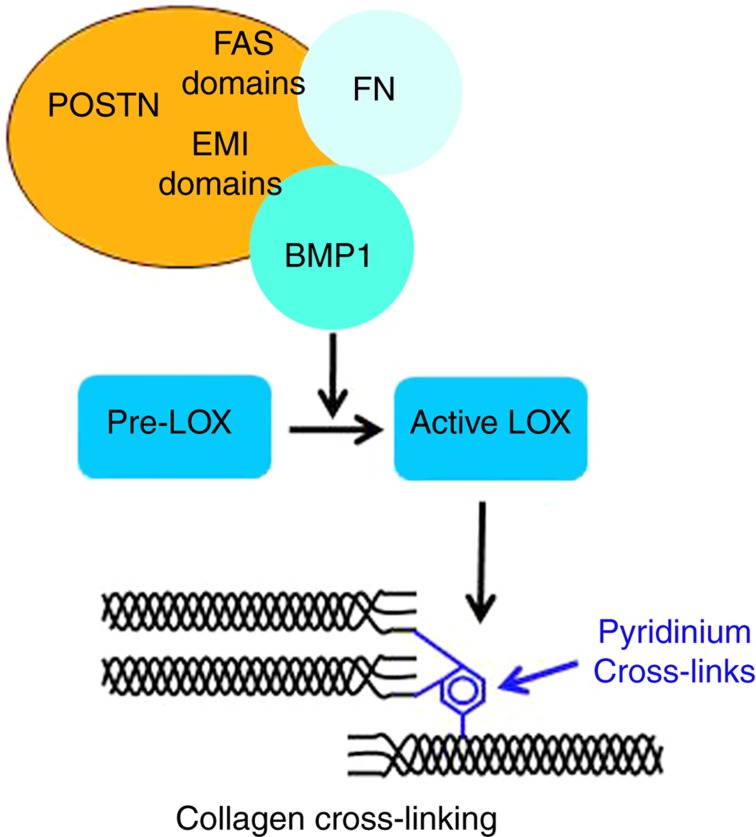
LOX is a copper metalloenzyme that requires pyridoxal phosphate (vitamin B6) and tyrosyl-lysine quinone as essential cofactors.4,5 Different factors influence the transcription and activation of LOX. Among positive regulators, TGFβ, insulin-like growth factor I, estrogens and 1.25-OH2D3 have been reported to increase the transcription and/or the activity of LOX.6,7,8 In contrast, homocysteine is believed to negatively affect LOX expression and activity.9 It was also demonstrated that homocysteine binds to the aldehyde groups of allysine and hydroxylysine, which are the precursors of enzymatic crosslinks and leads to a marked decrease in their levels.10 Other factors such as fibroblast growth factor and high dose of PGE2 and TNFα also inhibit LOX expression.11,12 Recently it has been demonstrated that the gamma-carboxyglutamic acid containing non-collagenous protein, periostin, has a major role in modulating the activity of LOX.13 Indeed LOX is synthesized as a non-active precursor, proLOX, which is activated by the cleavage of the propeptide mediated by BMP1, which is a procollagen peptidase. It has been shown that periostin binds to BMP1 and increase its deposition in the fibronectin matrix in close proximity of proLOX, thereby increasing its activation13 (Figure 2). Interestingly, we found that periostin null mice are characterized by decreased levels of the enzymatic divalent and trivalent crosslinks compared with wild-type animals.14 These findings suggest that besides its role in regulating bone formation, periostin may also alter the biomechanical properties of bone matrix.
Figure 2. Periostin is a key regulator of collagen cross-linking.
Periostin (POSTN) binds fibronectin (FN) through its EMI-domain and to BMP-1 through its FAS-1 domain, promoting the deposition of BMP-1 into the matrix. BMP-1 activates lysyl oxidase precursor (pro-LOX) to mature active form (LOX). LOX is responsible for the synthesis of pyridinium cross-links, further linking collagen fibers.70
Advanced glycation end products.
Non-enzymatic glycation is a post-translational modification of proteins that occurs through the Maillard reaction, where extracellular sugars spontaneously react with amino groups on proteins to form molecular entities, known as advanced glycation end products (AGEs) (for a review, see Paul and Bailey15). AGEs formed in bone are divided into two types, those making crosslinks between collagen molecules and those that are non-crosslinks. The first step in crosslinking AGEs is the reaction of the aldehyde of an open-chain form of glucose, ketase or other metabolic intermediate (such as glyoxal) with the ɛ-amino group of collagen-bound lysine to form a glycosyl-lysine via Schiff base formation. The hexosyl-lysine or histidine is then stabilized by spontaneous Amadori rearrangement. The Amadori adduct can then undergo further reactions with amino acids such as lysine or arginine in adjacent collagen molecules to form AGEs crosslinks (Figure 1). A typical example of crosslinking AGE is pentosidine, which is likely to be formed between helical lysine and arginine residues of two collagen molecules. There are numerous sources of AGEs in bone tissues, the majority of them have yet to be identified.
