Background: SIRT3 plays a major role in protecting against mitochondrial oxidative stress.
Results: Oxidative stress increases during keratinocyte differentiation, and SIRT3 can decrease differentiation by attenuating oxidative stress.
Conclusion: SIRT3-induced down-regulation of mitochondrial oxidative stress attenuates keratinocyte differentiation.
Significance: Understanding the regulation of keratinocyte differentiation is crucial for developing new therapies against dysregulated skin differentiation and aging.
Keywords: Differentiation, Keratinocytes, Mitochondria, Reactive Oxygen Species (ROS), Sirtuins, SIRT3
Abstract
Keratinocyte differentiation is a key process in the formation and maintenance of the protective skin barrier. Dysregulation in the balance of reactive oxygen species homeostasis may play a role in keratinocyte differentiation. We have identified the mitochondrial deacetylase SIRT3 as a key regulator of mitochondrial reactive oxygen species in keratinocytes. Our studies demonstrate that SIRT3 expression is down-regulated during keratinocyte differentiation, consistent with an increase in mitochondrial superoxide levels. Importantly, loss of SIRT3 expression in keratinocytes increased superoxide levels and promoted the expression of differentiation markers, whereas overexpression decreased superoxide levels and reduced the expression of differentiation markers. These findings identify a new role for SIRT3 in the suppression of epidermal differentiation via lowering oxidative stress.
Introduction
The epidermis functions as a protective barrier and is composed of four morphologically distinguished layers: basal, spinous, granular, and cornified. Keratinocyte differentiation is an essential process in the formation and maintenance of the protective skin barrier. Proliferating keratinocytes in the basal layer function to regenerate the epidermis and maintain attachment to the basal lamina. Maturing keratinocytes detach from the basal layer and start the differentiation process in the spinous layer by expressing early differentiation markers, such as keratin-1, keratin-10, involucrin, and transglutaminase. In the granular layer, keratinocytes produce cornified envelope proteins, such as the late differentiation markers loricrin and filaggrin. Finally, in the cornified layer, proteins are cross-linked, and lipids are extruded to form the epidermal barrier (1–3).
Mitochondrial function plays an important role during keratinocyte differentiation. For example, mitochondrial membrane potential declines during differentiation, and eventually, mitochondrial cell death pathways are activated to induce terminal differentiation (4). Recent studies reported that induction of oxidative stress up-regulates the expression of differentiation markers in keratinocytes and triggers apoptosis or terminal differentiation (5), whereas the loss of the antioxidant enzyme SOD2 (superoxide dismutase 2) induces senescence and differentiation in keratinocytes (6). Keratinocytes lacking mitochondrial transcription factor A cannot generate mitochondrial reactive oxygen species (ROS)2 and thus show impaired differentiation (7). However, few details are known about the molecular mechanisms responsible for controlling the levels of oxidative stress during normal differentiation.
The mitochondrial sirtuin deacetylase SIRT3 (sirtuin-3) plays a critical role in the generation and clearance of mitochondrial ROS (8). It binds and deacetylates several mitochondrial proteins that promote mitochondrial oxidative metabolism, such as electron transport chain subunits and fatty acid oxidation enzymes (9, 10). SIRT3 also dampens mitochondrial oxidative stress via deacetylation and subsequent activation of SOD2 and isocitrate dehydrogenase (8, 11–18).
As SIRT3 plays a pivotal role in ROS clearance, we sought to investigate the role of SIRT3 in regulating ROS and differentiation in keratinocytes. We show for the first time that during keratinocyte differentiation, SIRT3 expression is decreased and that the levels of the SIRT3 substrate NAD+ are reduced, whereas ROS are increased. We further demonstrate that a reduction in oxidative stress by the SOD mimetic manganese 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) or the antioxidant N-acetylcysteine (NAC) attenuates keratinocyte differentiation. In keratinocytes with reduced SIRT3 activity, we observed an increase in oxidative stress and an increase in expression of differentiation markers. We observed the opposite phenotypes in keratinocytes that overexpress SIRT3. Our findings identify a novel molecular mechanism controlling keratinocyte differentiation via ROS accumulation. SIRT3 lowers mitochondrial oxidative stress to decrease the rate of keratinocyte differentiation, whereas loss of SIRT3 induces keratinocyte differentiation due to aberrant ROS.
EXPERIMENTAL PROCEDURES
Cell Culture
The primary normal human epidermal keratinocyte strain N and the telomerase reverse transcriptase (TERT)-immortalized human epidermal keratinocyte line N/TERT-1 (19) were provided by J. Rheinwald and the Cell Culture Core of the Harvard Skin Disease Research Center (Boston, MA). The primary keratinocytes and TERT-immortalized keratinocytes were cultured in K-SFM (Invitrogen) supplemented with 25 μg/ml bovine pituitary extract, 100 ng/ml epidermal growth factor, 0.1 mm CaCl2 (Sigma), and 100 units/ml penicillin/streptomycin (Invitrogen). Cultures were maintained at low confluence to prevent differentiation. Density-dependent differentiation was induced by plating cells at ∼1 × 105/cm2 in high-density medium consisting of K-SFM and DMEM/nutrient mixture F-12 (1:1) supplemented with 25 μg/ml bovine pituitary extract, GlutaMAXTM I (Invitrogen), and 100 units/ml penicillin/streptomycin. Calcium-dependent differentiation was induced by adding 1.2 mm CaCl2 to the culture medium of subconfluent cultures.
