Background: Activating mutations of the calcium channel TRPC6 lead to adult onset genetic kidney disease.
Results: In acquired kidney diseases, increased TRPC6 expression protects kidney podocytes against complement-mediated injury.
Conclusion: The effect, protective or nocuous, of TRPC6 in podocytes is context dependent.
Significance: Pharmacologic inhibition of TRPC6 in acquired kidney disease may be detrimental.
Keywords: Calcium Signaling, CaMKII, Complement, Kidney, Podocytes, TRP Channels, TRPC6, Membranous Nephropathy, Nephrotoxic Serum Nephritis
Abstract
Gain-of-function mutations in the calcium channel TRPC6 lead to autosomal dominant focal segmental glomerulosclerosis and podocyte expression of TRPC6 is increased in some acquired human glomerular diseases, particularly in membranous nephropathy. These observations led to the hypothesis that TRPC6 overactivation is deleterious to podocytes through pathological calcium signaling, both in genetic and acquired diseases. Here, we show that the effects of TRPC6 on podocyte function are context-dependent. Overexpression of TRPC6 alone did not directly affect podocyte morphology and cytoskeletal structure. Unexpectedly, however, overexpression of TRPC6 protected podocytes from complement-mediated injury, whereas genetic or pharmacological TRPC6 inactivation increased podocyte susceptibility to complement. Mechanistically, this effect was mediated by Ca2+/calmodulin-dependent protein kinase II (CaMKII) activation. Podocyte-specific TRPC6 transgenic mice showed stronger CaMKII activation, reduced podocyte foot process effacement and reduced levels of proteinuria during nephrotoxic serum nephritis, whereas TRPC6 null mice exhibited reduced CaMKII activation and higher levels of proteinuria compared with wild type littermates. Human membranous nephropathy biopsy samples showed podocyte staining for active CaMKII, which correlated with the degree of TRPC6 expression. Together, these data suggest a dual and context dependent role of TRPC6 in podocytes where acute activation protects from complement-mediated damage, but chronic overactivation leads to focal segmental glomerulosclerosis.
Introduction
Gain-of-function mutations in transient receptor potential channel 6 (TRPC6),3 a calcium-permeable cation channel expressed in kidney podocytes, have recently been identified as a cause of autosomal dominant focal segmental glomerulosclerosis (FSGS) (1–3). Interestingly, glomerular expression of wild type TRPC6 was increased in acquired human and rodent glomerular diseases (4). This observation led to the hypothesis that increased TRPC6-mediated calcium signaling, either through hyperactive mutant channels or via overexpressed wild type channels, damages podocytes and causes glomerular disease (5). In support of this hypothesis, transgenic mice overexpressing either wild type or mutant TRPC6 selectively in podocytes developed glomerular lesions and proteinuria, although the phenotype was mild and had a late onset (6). The physiological function of podocyte TRPC6 remains largely elusive, and little is known about the downstream mechanisms of TRPC6 in podocytes. In cardiac myocytes (7) and cultured podocytes (8, 9), TRPC6 has been shown to activate calcineurin. Because calcineurin activation in podocytes causes proteinuria via dephosphorylation and subsequent cathepsin l-mediated proteolysis of the actin-associated protein synaptopodin (10), we hypothesized that TRPC6 overactivity induces calcineurin activation and subsequent synaptopodin degradation in podocytes, leading to actin rearrangement that results in podocyte damage and proteinuria.
Among acquired glomerular diseases, the highest levels of TRPC6 expression were found in membranous nephropathy (MN) (4). In MN, one of the most prevalent human glomerular diseases, subepithelial deposition of autoantibodies leads to characteristic morphological changes of the glomerular basement membrane along with podocyte injury and proteinuria (11). Whereas tremendous advances have been recently made in the identification of auto-antigens involved in MN (12), the mechanism by which antibody deposition causes podocyte injury remains incompletely understood. A central role of complement activation in MN has been postulated for decades (11). In sublytic concentrations, the terminal complement components C5b-9 lead to cytoskeletal rearrangements in podocytes (13). Several cellular signaling pathways have been shown to be induced in podocytes by complement (14), among them, phospholipase C activation leading to an increase in diacylglycerol (15), which is a well known endogenous activator of TRPC6 (16).
Given the above, we hypothesized that TRPC6 may be involved in the pathogenesis of podocyte injury during MN, potentially through a calcineurin-synaptopodin-cathepsin l-dependent pathway. Here, we overexpressed TRPC6 in cultured podocytes but were not able to detect any effects on synaptopodin expression levels or on the actin cytoskeleton. Unexpectedly, TRPC6 exhibited a CaMKII-dependent protective role in a model of complement-mediated podocyte injury. Mice with podocyte-specific overexpression of TRPC6 showed increased CaMKII activation and were less susceptible to nephrotoxic serum (NTS) nephritis, a rodent model of glomerulonephritis that is partially dependent on complement activation (17–21), whereas TRPC6−/− mice showed reduced CaMKII activation and higher degrees of proteinuria. These results suggest a dual role of TRPC6 in podocytes, providing protection from complement-mediated injury while inducing glomerulosclerosis upon chronic overactivity.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
The following primary antibodies were used: polyclonal rabbit anti-TRPC6 (Alomone Labs); Alexa Fluor 488- and 594-conjugated phalloidin (Sigma); M2 monoclonal mouse anti-FLAG (Sigma); polyclonal goat anti-synaptopodin (Santa Cruz Biotechnology; this antibody has been directly compared with our previously used rabbit polyclonal synaptopodin antibody (22), yielding identical results); polyclonal rabbit anti-total and anti-phospho(Thr-286)-CaMKII (Cell Signaling catalog nos. 3361 and 3362) for Western blot analysis and polyclonal rabbit anti-phospho(Thr-286)-CaMKIIα (Abcam ab5683) for immunohistochemistry; mouse monoclonal anti-GAPDH (Abcam); mouse monoclonal anti-paxillin (Millipore); rabbit anti-total and phospho-ERK (Cell Signaling); rabbit polyclonal anti-Neph1 (a kind gift of T. B. Huber, Albert-Ludwigs University Freiburg).
All chemicals were purchased from Sigma. Fresh frozen rat complement was purchased from Rockwell Sciences. Cobra venom factor (CVF) was purchased from Quidel.
Animal Studies
Transgenic mice overexpressing wild type TRPC6 under the NPHS2 promoter have been described previously (6) and were backcrossed onto a pure C57BL/6 background since the original report. TRPC6−/− mice (23) were maintained on a mixed 129Sv/J × C57BL/6J background. For each experiment, five mice per group were used. The NTS mouse model was induced as described (24) in male mice, aged 8–10 weeks (TRPC6 tg) or 11–13 weeks (TRPC6−/−). Briefly, mice were injected intraperitoneally with sheep anti-rabbit glomerular basement membrane antibody (10 mg/20 g body weight on 2 consecutive days). Urine was collected every other day starting on day 1 after the first injection. The “low dose” NTS nephritis model was induced by i.v. injection of 2 mg/20 g body weight of antibody, and urine was collected at base line and 4 h after injection. CVF was injected intraperitoneally, 12 units/mouse each 24 and 16 h before NTS injection. Urinary albumin excretion was determined by measurement of urinary albumin concentration by SDS-PAGE followed by Coomassie Brilliant Blue staining and densitometry using internal BSA standards and normalization to urinary creatinine concentration, measured using the alkaline picrate method (Cayman's urinary creatinine assay kit). For the “high dose” NTS model, mice were sacrificed 7 days after NTS injection for histological and biochemical analysis.
