Abstract
Objective
To develop HPTLC fingerprint profile of anti-inflammatory active extract fractions of Tribulus terrestris (family Zygophyllaceae).
Methods
The anti-inflammatory activity was tested for the methanol and its fractions (chloroform, ethyl acetate, n-butanol and aqueous) and chloroform extract of Tribulus terrestris (aerial parts) by injecting different groups of rats (6 each) with carrageenan in hind paw and measuring the edema volume before and 1, 2 and 3 h after carrageenan injection. Control group received saline i.p. The extracts treatment was injected i.p. in doses of 200 mg/kg 1 h before carrageenan administration. Indomethacin (30 mg/kg) was used as standard. HPTLC studies were carried out using CAMAG HPTLC system equipped with Linomat IV applicator, TLC scanner 3, Reprostar 3, CAMAG ADC 2 and WIN CATS-4 software for the active fractions of chloroform fraction of methanol extract.
Results
The methanol extract showed good antiedematous effect with percentage of inhibition more than 72%, indicating its ability to inhibit the inflammatory mediators. The methanol extract was re-dissolved in 100 mL of distilled water and fractionated with chloroform, ethyl acetate and n-butanol. The four fractions (chloroform, ethyl acetate, n-butanol and aqueous) were subjected to anti-inflammatory activity. Chloroform fraction showed good anti-inflammatory activity at dose of 200 mg/kg. Chloroform fraction was then subjected to normal phase silica gel column chromatography and eluted with petroleum ether-chloroform, chloroform-ethyl acetate mixtures of increasing polarity which produced 15 fractions (F1-F15). Only fractions F1, F2, F4, F5, F7, F9, F11 and F14 were found to be active, hence these were analyzed with HPTLC to develop their finger print profile. These fractions showed different spots with different Rf values.
Conclusions
The different chloroform fractions F1, F2, F4, F5, F7, F9, F11 and F14 revealed 4, 7, 7, 8, 9, 7, 7 and 6 major spots, respectively. The results obtained in this experiment strongly support and validate the traditional uses of this Sudanese medicinal plant.
Keywords: Tribulus terrestris, Finger print, Anti-inflammatory, Standardization, HPTLC
1. Introduction
Inflammation is a complex pathophysiological process mediated by a variety of signaling molecules produced by leukocytes, macrophages and mast cells as well as by the activation of complement factors, which bring about edema formation as a result of extravasation of fluid and proteins and accumulation of leukocytes at the inflammatory site. Resolution of inflammation requires the elimination of key inflammatory cells and the downregulation of pro-inflammatory mediators in the inflamed sites. This coordinated process is actively regulated by biochemical mediators which possess anti-inflammatory and/or pro-resolving effects[1]. Chronic inflammatory diseases remain one of the world's major health problems. Recent epidemiological studies highlight the key role of the type of consumed unsaturated fatty acid and the development of ulcerative colitis[2]. Many of the commonly used anti-inflammatory agents are becoming less acceptable due to serious adverse reactions such as gastric intolerance, sodium, potassium, and water retention, as well as an increase in blood urea nitrogen and creatinine, bleeding and bone marrow depression resulting from prolonged use[3]. Therefore, development of new, economic, potent and safe anti-inflammatory drug from natural source is the need of hour especially for developing countries. Tribulus terrestris (T. terrestris) is a flowering plant in the family Zygophyllaceae, native to warm temperate and tropical regions of the Old World in Southern Europe, Southern Asia, throughout Africa, Australia and Sudan. In Sudan, the plant widely used traditionally for treatment of inflammation and other related inflammatory symptoms[4]. Herbal medicines have stood the test of time for their safety, efficacy, cultural acceptability and lesser side effects. They are believed to have better compatibility with the human body[5]. In the recent years, HPTLC has become a conventional analytical approach for the standardization of herbal drugs due to its benefit of low operation cost, high sample throughput and need for minimum sample clean up[6],[7]. HPTLC is commonly applied for the identification, assay and the testing for purity, stability, dissolution or content uniformity of raw materials (herbal & animal extracts, fermentation mixtures, drugs and excipients) and formulated products (pharmaceuticals, cosmetics and nutrients)[8]. The main advantages of HPTLC over other conventional analytical methods, is that many samples can be run simultaneously using a little volume of mobile phase, thus reducing the time and cost per analysis[9]–[11]. These flexible and cost-effective techniques present the advantage of the simultaneous processing of standards and samples with versatile detection possibilities, including a great variety of post-chromatographic derivatization reagents. The aim of this study is to develop HPTLC fingerprint profile of active chloroform fractions of methanolic extract of aerial parts of T. terrestris.