Retroviral Transduction
Retroviral particles were produced by cotransfection of the pBABE-puro control plasmid or SIRT3-FLAG-containing pBABE-puro plasmid with gag/pol and vesicular stomatitis virus G packaging vectors into HEK293T cells using FuGENE®-6 transfection reagent (Roche Applied Science). Retroviral supernatant was harvested 36 and 60 h post-transfection and filtered through a 0.45-μm filter. N/TERT-1 cells were transduced with viral medium for 24 h. Selection was performed in the presence of puromycin (2.5 μg/ml) for 1 week.
Lentiviral Transduction
Lentiviral particles were produced by cotransfection of the empty or SIRT3 shRNA-containing pLKO.1-puro vector with pCMV-dR8.2 and pCMV-VSVG packaging plasmids into HEK293T cells with FuGENE-6. Lentiviral supernatant was harvested 36 and 60 h post-transfection and filtered through a 0.45-μm filter. N/TERT-1 cells were transduced with viral medium and incubated for 24 h. Selection was performed in the presence of puromycin (2.5 μg/ml) for 1 week.
Quantitative RT-PCR
Total RNA from cells was harvested using TRIzol® reagent (Invitrogen) and purified using an RNeasy kit (Qiagen). RNA purity and concentration were determined using a NanoDrop spectrophotometer. cDNA was synthesized from 1 μg of RNA using an iScriptTM cDNA synthesis kit (Bio-Rad). cDNA was used at a dilution of 1:250 per reaction with SYBR® Green Master Mix in a Roche LightCycler® 480 real-time PCR system. Serial dilutions of pooled samples were used to generate a standard curve and analyzed for each gene tested. Absolute RNA levels for each gene were calculated according to the standard curve and further normalized to housekeeping genes and control conditions as indicated. The primer sequences used in this study are summarized in Table 1 (RPLP0 (ribosomal protein, large, P0), SIRT3, loricrin, filaggrin, transglutaminase, involucrin, keratin-1, and keratin-10).
TABLE 1.
Sequences of primers used for quantitative RT-PCR
| Gene (function) | Primers |
|---|---|
| RPLP0 (housekeeping gene) | |
| Forward | ATCAACGGGTACAAACGAGTC |
| Reverse | CAGATGGATCAGCCAAGAAGG |
| SIRT3 (protein deacetylase) | |
| Forward | GGGCTTGAGAGAGTGTCGGGC |
| Reverse | TCACAACGCCGGTGCAGACC |
| Loricrin (late differentiation marker) | |
| Forward | TCATGATGCTACCCGAGGTTTG |
| Reverse | CAGAACTAGATGCAGCCGGAGA |
| Filaggrin (late differentiation marker) | |
| Forward | GGGCACTGAAAGGCAAAAAG |
| Reverse | CACCATAATCATAATCTGCACTACCA |
| Transglutaminase (early differentiation marker) | |
| Forward | ATCCTCATGGTCCACGTACACA |
| Reverse | CCCCCGCAATGAGATCTACA |
| Involucrin (early differentiation marker) | |
| Forward | GGGTGGTTATTTATGTTTGGGTGG |
| Reverse | GCCAGGTCCAAGACATTCAAC |
| Keratin-1 (early differentiation marker) | |
| Forward | ATTTCTGAGCTGAATCGTGTGATC |
| Reverse | CTTGGCATCCTTGAGGGCATT |
| Keratin-10 (early differentiation marker) | |
| Forward | TGATGTGAATGTGGAAATGAATGC |
| Reverse | GTAGTCAGTTCCTTGCTCTTTTCA |
Western Blotting
Cell lysate was obtained by scraping cells into radioimmune precipitation assay buffer containing 137 mm NaCl, 20 mm Tris-HCl (pH 7.4), 2.0 mm EDTA, 10% glycerol, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS supplemented with Complete mini protease inhibitor Tablets (Roche Applied Science) and Phosphatase Inhibitor Cocktails 2 and 3 (Sigma). Protein concentration was measured using the Bradford protein assay (Bio-Rad) according to the manufacturer's instructions. Protein samples were loaded at 20 μg/well into 10–20% Bio-Rad Criterion gels and transferred to nitrocellulose membranes. After blocking with 5% milk, membranes were incubated with rabbit polyclonal anti-loricrin (Covance) and mouse monoclonal α-tubulin (Sigma) antibodies. HRP-conjugated secondary antibodies (GE Healthcare) and Pierce ECL Western blotting substrate (Thermo Scientific) were used for chemiluminescence detection.
ROS Measurements
Steady-state levels of mitochondrial and cellular superoxide were assessed by measuring the oxidation of MitoSOXTM or dihydroethidium (both from Molecular Probes) according to the manufacturer's instructions. Briefly, cells were incubated overnight at 37 °C with MitoSOX (5 μm) or dihydroethidium (5 μm), harvested by trypsinization, and resuspended in Hanks' balanced salt solution containing 1% BSA. As a positive control and a negative control for ROS production, cells were treated with 100 μm H2O2 (Sigma), 100 μm MnTBAP (Calbiochem), or 2 mm NAC (Sigma) for the same amount of time as the dye was added. Afterward, cells were placed on ice and analyzed within 30 min using a FACSCalibur flow cytometer (BD Biosciences). The mean fluorescence intensity of cells detected at 488 nm excitation and 585 nm emission was determined.