AGE crosslinks are believed to alter collagen structure, and hence bone mechanical properties.16 In contrast, non-crosslink types of AGEs, such as carboxymeythyllysine, affect bone metabolism mainly by altering the osteoblast function via interaction with the cell surface receptor of AGE, RAGE.17 The effect of AGEs on osteoclastic bone resorption remains controversial. Indeed some studies report that AGEs may enhance osteoclast-induced bone resorption in a model where in vitro ribosylated bone particles are subcutaneously implanted in rats.18 In contrast, in another study in which human osteoclasts were seeded on bone slices, treated with a high dose of exogenous ribose, the activity of human osteoclasts was decreased.19 Such discrepancies are most likely related to differences in the model used, and also in the concentration of AGEs within the bone. A recent study that analyzed the relationship between AGEs and osteoclast activity in human cadaveric bone specimens showed that higher AGEs was associated with an increased number of larger resorption cavities.20 These data suggest that when present at physiological concentrations, AGEs most likely stimulate osteoclastic activity. Interestingly, we recently found that cathepsin K, the most important collagenolytic enzyme secreted by the osteoclasts, preferentially degrades matured bone matrix characterized by a higher degree of post-translational modifications, including pentosidine.21
Racemization and isomerization.
Racemization is the spontaneous conversion of the native L-enantiomeric form of amino acids or sugars to the rare D form. Accumulation of D isomer is common in tissues with low turnover such as dentin, dermis and cartilage. β-Isomerization is the transfer of the peptide bound between aspartic acid residues and the adjacent amino acid from the α-carboxyl group to the β- or γ-carboxyl group. This spontaneous transformation causes a kink in the peptide backbone, and may thus alter the properties of the molecule. In bone, racemization and isomerization have shown to involve the aspartic acid residue within the CTX of type I collagen in the so called CTX sequence.22,23
Age-related changes of bone collagen post-translational modifications
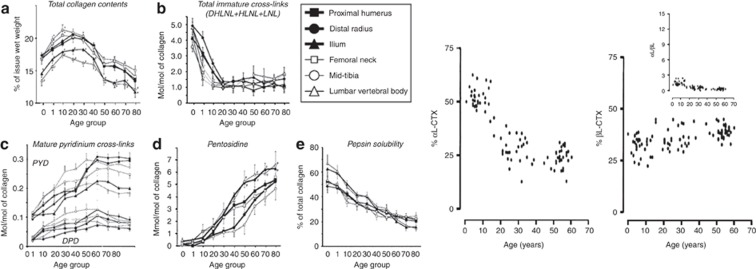
To analyze the association of collagen modifications with bone strength it is important to have in mind their physiological changes with age. Immature divalent enzymatic crosslinks decreases markedly between birth and 25 years, but persisted in a significant amount throughout adult life (Figure 3). This decrease of divalent crosslinks is accompanied by a gradual elevation of the enzymatic trivalent crosslinks with the largest increase occurring before the age 15 and then a slower increase up to age 30–40 years (Figure 3). Then the concentration of trivalent crosslinks stays virtually in the same range throughout adult life.24 In contrast, the AGE pentosidine markedly and continuously increases with age in human bone (Figure 3).24,25 Aspartic acid isomerization at 37 °C has been studied in vitro using synthetic CTX peptides and immature fetal bovine bone collagen extract that consists mainly of native α CTX isomer.23 At equilibrium of the reaction of isomerization, about 20% of CTX peptide remains in its original α form and 80% is β-isomerized.23 Thus, in contrast to AGEs, the relative concentration of isomerized β-CTX vs α-CTX cannot exceed the level reached at the equilibrium of the kinetic. The ratio between native and isomerized CTX (α/β-CTX) measured in urine by specific immunoassays gives an accurate estimate of the extent of type I collagen isomerization in bone tissue.23 Because of high bone remodeling in children, the equilibrium of isomerization cannot be achieved, resulting in a relatively higher proportion of α CTX compared with β CTX (high urinary α/β CTX ratio) (Figure 3). In adults, because on average the rate of bone remodeling is slower than isomerization, the equilibrium is achieved, resulting in a fairly constant α/β CTX ratio from the age of 20 years and above (Figure 3).
Figure 3. Age-related changes of enzymatic and non-enzymatic modifications in human bone and of CTX isomers in human urine.
(a) Total collagen contents; (b) total immature crosslinks; (c) mature pyridinium crosslinks (PYD and DPD); (d) pentosidine; (e) solubility of collagen assesed by pepsin digestion. Left panel: closed square: humerus; closed circle: distal radius; closed triangle: ilium; open square: femur; open circle: tibia; open triangle: vertebra. The data represent the concentration expressed as the mean (s.e.) value of the percent of tissue wet weight from normal cortical bone of 40 healthy male subjects, 0–84 years of age. *P<0.05 vs value of non-weight bearing bones; -P<0.05 vs value of extremity bones (from Saito71). Right panel: Relative contents of CTx isoforms in urine from healthy humans aged 0.2–74 years. The concentrations of the different CTx isoforms were determined in 114 healthy individuals. The relative content of each isoform (% of total CTx) and corresponding CTx ratios were calculated. Age-dependent variation of αL and βL (inset shows αL/βL ratio) (from Cloos and Fledelius23).
Collagen post-translational modifications: relationship with bone microstructure and mineralization
Several in vitro studies have analyzed the association of enzymatic and non-enzymatic crosslinks with bone microarchitecture. In trabecular bone from vertebral bodies, Banse et al.26 reported that biopsies with high pyrolle and low PYD content had thicker, but more disconnected two-dimensional trabecular architecture. Conversely, those with low pyrolle and high PYD had thinner trabeculae but numerous and a more complex network. Viguet-Carrin et al.27 found no significant relationship between three-dimensional assessment of trabecular microstructure at the vertebral bodies and the amount of PYD or DPD. More consistent associations were reported between AGEs and cortical bone structure. It has been reported that AGEs including pentosidine are more concentrated in older than younger osteons, with the highest levels being present in the interstitial bone, indicating that the accumulation of AGE in bone tissue is dependent not only on chronological age but also on local bone tissue maturation.20 In human vertebral bone, a recent study showed a significant positive association between pentosidine levels and trabecular number and connectivity density, and a negative association with trabecular separation.27 Thus, these data suggest that higher AGE content may be associated with denser and more complex rod-like trabecular architecture.