2. Materials and methods
2.1. Plant material
T. terrestris (aerial parts) was collected from Kordofan and Nuba Mountain (Western Sudan). The plant was authenticated at the Medicinal and Aromatic Plant Research Institute, Sudan and voucher specimens deposited in the Herbarium.
2.2. Preparation and extraction of plant material
The aerial parts of the plant was dried under shade and then powdered. The powdered plant material (300 g) was extracted by cold maceration method with sufficient quantity of chloroform and 80% methanol at room temperature for 48 h. The process of extraction was repeated twice to complete extraction. The extracts were filtered concentrated under reduced pressure which afforded 28 g methanol and 25 g chloroform concentrated extracts. Concentrated extracts were subjected to anti-inflammatory activity by carrageenan induced rat paw edema. Methanolic extract showed good anti-inflammatory activity was re-dissolved in 100 mL of distilled water and fractionated with chloroform, ethyl acetate and n-butanol sequentially (three time for each solvent). Different fractions were collected and concentrated and subjected for anti-inflammatory activity. Chloroform fractions of T. terrestris showed good anti- inflammatory activity, thus the chloroform extract was subjected to normal phase silica gel column chromatography and eluted with petroleum ether-chloroform and chloroform- ethyl acetate mixtures of increasing polarity to give 15 fractions.
2.3. HPTLC profile
2.3.1. Chromatographic method
HPTLC studies were carried out following the method of Harborne[12] and Wagner et al[13]. The protocol for preparing sample solutions was optimized for high quality fingerprinting. The fingerprinting of active chloroform fractions F1, F2, F4, F5, F7, F9, F11 and F14 were executed by spotting 10 µL of suitably diluted sample solution on a HPTLC plate. The plates were developed and scanned. The peak areas were recorded. Chromatography was performed on 20 cm×10 cm glass HPTLC plates precoated with 200 µm layers of silica gel 60F254 (E. Merck, Darmstadt, Germany). Sample of each fraction was applied as bands 6 mm wide and 8 mm apart by means of CAMAG (Muttenz, Switzerland) Linomat IV sample applicator equipped with a 25 µL syringe which was programmed through WIN CATS software.
2.3.2. Solvent system development
A number of solvent systems were tried, but the best resolution was obtained with the solvents, toluene: ethyl acetate: formic acid (8:2:1).
2.3.3. Detection of spots
The developed plate was dried at 100 °C in hot air oven for 3 min to evaporate solvents from the plate. The plate was kept in photo-documentation chamber (CAMAG reprostar 3) and images captured under UV light at 254 nm, respectively. The Rf values and finger print data were recorded by WIN CATS software.
2.4. Anti-inflammatory activity
The anti-inflammatory effect was evaluated by carrageenan induced rat paw edema according to the method Sudhir et al[4]. Each animal was marked on its right ankle in a circular manner using a non-erasable blue ink. The volume of each paw up to the ankle mark was then measured using Ugo-Basil plythesmometer using 0.45% sodium chloride solution as a displacement fluid. The volume of the immersed paw was then read in the digital display. Groups of six animals were injected, each with a dose of a different extract in a dose of 200 mg/kg intra peritoneal. After 60 min each rat was then injected intra plantarly with 0.2% aqueous carrageenan using a fine 1-mL hypodermic syringe. The control group was injected with the suspending agent solution intra peritoneally in a volume equivalent to the test volumes injected. Following carrageenan injections, paw volumes to the marked sites were read at 1, 2 and 3 h intervals. The volume of formed oedema was then calculated using the following formula:
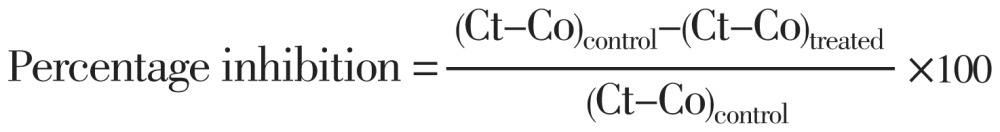 |
Where Co is volume of oedema in control group and Ct is volume of oedema in test group. Net edema volumes formed 2 h following injection of carrageenan were used to calculate the effect of the extracts on the induced oedema.