Immunohistochemistry and Immunofluorescence
Human biopsy specimens from patients with primary MN or FSGS and healthy human kidney tissue from tumor nephrectomy specimens were stained for TRPC6 and for active CaMKIIα using standard immunohistochemistry protocols. A semiquantitative grading system was used, and the intensity of staining was graded from 0 to 4+. Intensity of 1+ was considered as “weak”; 2–3 + was considered as “moderate” and 4+ was considered as “strong” staining intensity. The glomerulus displaying maximum staining intensity in the biopsy was used for the purpose of grading. Location of the staining, i.e. podocyte cytoplasm and/or foot processes, was also noted. The biopsies were assessed blinded by two independent pathologists and graded semiquantitatively. In cases where there was a discrepancy in grade assigned, the biopsies were reviewed again by both pathologists, and a consensus was reached. Cultured cells were fixed in ice-cold 4% PFA in PBS buffer for 10 min, permeabilized in PBS containing 0.3% Triton X-100 for 10 min, blocked (2% FBS, 2% BSA, and 0.2% fish gelatin), and stained with appropriate primary and secondary antibodies.
Morphological Kidney Analysis
Mouse kidneys were perfusion-fixed with 4% paraformaldehyde in phosphate-buffered saline and further processed for conventional histology (paraffin-embedding) or transmission electron microscopy (post-fixation in diluted Karnowsky's fixative, followed by Epon embedding). Immunoelectron microscopy of rat kidneys was done essentially as described previously (25). Briefly, ultra thin sections were blocked with 1% ovalbumin in PBS for 1 h, followed by incubation with the indicated antibody and an anti-goat 10-nm gold conjugate (1:50).
Immunoblotting
Cells or tissue were lysed in radioimmune precipitation assay (Boston BioProducts) or CHAPS (20 mm Tris, 500 mm NaCl, 0.5% CHAPS, pH 7.5) buffer containing protease and phosphatase inhibitor mixture (Roche Applied Science). Immunoblotting was performed according to standard protocols, using Invitrogen's NuPAGE Bis-Tris gel system and Immobilon-P PVDF membranes (Millipore) according to the manufacturer's instructions. Densitometric analysis was performed using ImageJ software (NIH).
Cell Culture
Conditionally immortalized mouse and human podocytes were cultured as described previously (26, 27). HEK 293T obtained from American Type Culture Collection (ATCC) were cultured according to the suppliers' instructions. All cell lines were regularly tested for mycoplasma infection by PCR.
Lentiviral Transduction of Podocytes
For overexpression, N-terminal FLAG- and C-terminal enhanced GFP-tagged wild type mouse and human TRPC6 cDNA was cloned into the VVPW lentiviral expression vector (kind gift of G. L. Gusella, Mount Sinai Hospital, New York, NY). FLAG-tagged dominant negative mutations of TRPC6 (dnTRPC6) were generated by PCR-mediated mutagenesis replacing three highly conserved amino acids (Leu-678-Trp-680) for alanine residues (28). For knockdown, we used the pLKO.1 vector (RNAi Consortium; supplied by Addgene). Eight shRNA sequences were tested, and the two most efficient sequences were used for further studies (kd1: AATCGAGGACCAGCATACATG; kd8: CGTCCAAATCTCAGCCGTTTA).
All constructs were confirmed by DNA sequencing. 80% confluent HEK 293T cells were transfected in antibiotic-free DMEM, 10% FBS with the VVPW or pLKO.1 plasmid, and the two helper plasmids psPAX2 and pCMV-VSVG (both from Addgene) in a ratio of 3:2:1 (for VVPW) or 10:9:1 (for pLKO.1) using FuGENE, according to the manufacturer's instructions. Control virus was produced using empty VVPW vector together with the same helper plasmids (for overexpression) or using pLKO.1 containing scramble shRNA (Addgene) for knockdown. After 16 h, the medium was changed to DMEM, 10% FBS, containing penicillin/streptomycin. Twenty four and 48 h thereafter, the virus containing cell culture supernatant was harvested and stored at 4 °C, the 24 and 48 h collections were pooled and centrifuged (600 × g, 5 min), and the supernatant was filtered through a 0.45-μm filter, aliquoted, and frozen at −80 °C. Podocytes were transduced with lentivirus 8–10 days after induction of differentiation in the presence of 4 μg/ml polybrene for 16 h and used for experiments 4 days later.
Sublytic Complement Assay
Differentiated conditionally immortalized mouse podocytes were pretreated with 2 mg/ml sheep anti-mouse podocyte IgG in complete growth medium for 30 min (37 °C) and washed once with medium, followed by the addition of 2–4% rat complement in ice-cold complete medium and incubation at 37 °C for 60 min. The cells were then washed with ice-cold PBS and either fixed with 4% PFA in PBS for 15 min (cells grown on coverslips for immunofluorescence) or lysed in radioimmune precipitation assay buffer containing protease and phosphatase inhibitors. The amount of antibody and complement used was titrated to achieve ∼5% cell lysis (as visualized by trypan blue exclusion). For quantification of the sublytic complement effect on podocytes, the mean cell area was calculated by measuring total area occupied by cells divided by the number of cell nuclei for six fields of vision (10× magnification) per condition using ImageJ software, and the reduction upon complement stimulation, as compared with cells not treated with complement, was calculated. Differentiated conditionally immortalized human podocytes were treated likewise with 4% rat complement after preincubation with growth medium containing 5% pooled serum from phospholipase A2 receptor (PLA2R) antibody-positive MN patients.
Cell Surface Protein Isolation
Cell surface biotinylation assays were performed using the Pierce cell surface protein isolation kit, following the manufacturer's instructions, with the following modification: due to the strong adherence of differentiated podocytes, cells were washed after biotinylation and lysed directly in the dishes rather than scraping off cells and centrifuging.
Isolation and Processing of Mouse Glomeruli
Mouse glomeruli were isolated using dynabead perfusion as described (29). Glomeruli were lysed using plastic pestles in radioimmune precipitation assay buffer containing protease and phosphatase inhibitor cocktails (50 μl per kidney).
Measurement of Intracellular Calcium
We used two different methods to measure podocyte calcium responses.
For calcium measurements in single cells that were used to validate functionality of overexpressed TRPC6 channels, we acquired images ratiometrically using Fura-2 optics, with an Olympus IX71 inverted fluorescence microscope and a 20× UAPO water immersion objective (numerical aperture of 0.70), optimized for UV excitation. A Lambda LS xenon lamp controlled by Lambda 10-3 controller (Sutter Instruments) provided fluorescence excitation at 340 and 380 nm. We acquired fluorescence using an Andor iXon EM CCD camera (Andor Technology). We carried out imaging analysis using imaging workbench 6 (Indec Biosystems), with Fura-2 emission ratio (340/380) taken as representative of cytoplasmic Ca2+ concentration ([Ca2+]i). Stimuli were applied with the bathing solution. Changes in the 340/380 fluorescence emission ratio over time were analyzed in individual podocytes.
For quantifying the calcium response to complement, we used the Fluo-4 Calcium Direct assay kit (Invitrogen), which enables in-cell measurement of calcium signaling through G protein-coupled receptors. Differentiated cultured mouse podocytes were trypsinized 2 days after lentiviral transduction, replated in 96-well plates, and cultured for 2 additional days before the experiment. Cells were then simultaneously preincubated with Fluo-4 and sheep anti-mouse podocyte antibody as described above and washed, and calcium signals were detected according to the manufacturer's instructions upon addition of complement.
Electrophysiology
Methods for whole-cell recordings of currents through TRPC6 channels in immortalized mouse podocyte cell lines were made using standard methods described previously in detail (30–32). Briefly, the external solution in the recording chamber contained 150 mm NaCl, 5.4 mm CsCl, 0.8 mm MgCl2, 5.4 mm CaCl2, and 10 mm HEPES, pH 7.4. Pipette solutions in all experiments contained 10 mm NaCl, 125 mm CsCl, 6.2 mm MgCl2, 10 mm HEPES, and 10 mm EGTA, pH 7.2. The recording chamber was superfused by gravity feed at a constant flow rate (0.3 ml/min). To monitor TRPC6, currents were periodically evoked by ramp voltage commands (−80 to +80 mV for 2.5 s) from a holding potential of −40 mV, before and during bath application of 50 μm OAG. Whole-cell currents were digitized and stored on hard disks for off-line analysis using PClampTM software (version 10.0, Molecular Devices). Currents at −80 mV and +80 mV were measured for statistical analysis.
Statistical Analysis
Statistical analysis was performed by Student's t test with the two-sided level of significance set at p < 0.05. Data are reported as mean values ± S.E. of the means unless otherwise stated.