NAD Assay
NAD+ levels were determined by enzymatic NADH recycling assay using an NAD+/NADH quantification kit (BioVision) according to the manufacturer's instructions. Cells were harvested by trypsinization and counted. 2 × 105 cells were lysed in NAD+/NADH extraction buffer, and another 2 × 105 cells were lysed in radioimmune precipitation assay buffer in parallel for normalization by protein content. Samples were immediately subjected to two freeze/thaw cycles on dry ice. The supernatant of the cells in NAD+/NADH extraction buffer was split into two sets; one set was used to measure the total NADH plus NAD+ (total NAD(H)) content, and the other set was used to carry out the thermal decomposition of NAD+ to measure only the NADH content of the cell. The cycling reaction was initiated in a 96-well plate in both sets and a set of NADH standard dilutions and stopped after 1 h. The absorbance at 450 nm was determined, and the NAD+ content could be calculated as NAD+ = total NAD(H) − NADH and was normalized to cellular protein content.
Immunofluorescence
Cells were grown on round cover glass and treated as indicated. They were fixed in 4% paraformaldehyde for 15 min at 4 °C, permeabilized in 0.3% Triton X-100 for 15 min at room temperature, blocked with 5% normal goat serum for 30 min at 37 °C, and then stained overnight at 4 °C with the indicated antibodies. For loricrin immunostaining, the rabbit polyclonal anti-loricrin antibody (Covance) was used (1:100 dilution). All slides were washed three times with PBS and incubated with Alexa Fluor 488-conjugated rabbit secondary antibodies for 1 h at room temperature. Nuclei were visualized by DAPI staining. Samples were rinsed, mounted on coverslips using ProLong® Gold antifade reagent (Invitrogen), and examined by fluorescence microscopy. All images were collected with a Nikon 80i upright microscope equipped with either a Plan Fluor 40×/1.3 numerical aperture objective lens or a Plan Apo 100×/1.4 numerical aperture objective lens and with epifluorescence illumination. Loricrin fluorescence was excited with a Chroma 480/40 filter, and emission was collected with a Chroma 535/50 filter. Images were acquired with a Hamamatsu C8484-03 monochrome CCD camera controlled with MetaMorph 7 software. Contrast and brightness were adjusted on displayed images (identically for compared image sets) using MetaMorph 7 software and Photoshop.
Statistical Analysis
All values are expressed as means ± S.E. Significant differences between two groups were evaluated by Student's unpaired t test.
RESULTS
SIRT3 Expression and Activity Are Down-regulated during Keratinocyte Differentiation
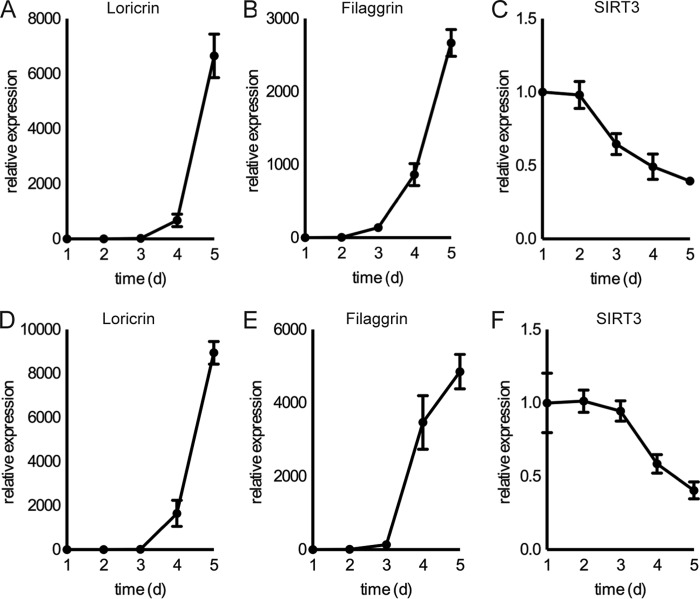
To examine changes in SIRT3 activity during normal keratinocyte differentiation, we used the normal human epidermal keratinocyte line N/TERT-1, a TERT-immortalized human keratinocyte cell line that retains the ability to differentiate in vivo and in vitro (19). We examined the expression of SIRT3 during keratinocyte culture with two established stimuli that induce commitment to terminal differentiation: culture confluence (Fig. 1, A–C) and elevation of extracellular calcium concentration (Fig. 1, D–F) (20). Under these conditions, SIRT3 expression decreased in N/TERT-1 keratinocytes. Additionally, the cellular levels of NAD(H), in particular in its oxidized state as NAD+, a co-substrate for sirtuin catalysis (21–23), decreased during keratinocyte differentiation, whereas the levels of NADH did not change significantly (Fig. 2, A–C). Taken together, these findings support the model that SIRT3 expression and activity decrease during keratinocyte differentiation.
FIGURE 1.