2.5. Statistical analysis
Results were analyzed using one way ANOVA and Student's t-test and presented as mean±SEM. Only results with P≤0.05 were regarded as significant[14].
3. Results
3.1. Anti-inflammatory activity methanol and chloroform extract of aerial parts of T. terrestris
Injection of carrageenan into a rat's hind paw resulted in swelling as early as 10 min after injection. This swelling reached a plateau by 30 min and was maintained for up to 240 min[15]. Treatment of rats (i.p) with CHCl3 and MeOH extracts in a dose of 200 mg/kg inhibited significantly (P<0.05, n=6), carrageenan induced inflammation by 61.9% and 72%, respectively (Table 1). Methanol extract showed significant inhibition of inflammation in comparison to standard indomethacin (82.3%).
Table 1. Anti-inflammatory activity of methanol and chloroform extract of T. terrestris.
| Treatment | Dose (mg/kg) | Paw edema volume (mL) (3 h after carageenan injection) | Inhibition (%) |
| Control (carageenan) | 0.1 mL | 0.68±0.06 | 0.0 |
| MeOH extract | 200 | 0.45±0.09** | 72.0 |
| CHCl3 extract | 200 | 0.40±0.02** | 61.9 |
| Indomethacin | 30 | 0.12±0.02** | 82.3 |
Values represent the mean±SEM, n=6. *: P<0.05.
3.2. Anti-inflammatory activity of different fractions of methanol extract of T. terrestris
Rats were treated with four fractions (chloroform, ethyl acetate, n-butanol and aqueous) of active methanol extract of T. terrestris (aerial parts). Out of these four fractions, chloroform and aqueous fractions significantly inhibited (P < 0.05) the carrageenan-induced inflammation at a dose of 200 mg/kg by 73.5% and 55.2%, respectively (Table 2).
Table 2. Anti-inflammatory activity of different fractions of methanol extract of T. terrestris.
| Treatment | Dose (mg/kg) | Paw edema volume (mL) (3 h after carageenan injection) | Inhibition (%) |
| Control (carageenan) | 0.1 mL | 0.68±0.06 | 0.0 |
| Cloroform fraction | 200 | 0.19±0.03** | 73.5 |
| Aqueous fraction | 200 | 0.75±0.04** | 55.2 |
| Ethyl acetate fraction | 200 | 0.65±0.04 | - |
| N-butanol fraction | 200 | 0.66±0.03 | - |
| Indomethacin | 30 | 0.13±0.02** | 80.8 |
Values represent the mean±SEM, n=6. *: P<0.05. -: not active.
The remaining two fractions i.e. ethyl acetate and n-butanol fractions were found to be inactive at a dose of 200 mg/kg. The standard drug indomethacin was found to inhibit the carrageenan-induced inflammation by 80.8% at a dose of 30 mg/kg.
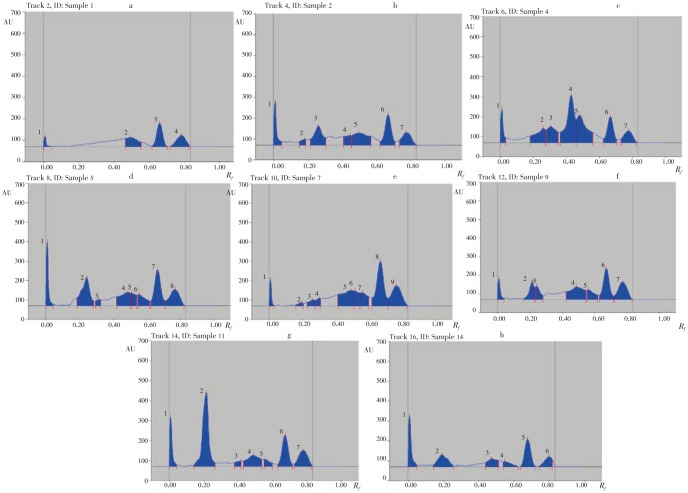
Active chloroform fractions with the help of column chromatography gave 15 fractions (F1-F15). Out of these fractions, 8 fractions (F1, F2, F4, F5, F7, F9, F11 and F14) were found to be pharmacologically active.