RESULTS
Overexpression of TRPC6 in Cultured Podocytes Does Not Affect Synaptopodin Levels and the Actin Cytoskeleton
Expression of wild type TRPC6 is increased in a number of both acquired human and experimental glomerular diseases (4). These disorders share as a common ultrastructural hallmark podocyte FP effacement, which is thought to be recapitulated in podocyte cell culture models by the loss of characteristic actin stress fibers (33). We therefore tested whether overexpression of wild type TRPC6 in cultured podocytes is sufficient to induce a loss or reduction of actin stress fibers. As a mechanism, we speculated that TRPC6-mediated calcium currents may induce calcineurin activation and lead to synaptopodin dephosphorylation, rendering synaptopodin susceptible to cathepsin l-mediated proteolytic cleavage (10).
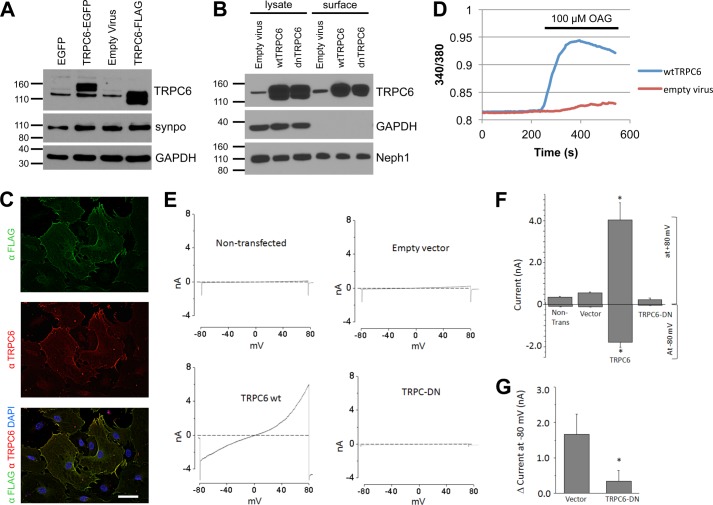
Using a lentiviral approach, we achieved high levels of enhanced GFP- or FLAG-tagged TRPC6 expression in differentiated cultured mouse podocytes (Fig. 1A and supplemental Fig. 1A). Correct localization of overexpressed channels could be demonstrated by both, cell surface biotinylation assays (Fig. 1B) and deconvolution fluorescence microscopy (Fig. 1C). Note that overexpression of TRPC6 produced a double band in the Western blot analysis, the lower band not being inserted into the cell membrane and likely representing a glycosylation-deficient variant (supplemental Fig. 1, B and C). Overexpressed wild type TRPC6 was functional as demonstrated by calcium imaging (Fig. 1D) and electrophysiology (Fig. 1, E and F). As in our previous studies on immortalized podocyte cell lines (30–32), we observed only small baseline cationic currents evoked by voltage ramps in non-infected and empty virus-infected podocytes. By contrast, cells overexpressing wild type TRPC6 exhibited very large rectifying cationic currents even prior to stimulation with OAG (Fig. 1, E and F). Application of 50 μm OAG evoked increases in cationic currents in all four groups. The increase in non-infected and empty virus-infected cells was comparable with those observed in our earlier studies on mouse podocyte cell lines (30–32), whereas it was very much larger in either absolute terms or as an ΔI in cells overexpressing wild type TRPC6. Indeed, we frequently observed that OAG application caused those cells to die suddenly during the recording. By contrast, OAG-evoked currents were significantly smaller in cells expressing dnTRPC6 compared with all of the other groups, although they were still discernible (Fig. 1G).
FIGURE 1.
Efficient overexpression of functional TRPC6 in differentiated cultured mouse podocytes. A, immunoblot analysis of differentiated cultured podocytes demonstrating highly efficient lentivirus mediated overexpression of FLAG- or enhanced GFP-tagged mouse wt TRPC6. synpo, synaptopodin. Note the thin band present in all lanes, which reflects endogenous TRPC6. B, cell surface biotinylation reveals localization of overexpressed FLAG-wtTRPC6 and FLAG-dnTRPC6 at the cell membrane. Neph1 serves as a positive control, and GAPDH serves as a negative control for cell surface expression. C, deconvolution microscopy demonstrating localization of overexpressed FLAG-TRPC6 at the membrane and specificity of the TRPC6 antibody (green, anti-FLAG; red, anti-TRPC6; scale bar, 50 μm). D, TRPC6-overexpressing podocytes show greatly enhanced calcium responses to direct agonist stimulation with OAG, demonstrating functionality of overexpressed TRPC6. E, base-line currents evoked by 2.5-s voltage ramps (−80 to +80 mV) in uninfected as well as empty vector-, FLAG-wtTRPC6, and FLAG-dnTRPC6 lentivirus-infected cells. Note large rectifying currents in podocytes overexpressing wild type TRPC6, even without ligand stimulation. F, mean ± S.E. (n = 6 cells per group) of currents observed in these groups at −80 mV (inward currents) and +80 mV (outward currents) as indicated. An asterisk indicates p < 0.001 by one-way analysis of variance followed by Tukey's honest significant difference post hoc test. G, changes in currents at +80 mV after bath application of 50 μm OAG. An asterisk indicates p < 0.05 by Student's unpaired t test, showing reduced responses to OAG in cells overexpressing dnTRPC6. Much larger OAG-evoked currents were seen in cells overexpressing wild type TRPC6 (> 4.0 nA), but many of these cells died upon OAG application before currents reached a steady level.
Contradicting our hypothesis, TRPC6 overexpression had no effects on synaptopodin levels (Fig. 1A), the actin cytoskeleton, and focal adhesions (Fig. 2A). Effects of TRPC6 overexpression on synaptopodin levels and the actin cytoskeleton were not dependent on the amount of TRPC6 overexpression, agonist stimulation (by hyperforin or OAG), or experimental conditions such as serum starvation (supplemental Fig. 2). Likewise, primary cultured podocytes derived from transgenic mice overexpressing wild type TRPC6 in podocytes did not exhibit reduced synaptopodin levels or a loss of actin stress fibers (supplemental Fig. 3). These data, although in disagreement with earlier more preliminary in vitro data (4), are in accordance with both human (2) and murine (6) in vivo data where genetic TRPC6 overactivity does not cause disease for months (mice) or decades (humans).
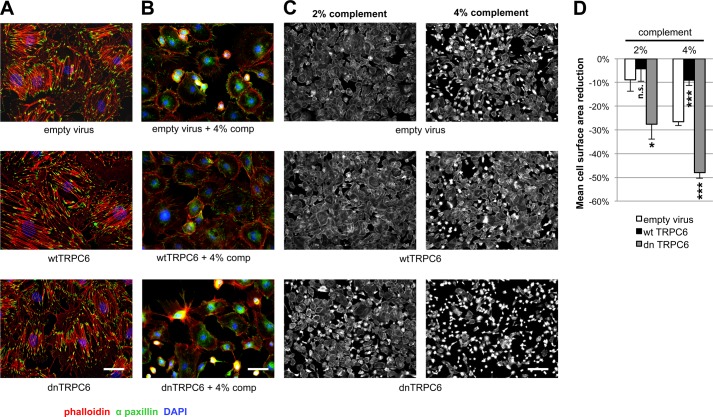
FIGURE 2.
TRPC6 does not affect synaptopodin levels and stress fibers in resting podocytes but protects them from complement-mediated injury. A, in the absence of complement attack, overexpression of wt or dnTRPC6 does not affect the actin cytoskeletal structure and focal adhesions of transduced podocytes (scale bar, 50 μm). B, complement (comp) treatment of anti-podocyte antibody-sensitized podocytes induces cell rounding, attenuation of actin stress fibers and loss of focal adhesions, an effect, which is attenuated by overexpression of wtTRPC6 and enhanced by dnTRPC6 (scale bar, 50 μm). C, low power magnification of phalloidin-stained antibody-sensitized podocytes exposed to 2% or 4% complement (scale bar, 200 μm). D, quantification of the experiment shown in B. The mean cell area was calculated by measuring total area occupied by cells divided by the number of cell nuclei for six fields of vision (10× magnification) per condition in two independent experiments and the reduction upon complement stimulation, as compared with cells not treated with complement, was calculated. *, p < 0.05 and ***, p < 0.001 for the comparison of wtTRPC6 or dnTRPC6 transduced podocytes compared with empty virus transduced podocytes treated with the same concentration of complement.