SIRT3 is differentially expressed during keratinocyte differentiation. RT-PCR analysis of N/TERT-1 keratinocytes cultured over 5 days after reaching confluence showed expression of SIRT3 and the differentiation markers loricrin and filaggrin during confluence-induced differentiation (A–C) and during calcium-induced differentiation with addition of 1.2 mm CaCl2 (D–F). Data are representative of three independent experiments. Data shown are from three biological replicates; error bars indicate S.E. d, days.
FIGURE 2.
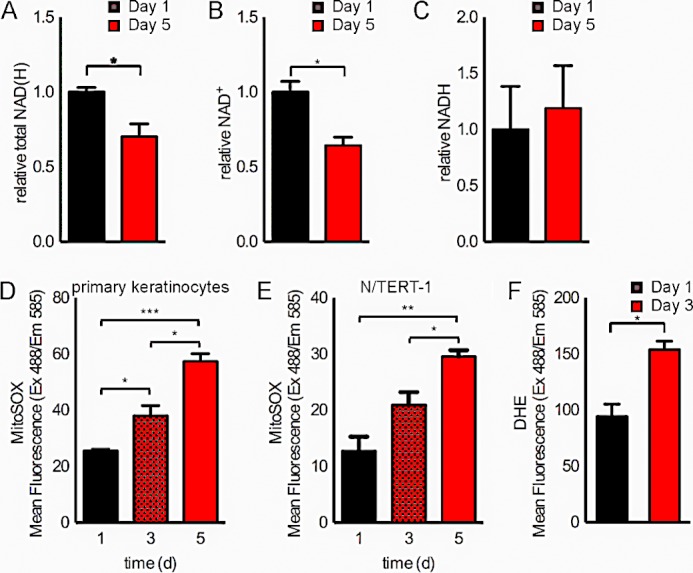
NAD+ levels decrease and mitochondrial superoxide levels increase during keratinocyte differentiation. Shown are relative total NAD(H) levels (A) and NAD+ (B) and NADH (C) measurements in N/TERT-1 keratinocytes at days 1 and 5 after reaching confluence. MitoSOX fluorescence was analyzed by flow cytometry of primary keratinocytes (D) and N/TERT-1 keratinocytes (E) at days 1, 3, and 5 after reaching confluence. Dihydroethidium (DHE) fluorescence was analyzed by flow cytometry of N/TERT-1 keratinocytes at days 1 and 3 after reaching confluence (F). Data are representative of two independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 3). d, days.
Mitochondrial and Cellular Superoxide Levels Increase during Keratinocyte Differentiation
As SIRT3 diminishes mitochondrial oxidative stress (8), we reasoned that a reduction in SIRT3 activity during keratinocyte differentiation may lead to increased mitochondrial ROS. Thus, we investigated alterations in the levels of mitochondrial superoxide during differentiation in N/TERT-1 keratinocytes. Notably, we detected an increase in mitochondrial superoxide levels during differentiation in both primary normal human epidermal keratinocyte strain N and the TERT-immortalized human keratinocyte cell line N/TERT-1 (Fig. 2, D and E). We further detected increased cellular superoxide levels during differentiation (Fig. 2F).
Loss of SIRT3 Affects Keratinocyte Morphology and Growth
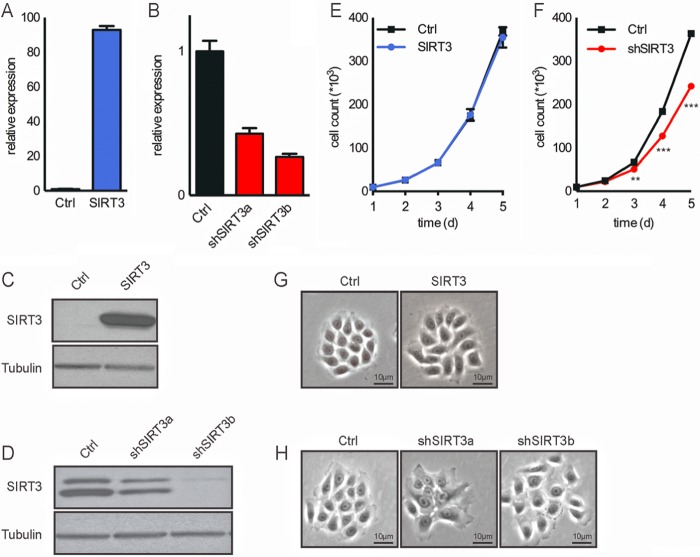
To directly probe the hypothesis that SIRT3 loss increases ROS to promote keratinocyte differentiation, we generated a series of stable N/TERT-1 cell lines in which SIRT3 was overexpressed or reduced. Using a pBABE-SIRT3 retroviral vector, we generated a stable cell line that overexpresses SIRT3 as confirmed by quantitative RT-PCR and Western blotting (Fig. 3, A and C). Additionally, using five different shRNA sequences targeting SIRT3 mRNA, we identified two shRNAs (shSIRT3a and shSIRT3b) that achieved efficient silencing as demonstrated by both quantitative RT-PCR and Western blotting (Fig. 3, B and D). The growth rate and morphology of the N/TERT-1 cells overexpressing SIRT3 were comparable to those of the control cell line (Fig. 3, E and G). In the case of the SIRT3-knockdown keratinocytes, loss of SIRT3 led to decreased proliferation (Fig. 3F), possibly shifting cells toward a differentiated morphology (Fig. 3H). The shSIRT3b cell line, with the most efficient silencing of SIRT3, stopped proliferating after four to five passages. For this reason, the shSIRT3a line was used for subsequent experiments and is referred to below as shSIRT3.