3.3. HPTLC profile of active fractions of chloroform fraction of T. terrestris methanol extract
Table 3 shows HPTLC fingerprinting of fractions (F1, F2, F4, F5, F7, F9, F11 and F14) of active chloroform extracts which revealed several peaks.
Table 3. HPTLC profile of active chloroform fractions F1, F2, F4, F5, F7, F9, F11 and F14.
| Fractions | Vol. applied | Peak | Rf | Height area | ||
| F1 | 1 | 0.01 | 476.1 | |||
| 2 | 0.49 | 2442.6 | ||||
| 10 µL | 3 | 0.66 | 3210.4 | |||
| 4 | 0.78 | 2417.6 | ||||
| F2 | 1 | 0.01 | 2582.8 | |||
| 2 | 0.18 | 711.1 | ||||
| 3 | 0.26 | 3954.9 | ||||
| 10 µL | 4 | 0.45 | 1587.2 | |||
| 5 | 0.50 | 4355.3 | ||||
| 6 | 0.67 | 4753.1 | ||||
| 7 | 0.77 | 2814.6 | ||||
| F4 | 1 | 0.01 | 2033.2 | |||
| 2 | 0.26 | 3694.6 | ||||
| 3 | 0.31 | 3972.3 | ||||
| 10 µL | 4 | 0.43 | 9388.4 | |||
| 5 | 0.48 | 7158.2 | ||||
| 6 | 0.67 | 4233.0 | ||||
| 7 | 0.78 | 2751.2 | ||||
| F5 | 1 | 0.01 | 3322.1 | |||
| 2 | 0.21 | 6043.6 | ||||
| 3 | 0.31 | 768.3 | ||||
| 10 µL | 4 | 0.48 | 3748.7 | |||
| 5 | 0.51 | 1698.3 | ||||
| 6 | 0.55 | 2207.3 | ||||
| 7 | 0.67 | 6102.1 | ||||
| 8 | 0.77 | 4262.2 | ||||
| F7 | 1 | 0.01 | 1223.0 | |||
| 2 | 0.19 | 476.2 | ||||
| 3 | 0.26 | 985.9 | ||||
| 10 µL | 4 | 0.30 | 899.5 | |||
| 5 | 0.49 | 5129.3 | ||||
| 6 | 0.51 | 1960.0 | ||||
| 7 | 0.56 | 2456.1 | ||||
| 8 | 0.67 | 8670.1 | ||||
| 9 | 0.76 | 5457.7 | ||||
| F9 | 1 | 0.01 | 1341.2 | |||
| 2 | 0.21 | 2408.5 | ||||
| 3 | 0.24 | 1921.3 | ||||
| 10 µL | 4 | 0.49 | 5233.9 | |||
| 5 | 0.56 | 2021.9 | ||||
| 6 | 0.67 | 5556.4 | ||||
| 7 | 0.77 | 4562.4 | ||||
| F11 | 1 | 0.01 | 2809.4 | |||
| 2 | 0.21 | 11471.1 | ||||
| 3 | 0.41 | 739.7 | ||||
| 10 µL | 4 | 0.49 | 3345.9 | |||
| 5 | 0.56 | 1027.7 | ||||
| 6 | 0.67 | 4890.4 | ||||
| 7 | 0.78 | 3815.9 | ||||
| F14 | 1 | 0.01 | 3316.8 | |||
| 2 | 0.19 | 3018.3 | ||||
| 10 µL | 3 | 0.47 | 1896.8 | |||
| 4 | 0.55 | 1043.1 | ||||
| 5 | 0.68 | 4099.1 | ||||
| 6 | 0.80 | 1814.6 | ||||
HPTLC profile of fractions under UV 254 nm was recorded. The corresponding HPTLC chromatograms are presented in Figure 1. The different Chloroform fractions F1, F2, F4, F5, F7, F9, F11 and F14 revealed 4, 7, 7, 8, 9, 7, 7 and 6 major spots, respectively with Rf values in the range of 0.01 to 0.78 for 10 µL application volume respectively (Table 3 and Figure 1). The purity of the sample was confirmed by comparing the absorption spectra at start, middle and end position of the band.