TRPC6 Protects Podocytes from Sublytic Complement Injury
Because overexpression of TRPC6 alone had no immediate effects on podocyte morphology, we speculated that it might modulate other nocuous stimuli. TRPC6 induction is most pronounced in MN (4), where subepithelial deposition of circulating antibodies against podocyte proteins such as PLA2R (34) or planted antigens such as cationic BSA (35) are thought to trigger complement-induced podocyte damage (11, 36). In vitro, anti-podocyte antibodies and sublytic concentrations of complement induce cytoskeletal rearrangements in podocytes (13) and activation of intracellular signaling cascades (14), including diacylglycerol production through phospholipase C (15). Because diacylglycerol is a direct activator of TRPC6 (16), we speculated that TRPC6 might be involved in mediating complement injury to podocytes.
Mouse podocytes preincubated with sheep anti-mouse podocyte IgG and exposed to sublytic rat complement concentrations exhibited characteristic cytoskeletal changes with cell membrane retraction between F-actin containing projections, leading to a star-like cell morphology. These morphological changes were variable between cells, ranging from minimal alterations to complete cell rounding (supplemental Fig. 4). Surprisingly, overexpression of wild type TRPC6 (wtTRPC6) in podocytes attenuated these complement-induced alterations, whereas podocytes overexpressing dnTRPC6 exhibited a markedly aggravated phenotype (Fig. 2, B–D). Similarly, pharmacologic inhibition of TRPC6 with the TRPC inhibitor SKF 96365 and lentiviral knockdown of TRPC6 both resulted in increased susceptibility to complement-mediated injury (supplemental Fig. 5).
The Protective Potential of TRPC6 in Complement Injury to Podocytes Is Mediated by CaMKII
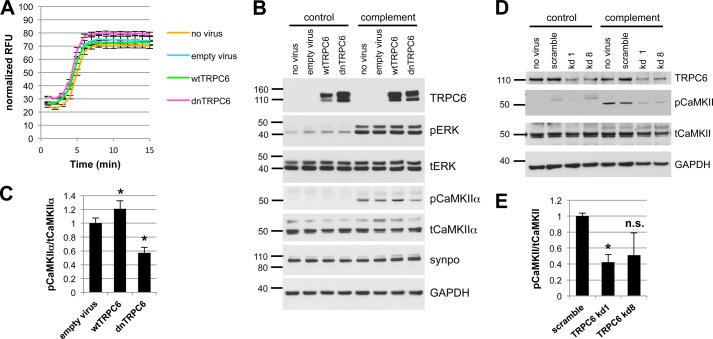
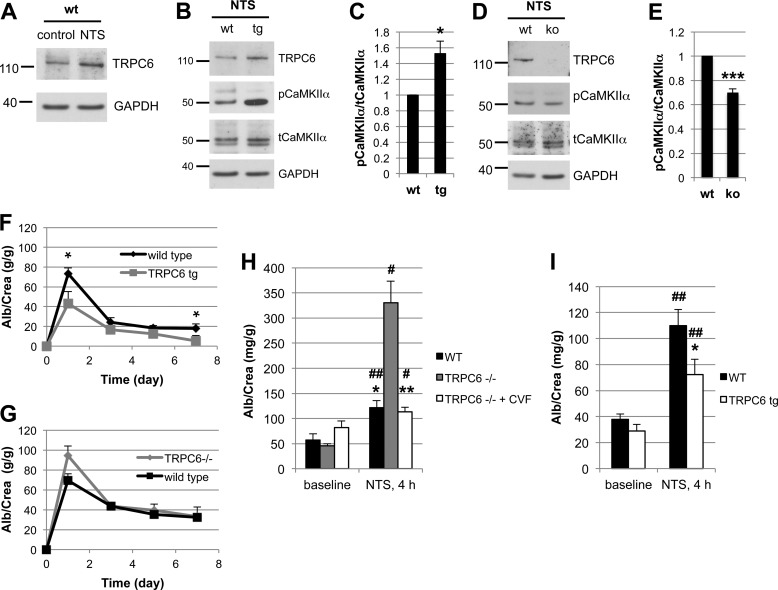
The overall level of complement activation as measured by C3a release and C9 membrane insertion was not altered in wtTRPC6- or dnTRPC6-overexpressing podocytes (data not shown), arguing against a direct effect of TRPC6 on complement activation. Sublytic complement injury in podocytes has been shown previously to induce a rapid and sustained increase in intracellular calcium concentration and buffering of intracellular calcium increased susceptibility of podocytes to complement (37). Interestingly, TRPC6 levels did not alter the overall calcium response of podocytes to complement (Fig. 3A). Calcium signaling is highly compartmentalized within cells and calcium-dependent pathways may be restricted to cellular microdomains (38). Hence, despite similar overall levels of intracellular calcium upon complement stimulation in control and wtTRPC6 or dnTRPC6 overexpressing podocytes, local TRPC6-dependent differences in intracellular calcium levels that escape detection using whole-cell calcium measurement might control spatially confined regulatory pathways. The ERK pathway is activated in response to complement injury (39) and has been implicated in TRPC6 effects on podocytes (40). We found prominent ERK activation in response to complement, but this activation was not dependent on TRPC6 (Fig. 3B). We next focused on CaMKII, which is expressed in podocytes (41), protects podocytes from injury (10), and can be activated via TRPC6.4 CaMKIIα was activated (as revealed by increased levels of autophosphorylated phospho(Thr-286)-CaMKIIα) upon complement stimulation and this activation was increased in wtTRPC6-overexpressing and reduced in dnTRPC6-overexpressing (Fig. 3, B and C) as well as TRPC6 knockdown podocytes (Fig. 3, D and E). Pharmacologic inhibition of CaMKII with KN-62 phenocopied the effect of TRPC6 inhibition in such a way that it increased podocyte susceptibility to complement (Fig. 4, A and B). The effect of CaMKII on complement-induced podocyte injury was not mediated by synaptopodin, which can be phosphorylated by CaMKII and thereby protected from cathepsin l-mediated degradation (10) because synaptopodin levels were unaffected by complement attack and independent of TRPC6 levels (Fig. 3B). We examined two additional downstream candidates for CaMKII-mediated phosphorylation and found that neither glycogen synthase kinase 3 nor the large GTPase dynamin are changed in phosphorylation under podocyte complement attack (supplemental Fig. 6). Immunogold electron microscopy localized CaMKII to the slit diaphragm in vivo (Fig. 4C), supporting the possibility of direct TRPC6-induced CaMKIIα activation within calcium microdomains, which play a central role in CaMKII regulation (42).
FIGURE 3.
CaMKII is activated upon complement stimulation in a TRPC6-dependent manner. A, overall calcium influx in response to antibody sensitization followed by 4% complement stimulation of control or wtTRPC6 or dnTRPC6 lentivirus transduced podocytes demonstrating equal overall calcium responses independent of TRPC6 levels. RFU, relative fluorescence units. B, Western blot analysis of ERK 1/2 and CaMKIIα activation upon complement stimulation demonstrating TRPC6 dependence of CaMKII autophosphorylation at Thr-286 (i.e. activation) but not ERK 1/2 activation. pERK, phosphorylated ERK; tERK, total ERK. Synaptopodin (synpo) levels were unchanged by complement and CaMKIIα. Note that endogenous TRPC6, which is not detectable in the presented blot, appeared after prolonged film exposure. C, densitometric quantification of Western blot results from three independent experiments. *, p < 0.05 for the comparison to empty vector. D and E, reduced CaMKII activation as shown in B and C for dnTRPC6 overexpression was confirmed in TRPC6 knockdown podocytes. n.s., not significant.