The data on the influence of post-translational collagen modifications on mineralization are limited. Banes et al.28 reported difference in the concentration of PYD crosslinks between mineralized and non-mineralized bone matrix of cortical bone, and found higher levels in this latter compartment. These data suggest that in non-mineralized tissue, PYD crosslinks may decrease the intermolecular distances among collagen chains in fibrils and impair mineralization.28 Using osteoblastic MC3T3 cell lines, overexpressing lysyl hydroxylase 2b, it was shown that compared with control cells, the diameter of collagen fibers were smaller and the number of mineralized nodules was lower, again suggesting that collagen crosslinks may alter mineralization.29 More recently the pattern of collagen crosslinks was investigated in bone of Phospho1 deficient mice.30 Phospho1 is a phosphoethanolamine/phosphocholine phosphatase involved in the generation of inorganic phosphate for bone mineralization. It is highly expressed at sites of mineralization in bone and cartilage. It was shown that although the total amount of immature (DHLNL and HLNL) and mature (PYD and DPD) collagen enzymatic crosslinks was not modified, there was an alteration of the DHLNL/HLNL and PYD/DPD ratios. This suggests that mineralization may alter the activity of the enzyme lysyl hydroxylase 1, which would lead to a greater proportion of hydroxylysine residues relative to lysine residues adjacent to the telopeptide aldehydes. Alternatively, mineralization itself may influence the ratio PYD/DPD.30
Collagen post-translational modifications and bone strength
Collagen crosslinking affects mainly the post-yield properties of bone, because pre-yield strength is mainly dependent on the mineral phase. Nevertheless, some studies suggest that enzymatic and AGE crosslinks affects not only bone toughness but also stiffness, and the elastic modulus independent of the mineral phase.
Role of enzymatic crosslinks in bone strength in animal and in vitro studies.
Because enzymatic crosslinks are mainly dependent on the regulated activity of LOX, marked changes in pyridinoline and pyrollic crosslinks cannot be observed physiologically. Thus, artificial models allowing changes in the concentration of enzymatic crosslinks have been used to assess the direct contribution of these crosslinks to bone strength. Oxlund et al.31 demonstrated that treatment of rats with beta aminoproprionitrile, which inhibits LOX induced a decrease of about 50% in pyridinium crosslinks that was associated with a 26 and 30% reduction of bending strength and modulus of cortical bone, respectively, compared with untreated animals. Using the same animal model, it was recently reported that beta aminoproprionitrile treatment induced a decrease in divalent and trivalent crosslinks, which was associated with a decrease of trabecular thickness and compressive energy to failure, while stiffness was not affected.32 Another rat study showed that increased homocysteinemia induced by a diet enriched in methiononine or homocysteine—which is likely to interfere with crosslink formation—for 3 months resulted in decreased bone formation, bone volume and bone strength at the distal femur.33 The impact of an increase of enzymatic crosslinks on bone strength can also be investigated using fetal bovine cortical bone, which is characterized by a low level of these molecules. By incubating bone samples at 37 °C for 60 days, we showed that the increase in PYD and DPD was positively associated with an increase of ultimate stress and post-yield energy absorption.34 Thus, all together these experimental data clearly indicate that marked changes in enzymatic trivalent crosslinks are associated with alteration in post-yield mechanical properties both in cortical and trabecular bone.
The contribution of enzymatic crosslinks on bone strength has also been investigated in a series of studies correlating their concentration to mechanical properties in human cadaveric bone specimens. Banse et al.35 showed that the ratio between the enzymatic crosslinks PYD/DPD, but not their actual concentration, was significantly associated with the compressive biomechanical properties of human vertebrae independent of BMD. There was no association of divalent immature crosslinks or pyrolle with mechanical properties. Another study failed to find a significant association between PYD and DPD, and compressive mechanical properties of the vertebral trabecular bone of elderly subjects.36 In iliac bone, there was also no correlation between enzymatic divalent or trivalent crosslinks and bone strength.37 Thus, the contribution of enzymatic crosslinks to bone strength when present at physiological levels remains to be determined, but is likely to be modest.
Role of ages on bone strength in in vitro studies
Several in vitro studies have shown that the non-enzymatic crosslinks AGEs have negative impact on post-yield properties of bone and toughness (for a review, see Vashishth et al.38). In models using glycation of bovine bone by exogenous ribose or glucose, it was shown that an increase of AGE content was associated with a decrease in post-yield strength and post-yield energy.39 Similar results were reported by Tang et al.40 in human cancellous bone incubated with ribose. 40More recently, using a high-resolution nonlinear finite element model that incorporates cohesive elements and micro-computed tomography-based three-dimensional meshes, it was shown that an increase of AGE crosslinks induces a 52% decrease in the propagation fracture toughness. Interestingly, when combined with increased porosity, AGE resulted in a further 88% decrease of propagation toughness.41 A recent study analyzed the effect of AGE on microcrack propagation. It was shown that in vitro ribosylation of human tibial cancellous bone increased microdamage morphology parameters when subjected to compressive loading.42 These findings indicate that accumulation of AGEs make the tissue more brittle,42 alters the quantity and morphology of microdamage and results in increased bone fragility.