Figure 1. The HPTLC chromatograms of different chloroform fractions F1, F2, F4, F5, F7, F9, F11 and F14, respectively.
4. Discussion
The quality of herbal medicines is defined in terms of the content of its bioactive compounds. Hence, HPTLC fingerprint profile of herbal products is such an important and powerful procedure which has often been employed for the determination of bioactive components of the herbal medicine. HPTLC fingerprinting is proved to be a liner, precise, accurate method for herbal identification and can be used further in authentication and standardization of the medicinally important plant. Such finger printing is useful in quality control of herbal products and checking for the adulterant. Therefore, it can be useful for the evaluation of different marketed pharmaceutical preparations and plant systematic studies[16]. CHCl3 and MeOH extract of aerial parts of T. terrestris possessed significant anti-inflammatory activities at a dose of 200 mg/kg. The active extract i.e. MeOH extract is fractionated with water, chloroform, ethyl acetate and n-butanol. The different fractions were concentrated and screened for anti-inflammatory activity. Among these four fractions only aqueous and chloroform fractions showed good activity and among these two fractions, chloroform fraction was best in reducing the inflammation. This active chloroform fraction was chromatographed on silica gel in column chromatography to get 15 more fractions. Out of these 15 fractions, eight fractions F1, F2, F4, F5, F7, F9, F11 and F14 were found to be pharmacologically active and analyzed by HPTLC fingerprint method. The fractions F1, F2, F4, F5, F7, F9, F11 and F14 revealed the presence of 4, 7, 7, 8, 9, 7, 7 and 6 spots at 10 µL applications, respectively in Rf range 0.01 to 0.78. Several peaks observed in this experiment indicate the diverse composition of T. terrestris.The marked anti-inflammatory activity showed by both methanol extract and its chloroform fraction revealed their potentials to be used in various inflammatory disorders after further safety studies. Consequently the HPTLC fingerprinting may serve as reference to check the quality of crude drugs and adulterations as well as pharmaceutical preparations containing the same plant. By exhaustive literature survey it is evident that there are no reports regarding fingerprinting analyses of methanol extract and its different fractions of aerial parts of T. terrestris, collected from Western Sudan. Hence, HPTLC fingerprinting is performed for the first time for this plant.
Acknowledgments
The authors are grateful to the Department of Pharmacognosy, Faculty of Pharmacy, University of Khartoum, Sudan and Deanship of Research in King Saud University, Riyadh, KSA. This research was funded by a grant from the Deanship of Research in King Saud University (Grant No. RGP-VPP-150).
Comments
Background
Many countries (including developed ones) suffer a big problem in standardizing and quality control of the herbal plants. This is due to many factors among which are the complex form of these products and the inability of the traditional methods to precisely estimate the quality of the herbs. It is reported that T. terrestris has many applications in various complications; hence it was an effort to check its anti-inflammatory effect and development of chromatographic profile for its anti-inflammatory active extract fractions which has been used for quality control and standardization.
Research frontiers
The present aim of this research work is to develop HPTLC fingerprint profile of anti-inflammatory active extract fractions of T. terrestris (family Zygophyllaceae).
Related reports
No similar reports were found in the literature regarding this plant specifically, since it is being evaluated using this tool for the first time as per literature review available in the resources.
Innovations and breakthroughs
The previous pharmacopeial methods for testing quality control concentrate on organoleptics, ash, and vague chemical screening which could not give precise about chemical contents. Chemical content is the driving force for the medicinal value of the plant. This is why using such new techniques assure not only identity, purity of the plant but even the activity against the claimed diseases. It may also help in quality control against adulterant and act as a biochemical marker for these medicinally important plants in the pharmaceutical industry and plant systematic studies.
Applications
It can help to sensitize other groups of researches to adapt similar methodology in quality studies of other plants. It can be utilized even for microbial fermentation products and the presence of the analyte of interest. This method may be used for quality control and standardization of T. terrestris extracts and marketed herbal formulations.
Peer review
This is a valuable research work in which author have developed HPTLC fingerprint profile of anti-inflammatory active extract fractions of T. terrestris. The research represents advanced methodological qualitative analysis of complex matrix such as herbal medicine where only very limited information can be acquisted using traditional techniques. The manuscript is well written. It has been organized according to well documented researches. The results are compatible with what the methods may generate. The results were discussed scientifically according to the results obtained.