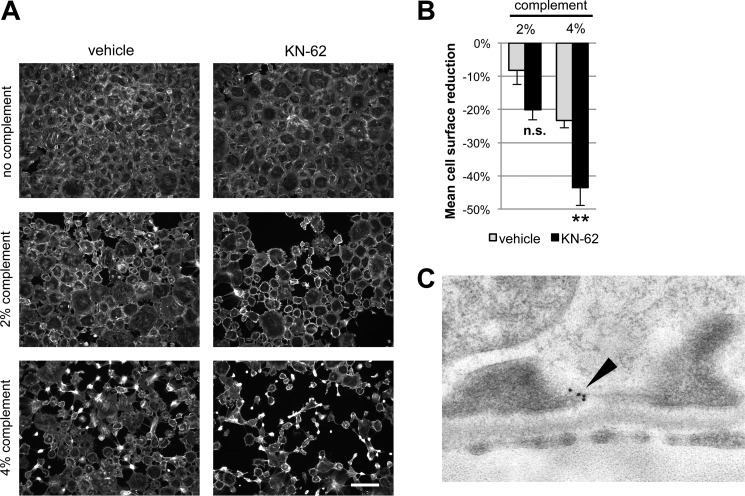
FIGURE 4.
CaMKII inhibition phenocopies the effect of reduced TRPC6 expression or activity upon complement stimulation of podocytes. A, cultured mouse podocytes pretreated with the CaMKII inhibitor KN-62 or vehicle were treated with anti-podocyte-antibody and rat complement (scale bar, 200 μm). B, quantification of the experiment shown in A (see Fig. 3 for explanation). **, p < 0.01 for the comparison of KN-62 treated to control cells treated with the same concentration of complement. C, Immunogold-EM localizing CaMKII in podocytes to the immediate vicinity of the slit diaphragm (see the arrowhead), where spatial compartmentalization with TRPC6 is likely. n.s., not significant.
TRPC6 Attenuates FP Effacement and Proteinuria in the Nephrotoxic Serum Nephritis Mouse Model
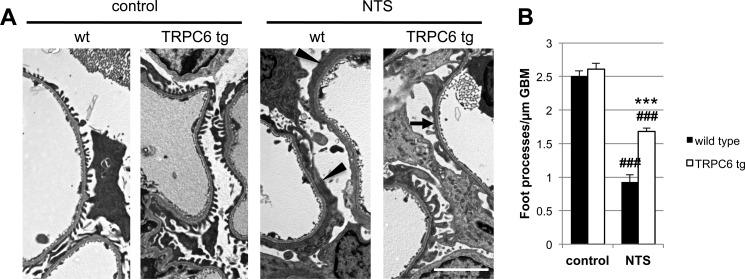
Given the lack of a robust mouse model for membranous nephropathy (43), we used the NTS nephritis model, which pathogenetically involves complement induced injury to podocytes (18, 21, 44) to evaluate the in vivo relevance of our findings. NTS injection in wild type mice resulted in severe proteinuria within 1 day that decreased thereafter but remained high (Fig. 5, F and G). NTS nephritis induced endogenous TRPC6 expression in glomeruli (Fig. 5A). Upon injection of NTS, mice that overexpress wtTRPC6 specifically in their podocytes exhibited increased CaMKIIα activity in isolated glomeruli (Fig. 5, B and C), whereas TRPC6−/− mice exhibited less CaMKII activation during NTS nephritis compared with their respective wild type littermates (Fig. 4, D and E). During the course of NTS, TRPC6-overexpressing mice exhibited less proteinuria (Fig. 4C) and reduced podocyte FP effacement (Fig. 6, A and B) compared with their wild type littermates. Of note, because TRPC6 transgenic mice develop spontaneous late-onset proteinuria and glomerular lesions, we used young mice with normal base-line protein excretion to induce NTS nephritis. In contrast, TRPC6−/− mice showed a trend toward increased proteinuria early during disease (Fig. 5G), whereas proteinuria and FP effacement at later stages were not different (data not shown). Because the NTS nephritis model is only partially complement dependent and the complement dependence is greater in early stages and using lower NTS doses (18), we repeated the NTS model using low dose NTS and analyzing urine as early as 4 h post injection. In this early phase of the low dose NTS model, TRPC6−/− mice showed significantly increased albuminuria, which could be prevented by complement depleting pretreatment of TRPC6−/− mice with CVF (Fig. 5H), whereas TRPC6 overexpression again conferred partial protection (Fig. 5I).
FIGURE 5.
TRPC6 activates CaMKII and reduces proteinuria in the mouse NTS model of glomerulonephritis. A, glomerular lysates obtained at day 7 after injection of NTS (high dose) into wt mice show increased expression of TRPC6 as compared with control animals. B and D, Western blot analysis of isolated glomeruli from podocyte-specific TRPC6 tg mice reveals increased CaMKIIα activation upon NTS disease induction compared with wt control mice, whereas CaMKIIα activation was reduced in TRPC6−/− mice. C and E, quantification of CaMKIIα activation from three independent Western blot analyses, one of which is shown in B and D. The ratio of pCaMKII to total CaMKII was set as 1 in the wt animals in each experiment for normalization. F and G, quantification of proteinuria by urinary albumin:creatinine ratio (Alb/Crea) from days 0–7 in NTS-injected wt and TRPC6 tg and in wt and TRPC6−/− mice, respectively. *, p < 0.05 for the comparison of tg with wt animals. H and I, upon injection of low dose NTS, TRPC6−/− mice exhibit significantly increased early proteinuria compared with their wt littermate controls, which was completely abolished by complement-depleting pretreatment with CVF, whereas TRPC6 tg mice developed less proteinuria than their wt littermate controls. *, p < 0.05; **, p < 0.01 for the comparison of NTS-injected wt and CVF-treated TRPC6−/− mice to their TRPC6−/− or TRPC6 tg littermate, respectively. #, p < 0.05; ##, p < 0.01 for the comparison of proteinuria 4 h after NTS injection to base-line proteinuria within an experimental animal group.
FIGURE 6.
TRPC6 overexpression reduces podocyte foot process effacement during NTS nephritis. A, electron micrographs of TRPC6 tg and wt mice before and 7 days after NTS injection. Podocytes displayed a variable degree of FP effacement ranging from minimal to severe (arrowheads) in wt mice and from minimal to moderate (arrow) in TRPC6 tg mice (scale bar, 5 μm). B, quantification of FP effacement by mean number of FPs per glomerular basement membrane (GBM) length in three capillary loops per glomerulus and five glomeruli per mouse (n = 2). Note that at base line, 8-week-old TRPC6 tg mice, which develop glomerular lesions starting at 3–5 months of age, did not yet exhibit FP effacement. ***, p < 0.001 for the comparison of NTS-injected TRPC6 tg with NTS-injected wt mice; ###, p < 0.001 for the comparison of NTS-injected with uninjected (control) mice of the same genotype.