The role of the non-enzymatic AGEs in bone fracture resistance has also been supported by a series of ex vivo cadaveric experiments. Wang et al.43 showed that higher levels of pentosidine in human cortical bone were associated with decreased resistance to fracture and, more specifically, toughness. Similar findings were reported in human vertebral bone tissue.44,45 High bone pentosidine content was also reported in patients with hip fractures.46
Effect of osteoporosis treatment on collagen crosslinks and bone strength
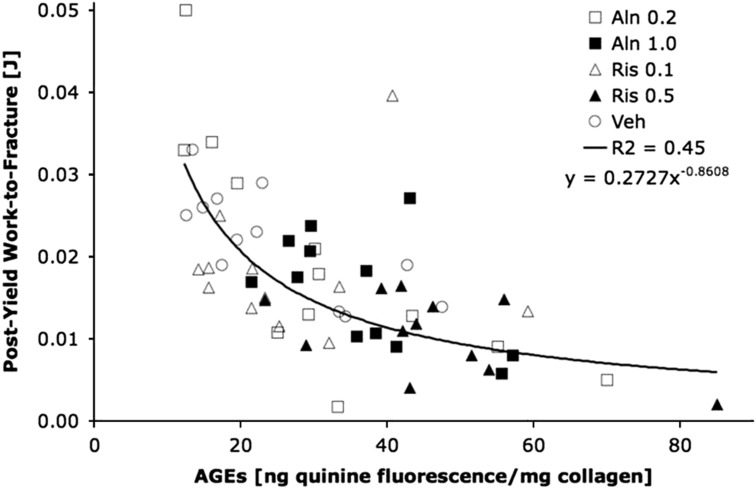
In vertebral trabecular bone from dogs it has been shown that the bisphosphonates risedronate and alendronate administered for 1 year at doses that were equivalent to the clinical doses used in postmenopausal osteoporosis induce an increase in the ratio PYD/DPD and of pentosidine (+34–58%), whereas α/β CTX ratio was decreased. This indicates that bisphosphonates increase bone collagen maturation.47 However, because the treated animals were normal, these results may or may not reflect what is occurring in osteoporosis. Interestingly, bone turnover rate was correlated with pentosidine content, indicating that higher the suppression of bone remodeling with bisphosphonate, larger is the accumulation of this AGE in bone matrix. In contrast, no effect was observed with raloxifene,47 probably because this drug reduces only moderately bone turnover. These effects on AGEs were not observed with the same bisphosphonates administrated at the same doses for the same duration on tibial cortical bone.48 These discrepant findings are likely to be due to the lower bone remodeling in cortical compared with trabecular bone.48 With higher doses, however (equivalent to Paget's disease clinical dose), both risedronate and alendronate induce a significant increase in total AGEs in cortical bone and a reduction of post-yield energy. The increased of AGEs was significantly and negatively associated with post-yield work to fracture (Figure 4). Saito et al.49 analyzed the long-term effect of incadronate on collagen crosslinks in cortical rib bone. They found that although the total amount of immature and mature enzymatic crosslinks was not altered after 3 years of treatment, the ratio of mature/immature dose dependently increases indicating an increase of collagen maturation. This increase of collagen maturation was also associated with a dose-dependent increase of pentosidine that was correlated with bone turnover rate.
Figure 4. Relationship between AGE content in tibial cortical bone and post-yield work-to-fracture in dogs treated with two doses of alendronate (Aln, 0.2 and 1.0 mg kg−1) and two doses of risedronate (0.1 and 0.5 mg kg−1).
Total AGE content was determined by fluorescence reading and using a quinine sulfate as a standard (from Tang et al.48).
More recently the effect of 18 months intermittent parathyroid hormone (PTH) 1–34 on bone structure, bone strength and collagen crosslinks have been investigated in ovariectomized monkeys.50 It was found that both low and high dose of PTH improved vertebral bone strength, bone mass and trabecular thickness, as expected. PTH also increased significantly the amount of total enzymatic crosslinks (immature and mature). Conversely, there was a decrease in the ratio of mature/immature pyridinium crosslinks and in pentosidine, suggesting that PTH treatment is associated with the formation of a bone matrix characterized by a lower degree of maturation. Interestingly, in a multivariate model it was shown that the content of immature and mature pyridinium crosslinks was an important determinant of stiffness, ultimate load and breaking energy independent of bone volume and trabecular thickness.50 Using Fourier transform infrared imaging to analyze the ratio of enzymatic crosslinks, similar results were obtained in monkeys treated with PTH with a decrease in the ratio of mature/immature collagen crosslinks.51
Systemic levels of enzymatic and non- enzymatic crosslinks and bone strength in clinical studies
Few studies have investigated the association between serum and urinary levels of collagen crosslinks and bone strength. One of the main limitations of the measurements of enzymatic crosslinks in blood and urine is that systemic levels reflect mainly the levels of bone turnover and not directly the alterations of their content per amount of collagen matrix. In addition, because most of collagen crosslinks including AGEs are not exclusively distributed in bone, there is also an issue of tissue specificity that is likely to impair the sensitivity of detecting significant associations. Although, high free PYD and DPD levels have been shown to be associated with increased risk of fracture in postmenopausal women (for a review see Garnero52), attempts to correlate the ratio of PYD/DPD with fracture risk have also been inconclusive. An indirect assessment of the influence of enzymatic crosslinks on bone strength may be gained from the measurements of homocysteine, which interferes with enzymatic collagen crosslinking. Epidemiological studies investigating the association between plasma homocysteine levels and fracture risk have been inconsistent. Increased plasma homocysteine levels were found to be associated with increased fracture risk in elderly men and women,53,54,55 with stronger association in men,53 especially when combined with low vitamin B12 levels.55 However, such associations were not confirmed in younger (mean age 62 years)56 and older (>75 years)57 postmenopausal women after adjustment for age.