Footnotes
Foundation Project: Supported by the Deanship of Research in King Saud University (Grant No. RGP-VPP-150).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Lee HN, Surh YJ. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochem Pharmacol. 2012;84(10):1340–1350. doi: 10.1016/j.bcp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Marion-Letellier R, Savoye G, Beck PL, Panaccione R, Ghosh S. Polyunsaturated fatty acids in inflammatory bowel diseases: a reappraisal of effects and therapeutic approaches. Inflamm Bowel Dis. 2013;19(3):650–661. doi: 10.1097/MIB.0b013e3182810122. [DOI] [PubMed] [Google Scholar]
- 3.Kuritzky L, Samraj GP. Nonsteroidal anti-inflammatory drugs in the treatment of low back pain. J Pain Res. 2012;5:579–590. doi: 10.2147/JPR.S6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sudhir S, Budhiraja RD, Miglani GP, Arora B, Gupta IC, Gorg KN. Pharmacological studies on leaves of Withania somnifera. Planta Med. 1986;1:61–63. doi: 10.1055/s-2007-969072. [DOI] [PubMed] [Google Scholar]
- 5.Chreiner M, Huyskens-Keil S. Phytochemicals in fruit and vegetables: health promotion and postharvest elicitors. Crit Rev Plant Sci. 2006;25:267–278. [Google Scholar]
- 6.Kaul N, Agrawal H, Patil B, Kakad A, Dhaneshwar SR. Application of stability-indicating HPTLC method for quantitative determination of metadoxine in pharmaceutical dosage form. Farmaco. 2005;60:351–360. doi: 10.1016/j.farmac.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Alqasoumi SI, Alam P, Alrehaily AJ, Shakeel F, Abdel-Kader MS. Stability-indicating densitometric HPTLC method for qualitative and quantitative analysis of hydroquinone in commercial whitening creams. J Planar Chromat. 2011;24:48–52. [Google Scholar]
- 8.Biringanine G, Chiarelli MT, Faes M, Duez P. A validation protocol for the HPTLC standardization of herbal products: application to the determination of acteoside in leaves of Plantago palmata Hook. f.s. Talanta. 2006;69:418–424. doi: 10.1016/j.talanta.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Faisal MS, Naz Z, Shakeel F, Ahmed S, Kohli K, Khar RK. A new TLC densitometric method for stability assessment of modafinil. Chem Anal-Warsaw. 2009;54:77–88. [Google Scholar]
- 10.Alam P, Alqasoumi SI, Shakeel F, Abdel-Kader MS. HPTLC densitometric analysis of arbutin in bulk drug and methanolic extracts of Arctostaphylos uva-ursi. Nat Prod Res. 2011;25(17):1671–1675. doi: 10.1080/14786419.2010.529447. [DOI] [PubMed] [Google Scholar]
- 11.Ali B, Mujeeb M, Aeri V, Mir SR, Ahmad S, Siddique NA, et al. et al. High-performance thin layer chromatographic quantification of bioactive psoralen and daidzein in leaves of Ficus carica L. Nat Prod Res. 2011;25:1666–1670. doi: 10.1080/14786419.2010.529446. [DOI] [PubMed] [Google Scholar]
- 12.Harborne JB. Phytochemical methods. 3rd ed. London: Thomson Science; 1998. pp. 44–46. [Google Scholar]
- 13.Wagner H, Baldt S, Zgainski EM. Plant drug analysis. Berlin: Springer; 1996. p. 85. [Google Scholar]
- 14.Daniel WW. Biostatics: a foundation for analysis in health sciences. 6th ed. New York: Wiley; 1995. pp. 273–303. [Google Scholar]
- 15.Cannon KE, Leurs R, Hough LB. Activation of peripheral and spinal histamine H3 receptors inhibits formalin-induced inflammation and nociception, respectively. Pharmacol Biochem Behav. 2007;88(1):122–129. doi: 10.1016/j.pbb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamunadevi M, Wesely EG, Johnson M. Chromatographic finger print analysis of steroids in Aerva lanata L. by HPTLC technique. Asian Pac J Trop Biomed. 2011;1:428–433. doi: 10.1016/S2221-1691(11)60094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]