FIGURE 3.
Generation of stable SIRT3-overexpressing and SIRT3-knockdown keratinocytes. Shown are the results from RT-PCR analysis (A and B) and Western blotting (C and D), the proliferation rate (E and F), and microscopic images (G and H) of control (Ctrl) and SIRT3-overexpressing (A, C, E, and G) and SIRT3-knockdown (B, D, F, and H) N/TERT-1 keratinocytes. **, p < 0.01; ***, p < 0.001 (n = 3). d, days.
SIRT3 Modulates Mitochondrial Superoxide Levels in Keratinocytes
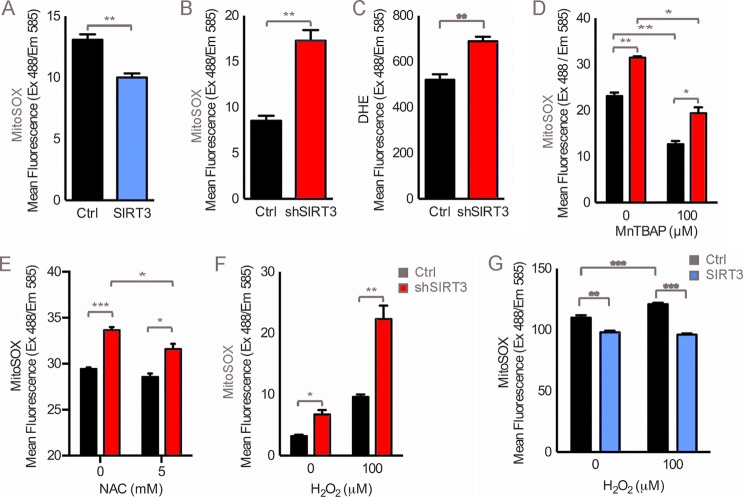
SIRT3 acts through several targets to both reduce superoxide production and enhance the oxidative stress response and detoxification mechanisms (8, 11–17). Consistent with the repression of mitochondrial oxidative stress by SIRT3, mitochondrial superoxide levels decreased in SIRT3-overexpressing keratinocytes (Fig. 4A) and increased with SIRT3 knockdown (Fig. 4, B and C). To test whether this increase in mitochondrial superoxide was due to reduced detoxification or enhanced ROS production, we tested the effect of two antioxidants, the SOD mimetic MnTBAP or NAC, as well as the effect of the oxidant hydrogen peroxide (H2O2) on SIRT3-deficient keratinocytes. Treatment with MnTBAP or NAC rescued SIRT3-deficient keratinocytes by boosting superoxide detoxification. These treatments diminished the increased superoxide levels that were produced due to SIRT3 silencing (Fig. 4, D and E). Furthermore, treatment with H2O2 elevated superoxide levels in SIRT3-deficient keratinocytes compared with similarly treated control keratinocytes, suggesting that SIRT3 deficiency also promotes the accumulation of superoxide in keratinocytes (Fig. 4F), whereas SIRT3 overexpression protects from H2O2-induced superoxide (Fig. 4G). These findings support a role for SIRT3 in reducing mitochondrial ROS by affecting both ROS production and detoxification.
FIGURE 4.
Effect of SIRT3 activity on superoxide levels in keratinocytes. Shown are the results from flow cytometry analysis of MitoSOX and dihydroethidium (DHE) fluorescence in control (Ctrl; black bars) and SIRT3-overexpressing (A, blue bars) or SIRT3-knockdown (B and C, red bars) N/TERT-1 keratinocytes and after overnight incubation with 100 μm MnTBAP (D), 5 mm NAC (E), or 100 μm H2O2 (F) in control or SIRT3-knockdown cells or SIRT3-overexpressing cells (G). Data are representative of three independent experiments. Error bars indicate S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 3).
Reducing Superoxide Levels Attenuates Differentiation
Recent studies showed that mitochondrial superoxide accumulates due to dysfunction or loss of antioxidant mechanisms in keratinocytes (6, 21). Furthermore, modulation of mitochondrial function can increase ROS and lead to keratinocyte senescence and increased terminal differentiation (5). Treatment with antioxidants, such as MnTBAP and NAC, has been shown to have beneficial effects on the phenotypes caused by deficient antioxidant mechanisms (22, 23). In our model, incubation with MnTBAP decreased superoxide levels in a dose-dependent manner (data not shown). To test whether pharmacological detoxification of mitochondrial superoxide affects the process of keratinocyte differentiation, we treated N/TERT-1 keratinocytes with various doses of MnTBAP over 5 days of confluence-induced differentiation. As expected, markers of differentiation, such as loricrin and filaggrin, increased significantly with time; however, the gene expression and protein level of the differentiation marker loricrin decreased with increasing doses of MnTBAP (Fig. 5A). In keratinocytes incubated with 5 μm MnTBAP during 5 days of confluence-induced differentiation, expression of both loricrin and filaggrin was attenuated by ∼50% (Fig. 5B). Importantly, treatment with 2 mm NAC also showed a similar attenuation of differentiation (Fig. 5C). Even in subconfluent proliferating keratinocyte cultures, where loricrin protein expression is low, incubation with 5 μm MnTBAP over 24 h lowered loricrin levels as detected by immunofluorescence (Fig. 5D). Thus, superoxide levels are an important factor in the induction of keratinocyte differentiation.