TRPC6 Modulates the Susceptibility of Human Podocytes to PLA2R-positive Human Membranous Nephropathy Sera
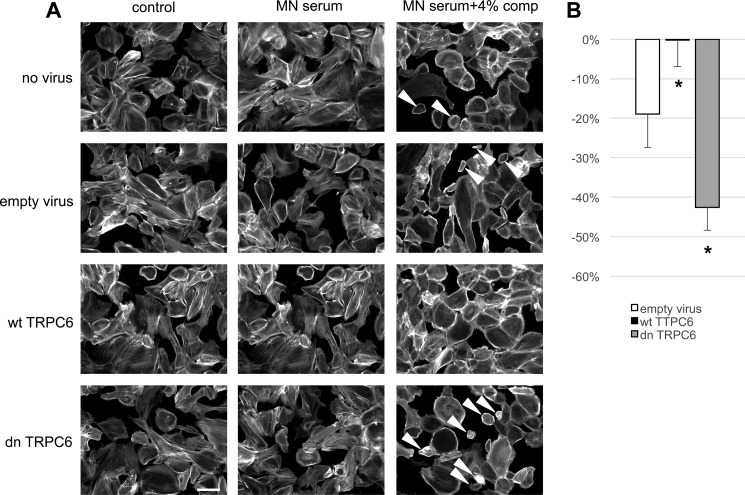
Human idiopathic membranous nephropathy has been recently linked to the presence of autoantibodies against M-type PLA2R in a majority of cases (34); however, the downstream pathways that lead to podocyte damage remain elusive. We wondered whether human podocytes sensitized with anti-PLA2R antibody-positive MN sera would exhibit a similar phenotype as mouse podocytes sensitized with sheep anti-mouse podocyte IgG upon exposure to rat complement and whether a protective role of TRPC6 could be found in human podocytes. Exposure of cultured differentiated human podocytes to growth medium containing 5% pooled sera from PLA2R antibody-positive MN patients did not affect cell morphology. Notably, human sera were not collected under complement-preserving conditions (i.e. immediate storage on ice until freezing). Addition of 4% rat complement after sensitizing cells with MN sera led to cell rounding of a fraction of podocytes (Fig. 7). This phenotype was clearly less prominent than that seen in mouse podocytes sensitized with sheep anti-mouse podocyte IgG, which may be due to the fact that the majority of PLA2R antibodies found in MN are IgG4, which do not directly activate complement (34). Again, overexpression of human wtTRPC6 attenuated the phenotype, whereas human dnTRPC6 increased sensitivity of human podocytes pretreated with MN sera to complement (Fig. 7).
FIGURE 7.
TRPC6 protects human podocytes from anti-PLA2R-antibody-mediated complement injury. A, differentiated human podocytes preincubated with anti-PLA2R-positive human MN sera developed cell rounding in response to complement treatment in a fraction of cells (arrowheads). This effect was absent in wtTRPC6-overexpressing cells but pronounced in dnTRPC6-overexpressing cells. B, quantification of the effect shown in A by comparing the average cell surface area reduction upon complement stimulation (for details, see Fig. 2; *, p < 0.05).
CaMKII Is Activated in Podocytes during Membranous Nephropathy in Vivo
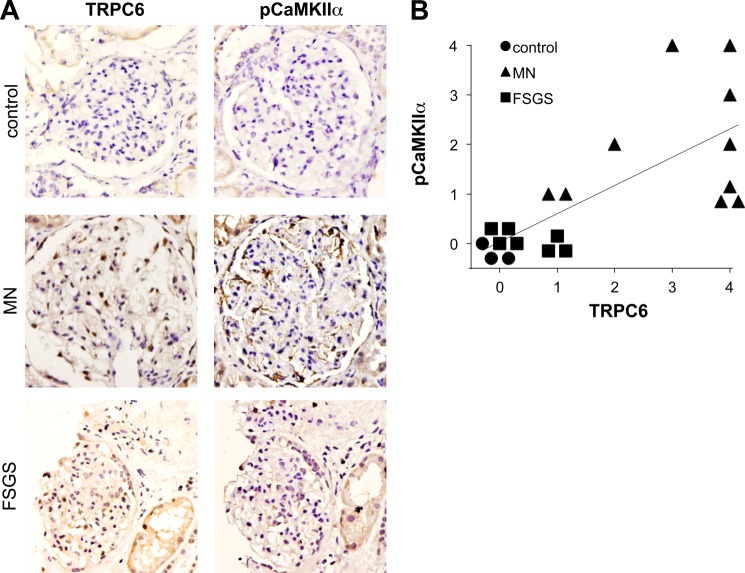
To test whether induced expression of TRPC6 in human MN (4) leads to CaMKII activation in vivo, we stained human biopsies for autophosphorylated (active) CaMKIIα. Immunohistochemistry of human MN biopsies with a phospho-CaMKII-specific antibody revealed moderate to strong staining of podocytes in all 10 biopsies tested. In contrast, we did not detect active CaMKII in three normal kidney tissue specimens (from tumor nephrectomies) and in seven FSGS biopsies (Fig. 8, A and B). Notably, TRPC6 expression was below detection levels in control kidneys and only slightly induced in FSGS glomeruli, whereas MN kidneys mostly showed moderate to strong positivity for TRPC6 (Fig. 8, A and B). Overall, active CaMKIIα staining was highly correlated with TRPC6 expression levels (Spearman's ρ = 0.829, p < 0.001).
FIGURE 8.
CaMKII activity correlates with TRPC6 expression in human MN. A, representative examples of human biopsy specimens from MN patients, FSGS patients, and normal kidney tissue from tumor nephrectomy samples stained for TRPC6 and phospho(Thr-286)-CaMKIIα. Note the linear pCaMKIIα staining along the glomerular capillaries in MN but not in controls (200× magnification). B, quantification of TRPC6 and pCaMKIIα staining in all analyzed samples shows a high degree of correlation between TRPC6 and active CaMKIIα (Spearman's ρ = 0.829, p < 0.001). For details on quantification of the stainings, see “Experimental Procedures.”
DISCUSSION
Many of the tremendous advances in our understanding of glomerular function that have been achieved during the past 15 years stem from human and rodent genetic studies. Although monogenic hereditary diseases account for only a minority of human glomerular diseases, it is generally assumed that acquired glomerulopathies can be mediated by alterations in those same pathways; hence, genetic studies may disclose targets for drug treatment of the much more frequent acquired glomerular diseases (33, 45). One such potential target is the cation channel TRPC6. Genetic hyperactivity of TRPC6 is a known cause of glomerulosclerosis in humans (1, 2) and mice (6) through as yet incompletely identified pathways, and the expression of wild type TRPC6 is induced in acquired glomerular disorders (4). This led us to hypothesize that TRPC6 overactivation, either caused by activating mutations or by increased expression of wild type channels, is deleterious to podocytes (5). Our data presented here, however, unexpectedly establish a dual and context dependent role for TRPC6 in podocytes.
Based on our results, TRPC6 does not seem to have an immediate effect on the actin cytoskeleton. The actin cytoskeleton was considered a potential target for the effects of TRPC6 activation (46), based on the importance of actin structure for podocyte morphology and function (47) and the fact that synaptopodin, an actin-associated protein (22) that stabilizes RhoA (48), and actin stress fibers (49) represent a substrate of calcium-dependent enzymes (10). Although we achieved very high levels of TRPC6 overexpression, this had no influence on synaptopodin levels and the podocyte actin cytoskeleton, irrespective of pharmacological TRPC6 activation and the level of overexpression. These data using podocytes differentiated for at least 9 days before viral infection and at least 12 days before analysis contrast earlier results, where transient transfection of podocytes differentiated for a shorter time period with a plasmid encoding for TRPC6 affected the actin cytoskeleton (4). The very low efficiency of conventional transfection methods in cultured podocytes and the fact that the cultured podocytes are not completely differentiated with actin fibers still developing at the time of transfection may explain the divergent result of those previous experiments. We believe that our lentivirus-based approach is more relevant and reflects better the in vivo situation because fully differentiated podocytes can be utilized. Of note, we confirmed our negative results shown in this work in human podocytes as well as in primary cultured mouse podocytes by investigating hundreds of TRPC6 infected cells, using various experimental conditions. Although in contrast to our initial hypothesis, this finding is well in line with previously available data: unlike many other FSGS genes, mutations in the TRPC6 gene do not lead to congenital or early childhood nephrotic syndrome but are characterized by late onset and incomplete penetrance (1, 2), and TRPC6 transgenic mice exhibit only low levels of proteinuria starting around the age of 3–5 months (6). Hence, sustained podocyte TRPC6 activation over extended periods of time is likely required for the development of glomerulosclerosis.
Acquired glomerular diseases usually develop over a much shorter period of time. Strikingly, our data indicate that induction of TRPC6 in murine NTS nephritis and in human MN might in fact not cause podocyte damage but rather play a protective role against complement-induced damage. Thus, the overall effect of TRPC6 in podocytes likely depends on the presence or absence of complement and on whether its induction is transient or sustained. In fact, it is interesting that no physiological role for TRPC6 in podocytes has so far been shown, and TRPC6−/− mice do not exhibit a renal phenotype (50). Our data are the first to show a potential beneficial role of podocyte TRPC6, which upon prolonged stimulation may turn into a detrimental signal.