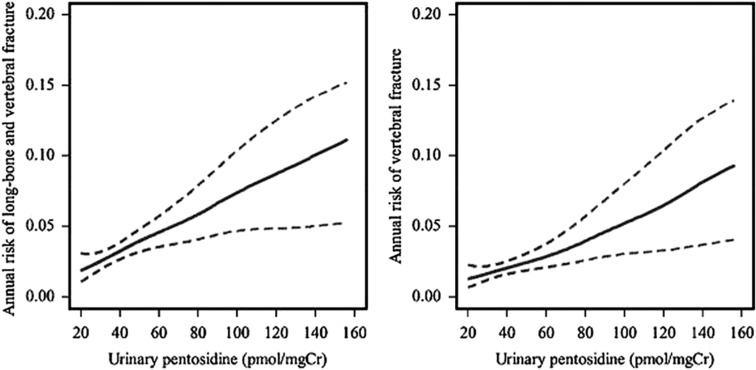
In 432 elderly Japanese women, increased urinary pentosidine was moderately associated with an increased risk of incident vertebral fracture independent of BMD and the systemic levels of bone turnover markers.58 Similar findings were recently reported in a larger cohort of Japanese postmenopausal women (Figure 5), with a moderate BMD-independent association of urinary pentosidine with incidence of vertebral and non-vertebral fractures.59 Although there was also a significant association of urinary pentosidine with the risk of all fractures in 396 healthy postmenopausal women from the French OFELY study, the odds ratio was not significant after adjustment for BMD.60 A clinical situation where AGEs may be particularly relevant is type 2 diabetes, a disease characterized by altered glucose metabolism and increased bone fragility despite increased or normal BMD. Two studies reported a strong association between increased serum or urinary pentosidine levels and prevalent and incident vertebral fracture in patients with type 2 diabetes, independent of several confounding factors such as levels of glycated albumin, BMD and renal function.61,62 Pentosidine is only one of the several AGEs present in bone matrix. Other AGEs have been shown to accumulate in bone tissue including vesperlysine, methylglyoxal-derived lysine dimer, glyoxal-derived lysine dimer, imidazolone and N(epsilon)-carboxymethyllysine,38 although their association with bone fracture resistance has not yet been investigated.
Figure 5. Annual risk (solid line) and 95% confidence intervals (broken lines) of long bone and vertebral fracture (left) and vertebral fracture only (right) in relation to urinary pentosidine estimated by multivariate generalized additive models in 765 postmenopausal women.
Data were adjusted for age, weight diabetes mellitus lumbar spine BMD, prior fracture and back pain (from Tanaka et al.59).
The contribution of collagen isomerization to fracture risk has also been investigated. In clinical situations characterized by a marked increase of bone remodeling in localized areas of the skeleton, such as in patients with Paget's disease63 and bone metastases,64 the equilibrium of isomerization cannot be achieved, resulting in a higher proportion of α vs β CTX as documented by histological studies. In patients with Paget's disease or bone metastases from breast cancer, alteration of collagen isomerization was shown to be associated with the presence of woven bone—a tissue characterized by disorganized collagen fibers and increased fragility. Another clinical situation characterized by a disorganized collagen matrix and increased bone fragility despite normal BMD is osteogenesis imperfect (OI). In adults with osteogenesis imperfect, we recently found increased urinary α/β CTX ratio65 compared with healthy controls, although to a lower magnitude than in Paget's disease of bone.
Alterations in type I collagen isomerization may also be of clinical relevance in postmenopausal and male osteoporosis. In the OFELY prospective study, we found that women with a urinary α/β CTX ratio in the highest quartile had an increased risk of incident fracture independent of both the level of hip BMD and of bone turnover rate measured by serum bone specific alkaline phosphatase.66 A strong association between high urinary α/β CTX ratio and the incidence of vertebral fracture was also more recently reported in older men participating in the Mr Os study.67
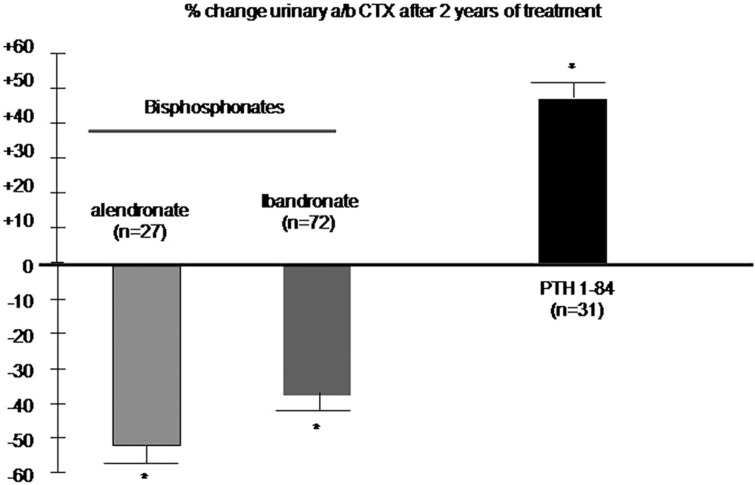
The fracture efficacy of anti-resorptive and anabolic therapies is only partly explained by changes in BMD and bone turnover, suggesting that other factors, including modifications of bone matrix properties may have a role. It has been reported that a decrease in the urinary α/β CTX ratio in postmenopausal women receiving alendronate at 10 or 20 mg per day and oral daily (2.5 mg) or intermittent ibandronate, but not in those receiving raloxifene or estradiol.68 Conversely, in women receiving PTH 1–84 for 2 years,69 we reported a marked increased of urinary α/β CTX, suggesting a decreased of collagen maturation (Figure 6). Whether these changes in collagen maturation observed with bisphosphonate and PTH therapy will translate into alterations of fracture resistance independent of BMD remains to be investigated.