FIGURE 5.
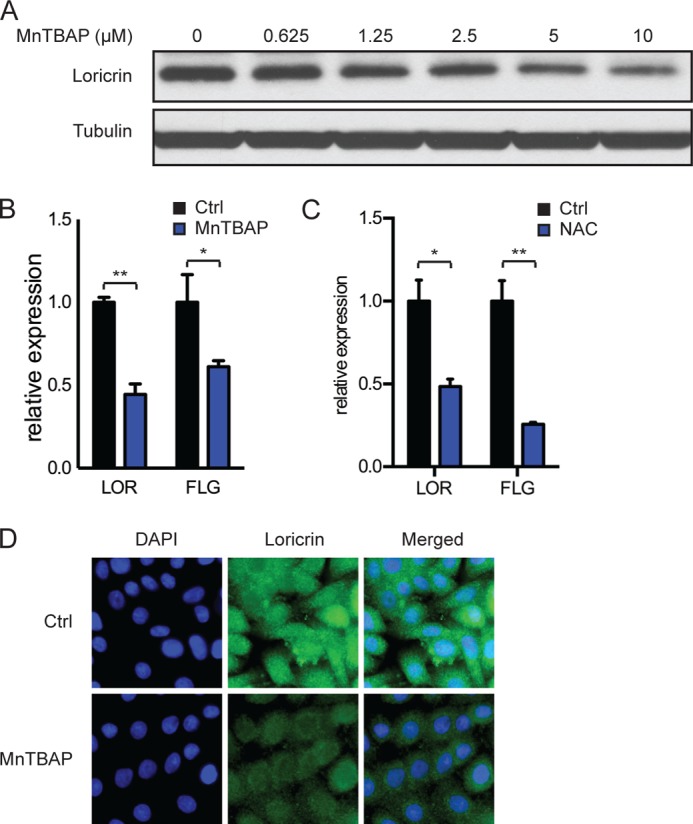
Effect of MnTBAP on differentiation in keratinocytes. A, Western blot of loricrin in N/TERT-1 keratinocytes incubated with MnTBAP over 5 days after reaching confluence. B and C, RT-PCR analysis of the differentiation markers loricrin (LOR) and filaggrin (FLG) in N/TERT-1 keratinocytes cultured with 5 μm MnTBAP over 5 days (B) or 2 mm NAC over 3 days (C) after reaching confluence. D, immunofluorescence staining for loricrin (green) of N/TERT-1 keratinocytes treated with 5 μm MnTBAP for 24 h before reaching confluence. Nuclei were stained with DAPI (blue). Data are representative of two independent experiments. Error bars indicate S.E. *, p < 0.05; **, p < 0.01 (n = 3). Ctrl, control.
SIRT3 Modulates Keratinocyte Differentiation
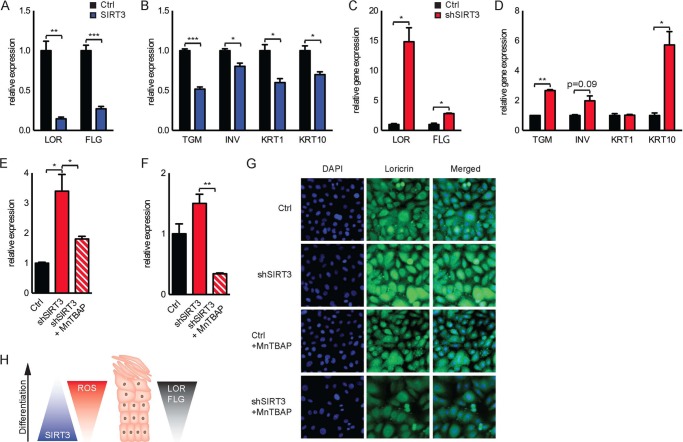
Next, we examined whether the SIRT3-mediated decrease in oxidative stress directly affects the differentiation characteristics of keratinocytes. Importantly, SIRT3 overexpression decreased the appearance of the late differentiation markers loricrin and filaggrin, as well as early differentiation markers, including transglutaminase, involucrin, and KRT1 and KRT10 proteins, whereas SIRT3 knockdown increased expression of these markers (Fig. 6, A–D). To investigate whether SIRT3 regulates differentiation via a mechanism of ROS, we performed similar studies in the presence of MnTBAP. Strikingly, incubation with MnTBAP over 3 days of confluence-induced differentiation attenuated the increased expression of loricrin and filaggrin in SIRT3-knockdown keratinocytes (Fig. 6, E and F) and also reduced loricrin protein expression in subconfluent SIRT3-knockdown keratinocytes (Fig. 6G). Diminished SIRT3 expression drove increased oxidative stress, stimulating the expression of keratinocyte differentiation (Fig. 6H). Taken together, our data reveal a novel mechanism by which reduced SIRT3 activity contributes to elevated ROS and promotes keratinocyte differentiation.