The effects of podocyte TRPC6 overexpression and knock out on the severity of NTS nephritis were limited, a fact that may be accounted for by a number of reasons. First, the NTS nephritis mouse model is only partially complement-dependent, and particularly at high antibody doses, complement-independent pathways contribute to glomerular damage (18). Consequently, we observed a much more pronounced effect of TRPC6 deficiency in a low dose NTS nephritis model at a very early stage. Second, the absence of TRPC6 both in vitro and in vivo resulted in a roughly 30–50% reduction of CaMKII autophosphorylation. Hence, TRPC6-independent pathways might also contribute to CaMKII activation and autophosphorylation. Third, the TRPC6-CaMKII pathway is most likely only one among others that regulate complement-mediated injury.
The overall intracellular calcium concentration in response to complement was not affected by TRPC6 levels to a detectable extent. Thus, the majority of overall calcium influx upon complement stimulation must be mediated by channels other than TRPC6, most likely through the pore-forming membrane attack complex C5b-9. It is well known that calcium signaling is highly compartmentalized within cells and calcium-dependent pathways may be restricted to microdomains (38). Hence, despite similar overall levels of intracellular calcium upon complement stimulation in wtTRPC6 and dnTRPC6-overexpressing podocytes, local TRPC6-dependent differences in intracellular calcium levels that escape detection using whole-cell calcium measurement might control spatially confined regulatory pathways. Likewise, the cellular effects of CaMKII activity largely depend on its subcellular targeting and the resulting proximity to substrates, which is regulated by multi-site phosphorylation (42). Our immunogold EM data showing subcellular localization of CaMKII in direct proximity to the slit diaphragm in vivo support the possibility of close functional interaction with TRPC6. The downstream effector pathways of CaMKII during complement injury in podocytes remain to be defined. We did not detect any effect on synaptopodin, which has been suggested as a target of CaMKII phosphorylation (10). However, podocytes express many known targets of CaMKII phosphorylation. In addition, CaMKII may act directly on the actin cytoskeleton (51). Furthermore, although our data provide evidence that CaMKII is involved in mediating the protective effects of TRPC6 on complement injury to podocytes, we cannot exclude the existence of additional pathways that mediate the downstream effects of TRPC6.
Our results have important potential clinical implications. First, they suggest CaMKII modulation as a potential therapeutic target in complement-mediated glomerular diseases. Second, and more importantly, they question whether TRPC6 represents an ideal drug target for the treatment of acquired glomerular diseases, such as MN, diabetic nephropathy, and non-genetic FSGS. Whether such pharmacological interventions would be beneficial or detrimental may critically depend on the pathophysiological context, including the presence or absence of complement activation.
Acknowledgments
Human MN sera were kindly provided by David Salant and Laurence Beck (both from Boston University). We thank Madhusudhan Bhat (All India Institute of Medical Sciences) for help with immunohistochemistry standardization and Alejandro Caicedo (University of Miami) for assistance with calcium imaging.
This work was supported in part by Grants DK073495, DK089394, and DK101350 from the National Institutes of Health (to J. R.).

This article contains supplemental Figs. 1–6.
A. D. Kistler, G. Singh, M. M. Altintas, H. Yu, I. C. Fernandez, C. Gu, C. Wilson, S. K. Srivastava, A. Dietrich, K. Walz, D. Kerjaschki, P. Ruiz, S. Dryer, S. Sever, A. K. Dinda, C. Faul, and J. Reiser, unpublished observations.
- TRPC6
- transient receptor potential channel 6
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- FP
- foot process
- MN
- membranous nephropathy
- NTS
- nephrotoxic serum
- OAG
- 1-oleoyl-2-acetylglycerol
- tg
- transgenic
- CVF
- cobra venom factor
- dn
- dominant negative
- PLA2R
- phospholipase A2 receptor.
REFERENCES
- 1. Winn M. P., Conlon P. J., Lynn K. L., Farrington M. K., Creazzo T., Hawkins A. F., Daskalakis N., Kwan S. Y., Ebersviller S., Burchette J. L., Pericak-Vance M. A., Howell D. N., Vance J. M., Rosenberg P. B. (2005) A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 [DOI] [PubMed] [Google Scholar]
- 2. Reiser J., Polu K. R., Möller C. C., Kenlan P., Altintas M. M., Wei C., Faul C., Herbert S., Villegas I., Avila-Casado C., McGee M., Sugimoto H., Brown D., Kalluri R., Mundel P., Smith P. L., Clapham D. E., Pollak M. R. (2005) TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat. Genet. 37, 739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heeringa S. F., Möller C. C., Du J., Yue L., Hinkes B., Chernin G., Vlangos C. N., Hoyer P. F., Reiser J., Hildebrandt F. (2009) A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4, e7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Möller C. C., Wei C., Altintas M. M., Li J., Greka A., Ohse T., Pippin J. W., Rastaldi M. P., Wawersik S., Schiavi S., Henger A., Kretzler M., Shankland S. J., Reiser J. (2007) Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 18, 29–36 [DOI] [PubMed] [Google Scholar]
- 5. Möller C. C., Flesche J., Reiser J. (2009) Sensitizing the slit diaphragm with TRPC6 ion channels. J. Am. Soc. Nephrol. 20, 950–953 [DOI] [PubMed] [Google Scholar]
- 6. Krall P., Canales C. P., Kairath P., Carmona-Mora P., Molina J., Carpio J. D., Ruiz P., Mezzano S. A., Li J., Wei C., Reiser J., Young J. I., Walz K. (2010) Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One 5, e12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuwahara K., Wang Y., McAnally J., Richardson J. A., Bassel-Duby R., Hill J. A., Olson E. N. (2006) TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 116, 3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlöndorff J., Del Camino D., Carrasquillo R., Lacey V., Pollak M. R. (2009) TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am. J. Physiol. Cell Physiol. 296, C558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nijenhuis T., Sloan A. J., Hoenderop J. G., Flesche J., van Goor H., Kistler A. D., Bakker M., Bindels R. J., de Boer R. A., Möller C. C., Hamming I., Navis G., Wetzels J. F., Berden J. H., Reiser J., Faul C., van der Vlag J. (2011) Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am. J. Pathol. 179, 1719–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faul C., Donnelly M., Merscher-Gomez S., Chang Y. H., Franz S., Delfgaauw J., Chang J. M., Choi H. Y., Campbell K. N., Kim K., Reiser J., Mundel P. (2008) The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glassock R. J. (2010) The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am. J. Kidney Dis. 56, 157–167 [DOI] [PubMed] [Google Scholar]
- 12. Glassock R. J. (2012) The pathogenesis of membranous nephropathy: evolution and revolution. Curr. Opin. Nephrol. Hypertens. 21, 235–242 [DOI] [PubMed] [Google Scholar]
- 13. Topham P. S., Haydar S. A., Kuphal R., Lightfoot J. D., Salant D. J. (1999) Complement-mediated injury reversibly disrupts glomerular epithelial cell actin microfilaments and focal adhesions. Kidney Int. 55, 1763–1775 [DOI] [PubMed] [Google Scholar]
- 14. Cybulsky A. V., Quigg R. J., Salant D. J. (2005) Experimental membranous nephropathy redux. Am. J. Physiol. Renal Physiol. 289, F660-F671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cybulsky A. V., Salant D. J., Quigg R. J., Badalamenti J., Bonventre J. V. (1989) Complement C5b-9 complex activates phospholipases in glomerular epithelial cells. Am. J. Physiol. 257, F826-F836 [DOI] [PubMed] [Google Scholar]
- 16. Hofmann T., Obukhov A. G., Schaefer M., Harteneck C., Gudermann T., Schultz G. (1999) Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 [DOI] [PubMed] [Google Scholar]