Figure 6. Effect of bisphosphonates and intermittent PTH on type I collagen isomerisation in postmenopausal women with osteoporosis.
The graphs show the mean (s.e.) of the percent change from baseline at 2 years of the urinary α/β CTX ratio. In the PTH study, patients were treated for one year with PTH (1–84) followed by 1 year of placebo Please note that the data were not derived from head to head comparison studies (from Byrjalsen et al.68 and Garnero et al.69). *P<0.001 vs baseline.
Altogether, the data reviewed above indicate that the degree of post-translational modifications—more specifically non-enzymatic age-related-modifications of collagen—has an independent role in determining the bone mechanical competence, and that the ratio of α/β CTX may provide an in vivo marker of bone matrix maturation.
Footnotes
The author declares no conflict of interest.
References
- Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E et al. Fracture incidence and association with bone mineral density in elderly men and women: The Rotterdam Study. Bone 2004;34:195–202. [DOI] [PubMed] [Google Scholar]
- Sornay-Rendu E, Munoz F, Garnero P, Duboeuf F, Delmas PD. Identification of osteopenic women at high risk of fracture: the OFELY Study. J Bone Miner Res 2005;20:1813–1819. [DOI] [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med 2004;15:126–136. [DOI] [PubMed] [Google Scholar]
- Bird TA, Levene CI. Lysyl oxidase: evidence that pyridoxal phosphate is a co-factor. Biochem Biophys Res Commun 1982;108:1172–1180. [DOI] [PubMed] [Google Scholar]
- Wang SX, Mure M, Medzihradszky KF, Burlingame AL, Brown DE, Dooley DM et al. A crosslinked cofactor in lysyl oxidase: redox function for amino acid side chains. Science 1996;273:1078–1084. [DOI] [PubMed] [Google Scholar]
- Feres-Filho EJ, Choi YJ, Han X, Takala TE, Trackman PC. Pre and post translational regulation of lysyl oxidase by transforming growth factor-beta 1 in osteoblastic MC3T3-E cells. J Biol Chem 1995;270:307978–330803. [DOI] [PubMed] [Google Scholar]
- Reiser K, Summers P, rano JF, Rucker R, Last J, McDonald R. Effects of elevated circulating IGF-1 on the extracellular matrix in high growth C57BL/6J mice. Am J Physiol 1996;271:R696–R703. [DOI] [PubMed] [Google Scholar]
- Ozasa H, Tominaga T, Nishimura T, Takeda T. Lysyl oxidase activity in the mouse uterine cervix is physiologcally regulated by estrogens. Biochim Biophys Acta 1978;541:408–413.27234 [Google Scholar]
- Liu G, Nellaiappan K, Kagan HM. Irreversible inhibition of lysil oxidase by homocysteine thiolactone and its selenium and oxygen analogues. Implications for homocysteinuria. J Biol Chem 2004;177:1–8. [DOI] [PubMed] [Google Scholar]
- Kang HA, Trelstad RL. A collagen defect in homocystinuria. J Clin Invest 1973;52:2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feres-Filho EJ, Menassa GB, Tackman PC. Regulation of lysyl oxidase by basic fibroblast growth factor in osteoblastic MC3T3-E1 osteoblasts. Calcif Tissue Int 1996;75:384–395. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Alcudia JF, Martinez-Gonzalez J, Raposo B, Navarro MA, Badimon L. Lysyl oxidase (LOX) down regulation by TNF alpha: a new mechanism underlying TNF alpha-induced endothelial dysfunction. Atherosclerosis 2008;196:558–564. [DOI] [PubMed] [Google Scholar]
- Maruhashi T, Kii I, Saito M, Kudo A. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem 2010;285:13294–13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gineyts E, Ferrari SL, Bonnet N, Garnero P. Periostin, a matricellular glutamic-acid (Gla) protein influences crosslinking of bone collagen. J Bone Miner Res 2011;25:(Suppl 1): S390. [Google Scholar]
- Paul RG, Bailey AJ. Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol 1996;28:1297–1310. [DOI] [PubMed] [Google Scholar]
- Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep 2007;5:62–66. [DOI] [PubMed] [Google Scholar]
- Cortizo AM, Lettieri MG, Barrio DA, Mercer N, Etcheverry SB, McCarthy AD. Advanced glycation end products (AGEs) induce concerted changes in the osteoblastic expression of their receptor RAGE and in the activation of extracellular signal-regulated kinases (ERK). Mol Cell Biochem 2003;250:1–10. [DOI] [PubMed] [Google Scholar]
- Miyata T, Notoya K, Yoshida K, Horie K, Maeda K, Kurokawa K et al. Advanced glycation end products enhance osteoclast-induced bone resorption in culture mouse unfractionated bone cells and in rats subcutaneously with devitalized bone particles. J Am Soc Nephrol 197;8:260–270. [DOI] [PubMed] [Google Scholar]
- Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem 2007;282:5691–5703. [DOI] [PubMed] [Google Scholar]
- Dong XN, Qin A, Xu J, Wang X. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone 2011;49:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel O, Gineyts E, Bertholon C, Garnero P. Cathepsin K preferentially solubilizes matured bone matrix. Calcif Tissue Int 2012;91:32–39. [DOI] [PubMed] [Google Scholar]