FIGURE 6.
Effect of SIRT3 activity on differentiation in keratinocytes. Shown are the results from RT-PCR analysis of the late differentiation markers loricrin (LOR) and filaggrin (FLG) (A) and the early differentiation markers transglutaminase (TGM), involucrin (INV), keratin-1 (KRT1), and keratin-10 (KRT10) (B) in control (Ctrl) and SIRT3-overexpressing N/TERT-1 keratinocytes cultured over 5 days after reaching confluence. Also shown are the results from RT-PCR analysis of the late differentiation markers loricrin (LOR) and filaggrin (FLG) (C) and the early differentiation markers transglutaminase, involucrin, keratin-1 (KRT1), and keratin-10 (KRT10) (D) in control and SIRT3-knockdown N/TERT-1 keratinocytes cultured for 3 days after reaching confluence. Quantitative RT-PCR analysis of control or SIRT3-knockdown N/TERT-1 keratinocytes cultured with 5 μm MnTBAP over 3 days after reaching confluence demonstrated expression of the late differentiation markers loricrin (E) and filaggrin (F). G, immunofluorescence staining for loricrin (green) of control or SIRT3-knockdown N/TERT-1 keratinocytes treated with 5 μm MnTBAP for 24 h before reaching confluence. Nuclei were stained with DAPI (blue). Data are representative of two or three independent experiments. Error bars indicate S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 3). H, schematic of changes in SIRT3 and ROS levels during keratinocyte differentiation.
DISCUSSION
This study reveals important and novel mechanistic insights into how oxidative stress levels change during normal keratinocyte differentiation. First, we showed that SIRT3 expression and the levels of the SIRT3 co-substrate NAD+ are down-regulated during keratinocyte differentiation. Decreased availability of the sirtuin co-substrate NAD+ may lead to decreased SIRT3 activity in keratinocytes during differentiation. Given the well established role of SIRT3 as a regulator of mitochondrial oxidative stress (8, 11–18), mitochondrial superoxide levels increased in both primary and immortalized keratinocytes with differentiation. We identified SIRT3 as a key regulator of this phenotype; constitutive SIRT3 deficiency resulted in increased mitochondrial superoxide levels, whereas constitutive SIRT3 expression reduced mitochondrial ROS.
The mitochondrial sirtuin SIRT3 is uniquely poised to modulate both the generation of ROS and the detoxification of harmful free radicals, protecting cells from mitochondrial oxidative stress (8, 11, 17, 18). Loss of SIRT3 triggers ROS and oxidative damage, affecting distinct cellular processes and promoting phenotypes of aging (13, 14). Our data define a key role for SIRT3 in keratinocyte differentiation through regulation of ROS. Increased SIRT3 activity prevents expression of differentiation markers, and loss of SIRT3 induces keratinocyte differentiation. Importantly, addition of the antioxidant MnTBAP counteracts differentiation in SIRT3-knockdown keratinocytes, suggesting that the loss of SIRT3 induces differentiation through increased ROS. This conclusion is supported by studies showing that oxidative stress induces keratinocyte differentiation and senescence (4, 6, 21). For example, defects in antioxidant capacity, such as an SOD deficiency, may lead to keratinocyte senescence and decreased thickness of the epidermis (6).
It remains unclear how mitochondrial oxidative stress signaling induces and modulates keratinocyte differentiation. Previous studies suggest that ROS signaling might induce keratinocyte differentiation through the PKC/AP-1 pathway (24, 25). Interestingly, SIRT3 gene expression might in turn also be regulated through AP-1 transcription factors (26), suggesting a possible regulatory pathway. Elucidating the mechanisms involved in normal epidermal keratinocyte differentiation will be crucial for understanding a number of skin diseases with abnormal epidermal keratinocyte differentiation, as well as aging. Our studies highlight keratinocyte SIRT3 as a new target that could potentially be developed to regulate disorders resulting from abnormal oxidative stress and epidermal differentiation.
Acknowledgments
We thank members of the Haigis laboratory, especially Jaewon Lee and Natalie German, for critical comments on the manuscript. Primary keratinocytes and the normal primary human epidermal keratinocyte line N/TERT-1 were provided by J. Rheinwald and the Cell Culture Core of the Harvard Skin Disease Research Center. We thank the Nikon Imaging Center at Harvard Medical School for training and assistance with fluorescence microscopy.
This work was supported by The Estee Lauder Companies Inc. (to M. C. H.), the German Academic Exchange Service (to A. S. B.), and the Glenn Foundation for Medical Research (to the Haigis laboratory).
- ROS
- reactive oxygen species
- MnTBAP
- manganese 5,10,15,20-tetrakis(4-benzoic acid)porphyrin
- NAC
- N-acetylcysteine
- TERT
- telomerase reverse transcriptase.