- 17. Quigg R. J., Kozono Y., Berthiaume D., Lim A., Salant D. J., Weinfeld A., Griffin P., Kremmer E., Holers V. M. (1998) Blockade of antibody-induced glomerulonephritis with Crry-Ig, a soluble murine complement inhibitor. J. Immunol. 160, 4553–4560 [PubMed] [Google Scholar]
- 18. Hébert M. J., Takano T., Papayianni A., Rennke H. G., Minto A., Salant D. J., Carroll M. C., Brady H. R. (1998) Acute nephrotoxic serum nephritis in complement knockout mice: relative roles of the classical and alternate pathways in neutrophil recruitment and proteinuria. Nephrol. Dial. Transplant. 13, 2799–2803 [DOI] [PubMed] [Google Scholar]
- 19. Lin F., Emancipator S. N., Salant D. J., Medof M. E. (2002) Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab. Invest. 82, 563–569 [DOI] [PubMed] [Google Scholar]
- 20. Lin F., Salant D. J., Meyerson H., Emancipator S., Morgan B. P., Medof M. E. (2004) Respective roles of decay-accelerating factor and CD59 in circumventing glomerular injury in acute nephrotoxic serum nephritis. J. Immunol. 172, 2636–2642 [DOI] [PubMed] [Google Scholar]
- 21. Turnberg D., Botto M., Warren J., Morgan B. P., Walport M. J., Cook H. T. (2003) CD59a deficiency exacerbates accelerated nephrotoxic nephritis in mice. J. Am. Soc. Nephrol. 14, 2271–2279 [DOI] [PubMed] [Google Scholar]
- 22. Mundel P., Heid H. W., Mundel T. M., Krüger M., Reiser J., Kriz W. (1997) Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol. 139, 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dietrich A., Mederos Y Schnitzler M., Gollasch M., Gross V., Storch U., Dubrovska G., Obst M., Yildirim E., Salanova B., Kalwa H., Essin K., Pinkenburg O., Luft F. C., Gudermann T., Birnbaumer L. (2005) Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol. Cell Biol. 25, 6980–6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ophascharoensuk V., Pippin J. W., Gordon K. L., Shankland S. J., Couser W. G., Johnson R. J. (1998) Role of intrinsic renal cells versus infiltrating cells in glomerular crescent formation. Kidney Int. 54, 416–425 [DOI] [PubMed] [Google Scholar]
- 25. Kerjaschki D., Exner M., Ullrich R., Susani M., Curtiss L. K., Witztum J. L., Farquhar M. G., Orlando R. A. (1997) Pathogenic antibodies inhibit the binding of apolipoproteins to megalin/gp330 in passive Heymann nephritis. J. Clin. Invest. 100, 2303–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mundel P., Reiser J., Zúñiga Mejía Borja A., Pavenstädt H., Davidson G. R., Kriz W., Zeller R. (1997) Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 236, 248–258 [DOI] [PubMed] [Google Scholar]
- 27. Saleem M. A., O'Hare M. J., Reiser J., Coward R. J., Inward C. D., Farren T., Xing C. Y., Ni L., Mathieson P. W., Mundel P. (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 13, 630–638 [DOI] [PubMed] [Google Scholar]
- 28. Hofmann T., Schaefer M., Schultz G., Gudermann T. (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. U.S.A. 99, 7461–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takemoto M., Asker N., Gerhardt H., Lundkvist A., Johansson B. R., Saito Y., Betsholtz C. (2002) A new method for large scale isolation of kidney glomeruli from mice. Am. J. Pathol. 161, 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim E. Y., Anderson M., Dryer S. E. (2012) Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidases and reactive oxygen species. Am. J. Physiol. Renal Physiol. 302, F298–F307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim E. Y., Anderson M., Wilson C., Hagmann H., Benzing T., Dryer S. E. (2013) NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: Essential role of podocin in formation of this complex. Am. J. Physiol. Cell Physiol. 305, C960–C971 [DOI] [PubMed] [Google Scholar]
- 32. Anderson M., Kim E. Y., Hagmann H., Benzing T., Dryer S. E. (2013) Opposing effects of podocin on the gating of podocyte TRPC6 channels evoked by membrane stretch or diacylglycerol. Am. J. Physiol. Cell Physiol. 305, C276–289 [DOI] [PubMed] [Google Scholar]
- 33. Reiser J., Gupta V., Kistler A. D. (2010) Toward the development of podocyte-specific drugs. Kidney Int. 77, 662–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beck L. H., Jr., Bonegio R. G., Lambeau G., Beck D. M., Powell D. W., Cummins T. D., Klein J. B., Salant D. J. (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Debiec H., Lefeu F., Kemper M. J., Niaudet P., Deschênes G., Remuzzi G., Ulinski T., Ronco P. (2011) Early-childhood membranous nephropathy due to cationic bovine serum albumin. N. Engl. J. Med. 364, 2101–2110 [DOI] [PubMed] [Google Scholar]
- 36. Cybulsky A. V. (2011) Membranous nephropathy. Contrib. Nephrol. 169, 107–125 [DOI] [PubMed] [Google Scholar]
- 37. Cybulsky A. V., Bonventre J. V., Quigg R. J., Lieberthal W., Salant D. J. (1990) Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int. 38, 803–811 [DOI] [PubMed] [Google Scholar]
- 38. Laude A. J., Simpson A. W. (2009) Compartmentalized signalling: Ca2+ compartments, microdomains and the many facets of Ca2+ signalling. FEBS J. 276, 1800–1816 [DOI] [PubMed] [Google Scholar]
- 39. Takano T., Cybulsky A. V. (2000) Complement C5b-9-mediated arachidonic acid metabolism in glomerular epithelial cells: role of cyclooxygenase-1 and -2. Am. J. Pathol. 156, 2091–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chiluiza D., Krishna S., Schumacher V. A., Schlöndorff J. (2013) Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular-signal-regulated kinases Erk1/2. J. Biol. Chem. 288, 18407–18420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giardino L., Armelloni S., Corbelli A., Mattinzoli D., Zennaro C., Guerrot D., Tourrel F., Ikehata M., Li M., Berra S., Carraro M., Messa P., Rastaldi M. P. (2009) Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J. Am. Soc. Nephrol. 20, 1929–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Skelding K. A., Rostas J. A. (2009) Regulation of CaMKII in vivo: the importance of targeting and the intracellular microenvironment. Neurochem. Res. 34, 1792–1804 [DOI] [PubMed] [Google Scholar]
- 43. Jefferson J. A., Pippin J. W., Shankland S. J. (2010) Experimental models of membranous nephropathy. Drug Discov. Today Dis. Models 7, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quigg R. J., He C., Lim A., Berthiaume D., Alexander J. J., Kraus D., Holers V. M. (1998) Transgenic mice overexpressing the complement inhibitor crry as a soluble protein are protected from antibody-induced glomerular injury. J. Exp. Med. 188, 1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lavin P. J., Gbadegesin R., Damodaran T. V., Winn M. P. (2008) Therapeutic targets in focal and segmental glomerulosclerosis. Curr. Opin. Nephrol. Hypertens. 17, 386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schlöndorff J. S., Pollak M. R. (2006) TRPC6 in glomerular health and disease: what we know and what we believe. Semin. Cell. Dev. Biol. 17, 667–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Faul C., Asanuma K., Yanagida-Asanuma E., Kim K., Mundel P. (2007) Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 [DOI] [PubMed] [Google Scholar]
- 48. Asanuma K., Yanagida-Asanuma E., Faul C., Tomino Y., Kim K., Mundel P. (2006) Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat. Cell Biol. 8, 485–491 [DOI] [PubMed] [Google Scholar]
- 49. Asanuma K., Kim K., Oh J., Giardino L., Chabanis S., Faul C., Reiser J., Mundel P. (2005) Synaptopodin regulates the actin-bundling activity of α-actinin in an isoform-specific manner. J. Clin. Invest. 115, 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eckel J., Lavin P. J., Finch E. A., Mukerji N., Burch J., Gbadegesin R., Wu G., Bowling B., Byrd A., Hall G., Sparks M., Zhang Z. S., Homstad A., Barisoni L., Birbaumer L., Rosenberg P., Winn M. P. (2011) TRPC6 enhances angiotensin II-induced albuminuria. J. Am. Soc. Nephrol. 22, 526–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shen K., Teruel M. N., Subramanian K., Meyer T. (1998) CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron 21, 593–606 [DOI] [PubMed] [Google Scholar]