- Fledelius C, Johnsen AH, Cloos PA, Bode M, Qvist P. Characterization of urinary degradation products derived from type I collagen. Identification of a beta-isomerized Asp-Gly sequence within the C-terminal telopeptides (alpha 1) region. J Biol Chem 1997;272:9755–9763. [DOI] [PubMed] [Google Scholar]
- Cloos PA, Fledelius C. Collagen fragments in urine derived from bone resorption are highly racemized and isomerized: a biological clock of protein aging with clinical potential. Biochem J 2000;345:473–480. [PMC free article] [PubMed] [Google Scholar]
- Saito M, Marumo K, Fujii K, Ishioka N. Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem 1997;253:26–32. [DOI] [PubMed] [Google Scholar]
- Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M et al. Advanced glycation end products and bone loss during aging. Ann NY Acad Sci 2005;1043:710–717. [DOI] [PubMed] [Google Scholar]
- Banse X, Devogelaer JP, Lafosse A, Sims TJ, Grynpas M, Bailey AJ. Cross-link profile of bone collagen correlates with structural organization of trabeculae. Bone 2002;31:70–76. [DOI] [PubMed] [Google Scholar]
- Viguet-Carrin S, Follet H, Gineyts E, Roux JP, Munoz F, Chapurlat R et al. Association between collagen cross-links and trabecular microarchitecture properties of human vertebral bone. Bone 2010;46:342–347. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Yamauchi M, Mechanic GL. Nonmineralized and mineralized compartments of bone: the role of pyridinoline in nonmineralized collagen. Biochem Biophys Res Commun 1983;113:975–981. [DOI] [PubMed] [Google Scholar]
- Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Overexpression of lysyl hydroxylase-2b leads to defective collagen fibrillogenesis and matrix mineralization. J Bone Miner Res 2005;20:81–87. [DOI] [PubMed] [Google Scholar]
- Huesa C, Yadav MC, Finnilä MA, Goodyear SR, Robins SP, Tanner KE et al. PHOSPHO1 is essential for mechanically competent mineralization and the avoidance of spontaneous fractures. Bone 2011;48:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxlund H, Barckman M, Ortoft G, Andreassen TT. Reduced concentrations of collagen crosslinks are associated with reduced strength of bone. Bone 1995;17:365S–371S. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Tatakis DN, Robins S, Fratzl P, Manjubala I, Zoehrer R et al. Lathyrism-induced alterations in collagen crosslinks influence mechanical properties of bone material without affecting the mineral. Bone 2011;49:1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, Wildemann B, Claes L, Klohs S, Ohnmacht M, Taban-Shomal O et al. Experimental hyperhomocysteinemia reduces bone quality in rats. Clin Chem 2007;53:1455–1461. [DOI] [PubMed] [Google Scholar]
- Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML. Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 2006;38:300–309. [DOI] [PubMed] [Google Scholar]
- Banse X, Sims TJ, Bailey AJ. Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross_links. J Bone Miner Res 2002;17:1621–1628. [DOI] [PubMed] [Google Scholar]
- Follet H, Viguett-Carrin S, Burt-Pichat B, Dépalle B, Bala Y, Gineyts E et al. Effects of preexisting microdamage, collagen cross-links, degree of mineralization, age, and architecture on compressive mechanical properties of elderly human vertebral trabecular bone. J Orthop Res 2011;29:481–488. [DOI] [PubMed] [Google Scholar]
- Bailey AJ, Sims TJ, Ebbesen EN. Age related changes in the biochemical properties of human cancellous bone collagen: relationships to bone strength. Calcif Tissue Int 1999;65:203–210. [DOI] [PubMed] [Google Scholar]
- Vashishth D, Gibson GJN, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 2001;28:195–201. [DOI] [PubMed] [Google Scholar]
- Tang SY, Zeenath U, Vashishth D. Effects of on enzymatic glycation on cancellous bone fragility. Bone 2007;40:1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SY, Vashishth D. The relative contributions of non-enzymatic glycation and cortical poroosty on the fracture toughness of aging bone. J Biomech 2011;44:330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SY, Vashishth D. Non enzymatic glycation alters microdamage formation in human cancellous bone. Bone 2010;46:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanese J, Bank R, Tekoppele J, Keaveny T. Increased cross-linking by non-enzymatic glycation reduces the ductility of bone and bone collagen. Proc ASME Bioeng Conf 1999;42:267–268. [Google Scholar]
- Wang X, Shen X, Li X, Agrawal CM. Age-related changes in collagen network and toughness of bone. Bone 2002;31:1–7. [DOI] [PubMed] [Google Scholar]
- Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der F et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 2005;37:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I et al. Contribution of advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone 2006;39:1073–1079. [DOI] [PubMed] [Google Scholar]
- Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int 2006;79:160–168. [DOI] [PubMed] [Google Scholar]
- Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross_linking and isomerization in beagle dog vertebra. Osteoporos Int 2008;19:329–337. [DOI] [PubMed] [Google Scholar]