REFERENCES
- 1. Fuchs E., Green H. (1980) Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19, 1033–1042 [DOI] [PubMed] [Google Scholar]
- 2. Kalinin A., Marekov L. N., Steinert P. M. (2001) Assembly of the epidermal cornified cell envelope. J. Cell Sci. 114, 3069–3070 [DOI] [PubMed] [Google Scholar]
- 3. Steven A. C., Steinert P. M. (1994) Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. 107, 693–700 [PubMed] [Google Scholar]
- 4. Allombert-Blaise C., Tamiji S., Mortier L., Fauvel H., Tual M., Delaporte E., Piette F., DeLassale E. M., Formstecher P., Marchetti P., Polakowska R. (2003) Terminal differentiation of human epidermal keratinocytes involves mitochondria- and caspase-dependent cell death pathway. Cell Death Differ. 10, 850–852 [DOI] [PubMed] [Google Scholar]
- 5. Tamiji S., Beauvillain J. C., Mortier L., Jouy N., Tual M., Delaporte E., Formstecher P., Marchetti P., Polakowska R. (2005) Induction of apoptosis-like mitochondrial impairment triggers antioxidant and Bcl-2-dependent keratinocyte differentiation. J. Invest Dermatol. 125, 647–658 [DOI] [PubMed] [Google Scholar]
- 6. Velarde M. C., Flynn J. M., Day N. U., Melov S., Campisi J. (2012) Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging 4, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamanaka R. B., Glasauer A., Hoover P., Yang S., Blatt H., Mullen A. R., Getsios S., Gottardi C. J., DeBerardinis R. J., Lavker R. M., Chandel N. S. (2013) Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal. 6, ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bause A. S., Haigis M. C. (2013) SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 48, 634–639 [DOI] [PubMed] [Google Scholar]
- 9. Finley L. W., Haas W., Desquiret-Dumas V., Wallace D. C., Procaccio V., Gygi S. P., Haigis M. C. (2011) Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS ONE 6, e23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., Stevens R. D., Li Y., Saha A. K., Ruderman N. B., Bain J. R., Newgard C. B., Farese R. V., Jr., Alt F. W., Kahn C. R., Verdin E. (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Y., Zhang J., Lin Y., Lei Q., Guan K. L., Zhao S., Xiong Y. (2011) Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 12, 534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs K. M., Pennington J. D., Bisht K. S., Aykin-Burns N., Kim H. S., Mishra M., Sun L., Nguyen P., Ahn B. H., Leclerc J., Deng C. X., Spitz D. R., Gius D. (2008) SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci. 4, 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qiu X., Brown K., Hirschey M. D., Verdin E., Chen D. (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12, 662–667 [DOI] [PubMed] [Google Scholar]
- 14. Someya S., Yu W., Hallows W. C., Xu J., Vann J. M., Leeuwenburgh C., Tanokura M., Denu J. M., Prolla T. A. (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sundaresan N. R., Gupta M., Kim G., Rajamohan S. B., Isbatan A., Gupta M. P. (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 119, 2758–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tao R., Coleman M. C., Pennington J. D., Ozden O., Park S. H., Jiang H., Kim H. S., Flynn C. R., Hill S., Hayes McDonald W., Olivier A. K., Spitz D. R., Gius D. (2010) Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell 40, 893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu W., Dittenhafer-Reed K. E., Denu J. M. (2012) SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 287, 14078–14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bell E. L., Guarente L. (2011) The SirT3 divining rod points to oxidative stress. Mol. Cell 42, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dickson M. A., Hahn W. C., Ino Y., Ronfard V., Wu J. Y., Weinberg R. A., Louis D. N., Li F. P., Rheinwald J. G. (2000) Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20, 1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poumay Y., Pittelkow M. R. (1995) Cell density and culture factors regulate keratinocyte commitment to differentiation and expression of suprabasal K1/K10 keratins. J. Invest. Dermatol. 104, 271–276 [DOI] [PubMed] [Google Scholar]
- 21. Hornig-Do H. T., von Kleist-Retzow J. C., Lanz K., Wickenhauser C., Kudin A. P., Kunz W. S., Wiesner R. J., Schauen M. (2007) Human epidermal keratinocytes accumulate superoxide due to low activity of Mn-SOD, leading to mitochondrial functional impairment. J. Invest. Dermatol. 127, 1084–1093 [DOI] [PubMed] [Google Scholar]
- 22. Melov S., Schneider J. A., Day B. J., Hinerfeld D., Coskun P., Mirra S. S., Crapo J. D., Wallace D. C. (1998) A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 18, 159–163 [DOI] [PubMed] [Google Scholar]
- 23. Benrahmoune M., Therond P., Abedinzadeh Z. (2000) The reaction of superoxide radical with N-acetylcysteine. Free Radic. Biol. Med. 29, 775–782 [DOI] [PubMed] [Google Scholar]
- 24. Bose A., Teh M. T., Hutchison I. L., Wan H., Leigh I. M., Waseem A. (2012) Two mechanisms regulate keratin K15 expression in keratinocytes: role of PKC/AP-1 and FOXM1 mediated signalling. PLoS ONE 7, e38599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rutberg S. E., Saez E., Glick A., Dlugosz A. A., Spiegelman B. M., Yuspa S. H. (1996) Differentiation of mouse keratinocytes is accompanied by PKC-dependent changes in AP-1 proteins. Oncogene 13, 167–176 [PubMed] [Google Scholar]
- 26. Bellizzi D., Covello G., Di Cianni F., Tong Q., De Benedictis G. (2009) Identification of GATA2 and AP-1 activator elements within the enhancer VNTR occurring in intron 5 of the human SIRT3 gene. Mol. Cells 28, 87–92 [DOI] [PubMed] [Google Scholar]