- Tang SY, Allen R, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int 2009;20:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Mori S, Mashiba T, Komatsubara S, Marumo K. Collagen maturity, glycation induced-pentosidien, and mineralization are increased following 3 year treatment with incadronate in dogs. Osteoporos Int 2008;19:1343–1354. [DOI] [PubMed] [Google Scholar]
- Saito M, Marumo K, Kida Y, Ushiku C, Kato S, Takao-Kawabata R et al. Changes in the contents of enzymatic immature, mature and non-enzymatic senescent crosslinks of collagen after once-weekly treatment with human parathyroid hormone (1-34) for 18 months contribute to improvement of bone strength in ovariectomized monkeys. Osteoporos Int 2011;22:2373–2383. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Burr DB, Mendelsohn R, Hock JM, Boskey AL. Bone mineral and collagen quality in humeri of ovariectomized cynomolgus monkeys given rhPTH(1-34) for 18 months. J Bone Miner Res 2003;18:769–775. [DOI] [PubMed] [Google Scholar]
- Garnero P. Markers of bone turnover for the prediction of fracture risk. Osteoporos Int 2000;11 (Suppl 6): S55–S65. [DOI] [PubMed] [Google Scholar]
- McLean RR, Jacques PF, Selhub J, Tucker KL, Samelson EJ, Broe KE et al. Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 2004;350:2042–2049. [DOI] [PubMed] [Google Scholar]
- van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, van der Klift M, de Jonge R, Lindemans J et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med 2004;350:2033–2041. [DOI] [PubMed] [Google Scholar]
- Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res 2005;20:921–929. [DOI] [PubMed] [Google Scholar]
- Périer MA, Gineyts E, Munoz F, Sornay-Rendu E, Delmas PD. Homocysteine and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int 2007;18:1239–1236. [DOI] [PubMed] [Google Scholar]
- Gerdhem P, Ivaska KK, Isaksson A, Pettersson K, Väänänen HK, Obrant KJ et al. Associations between homocysteine, bone turnover, BMD, mortality, and fracture risk in elderly women. J Bone Miner Res 2007;22:127–134. [DOI] [PubMed] [Google Scholar]
- Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab 2008;26:93–100. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kuroda T, Saito M, Shiraki M. Urinary penstosidien improves risk classification using fracture assessment tools for postmenopausal lwoen. J Bone Miner Res 2011;26:2778–2784. [DOI] [PubMed] [Google Scholar]
- Gineyts E, Munoz F, Bertholon C, Sornay-Rendu E, Chapurlat R. Urinary levels of pentosidien and the risk of fracture in postmenopausal women: the OFELY study. Osteoporos Int 2010;21:243–250. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 2008;93:1013–1019. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Garnero P, Hillier T, Sellmeyer DE, Strotmeyer ES, Feingold KR et al. Pentosdine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab 2009;94:2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnero P, Fledelius C, Gineyts E, Serre CM, Vignot E, Delmas PD. Decreased beta-isomerization of the C-terminal telopeptide of type I collagen alpha 1 chain in Paget's disease of bone. J Bone Miner Res 1997;12:1407–1415. [DOI] [PubMed] [Google Scholar]
- Leeming DJ, Delling G, Koizumi M, Henriksen K, Karsdal MA, Li B et al. Alpha CTX as a biomarker of skeletal invasion of breast cancer: immunolocalization and the load dependency of urinary excretion. Cancer Epidemiol Biomarkers Prev 2006;15:1392–1395. [DOI] [PubMed] [Google Scholar]
- Garnero P, Schott AM, Prockop D, Chevrel G. Bone turnover and type I collagen C-telopeptide isomerization in adult osteogenesis imperfecta: associations with collagen gene mutations. Bone 2009;44:461–466. [DOI] [PubMed] [Google Scholar]
- Garnero P, Cloos P, Sornay-Rendu E, Qvist P, Delmas PD. Type I collagen racemization and isomerization and the risk of fracture in postmenopausal women: the OFELY prospective study. J Bone Miner Res 2002;17:826–833. [DOI] [PubMed] [Google Scholar]
- Bauer DC, Garnero P, Litwack S, Cauley JA, Ensrud K, Eastell R et al. Type I collagen isomerization (alpha/beta CTX ratio) and the risk of new vertebral fracture in men: a prospective study. J Bone Miner Res 2010;25 (Suppl 1): 1024. [Google Scholar]
- Byrjalsen I, Leeming DJ, Qvist P, Christiansen C, Karsdal MA. Bone turnover and bone collagen maturation in osteoporosis: effects of antiresorptive therapies. Osteoporos Int 2008;19:339–348. [DOI] [PubMed] [Google Scholar]
- Garnero P, Bauer D, Mareau E, Bilezikian JP, Greenspan SL, Rosen C et al. Effects of PTH and alendronate on Type I collagen isomerization in postmenopausal women with osteoporosis: the PaTH Study. J Bone Miner Res 2008;23:1442–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle B, Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos Int 2012;23:1199–1212. [DOI] [PubMed] [Google Scholar]
- Saito M. Age-related changes in biochemical characteristics of collagen from human weight-bearing and non weight-bearing bone. Tokyo Jikeika Ika Daigaku Zasshi 1999;114:327–337. [Google Scholar]