Abstract
Obesity is characterized by an increased recruitment of proinflammatory macrophages to the adipose tissue (AT), leading to systemic inflammation and metabolic disease. The pathogenesis of this AT inflammation, however, remains to be elucidated. The circulating adipokine leptin is increased in obesity and is involved in immune cell function and activation. In the present study, we investigated the role of leptin in the induction of obesity-associated inflammation. We generated radiation chimeric C57BL/6J mice reconstituted with either leptin receptor-deficient (db/db) or wild-type (WT) bone marrow and challenged them with a high-fat diet (HFD) for 16 weeks. Mice reconstituted with db/db bone marrow (WT/db), had significantly lower body weight and adiposity compared with mice with WT bone marrow (WT/WT). Gonadal AT in WT/db mice displayed a 2-fold lower expression of the inflammatory genes Tnfa, Il6, and Ccl2. In addition, gonadal fat of WT/db mice contained significantly fewer crown-like structures compared with WT/WT mice, and most of their AT macrophages expressed macrophage galactose-type C type lectin 1 (MGL1) and were C-C chemokine receptor type 2 (CCR2)-negative, indicative of an anti-inflammatory phenotype. Moreover, WT/db mice exhibited greater insulin sensitivity compared with WT/WT mice. These data show that disrupted leptin signaling in bone marrow-derived cells attenuates the proinflammatory conditions that mediate many of the metabolic complications that characterize obesity. Our findings establish a novel mechanism involved in the regulation of obesity-associated systemic inflammation and support the hypothesis that leptin is a proinflammatory cytokine.
Obesity is positively correlated with a plethora of inflammatory diseases and a higher susceptibility to infections (1). Therefore, investigating the link between obesity and immune system function is of utmost importance.
The close interplay between metabolic and inflammatory pathways is beneficial under physiological conditions but becomes deleterious with either nutrient scarcity or nutrient excess (2). Indeed, obesity is characterized by a state of low-grade inflammation (3) in which increased levels of inflammatory markers and leptin prevail in the expanding adipose tissue (AT) (4, 5). This chronic low-grade inflammation is believed to be the origin of most the obesity-associated diseases (2).
Macrophages have been identified as the major secretory cells in AT, contributing to systemic and local inflammation in obesity (6, 7). AT macrophage (ATM) recruitment is increased dramatically with the expansion of fat mass, comprising up to 50% of AT cells in the obese state (6). In addition to being more numerous in obese subjects, ATMs differ in localization, phenotype, and function between lean and obese individuals. In the lean state, resident (type II) macrophages display an anti-inflammatory phenotype with high gene expression of Il10, Mgl1 (macrophage galactose-type-C-type lectin 1), and Arg1 (8). Type II macrophages are mouse EGF-like module containing mucin-like hormone receptor-like 1 positive (F4/80+), cluster of differentiation molecule 11c negative (CD11c−), MGL1+ (CD301) (9), and dispersed in the interstitial space between adipocytes (10) where they are believed to play a role in preserving AT integrity and maintenance. Conversely, obesity-recruited (type I) macrophages display a proinflammatory phenotype (9), have a high level of expression of Tnfa, Il6 and Nos2 (11) transcripts, and are F4/80+, CD11c+, and MGL1− (8, 12). These cells form what is known as crown-like structures (CLSs) surrounding adipocytes and are involved in the pathogenesis of insulin resistance (13).
Although macrophage involvement in AT inflammation with obesity is well characterized, little is known about the mechanistic basis for ATM accumulation. Leptin is involved in both innate and adaptive immunity (14). The long leptin receptor isoform (LepRb), responsible for leptin's physiological activities (15), has been identified on most immune cells including monocytes and macrophages (16–19). Leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice exhibit less macrophage infiltration and inflammatory gene expression in AT, despite increased weight gain and adiposity (7). Moreover, obesity is accompanied by sustained elevated circulating concentrations of leptin, resulting in leptin resistance (20).
In the present study, we investigated whether leptin signaling in white blood cells was involved in the recruitment of proinflammatory ATMs in diet-induced obesity. We demonstrated that the absence of leptin-receptor signaling in white blood cells led to reduced weight gain and adiposity and improved insulin sensitivity in high-fat diet (HFD)-fed db/db bone marrow-transplanted wild-type (WT) mice compared with HFD-fed mice that received WT bone marrow transplants. Mice that received db/db bone marrow also exhibited decreased AT inflammation.
Materials and Methods
Animals
Five-week-old WT male C57BL/6J recipient mice, 10-week-old male WT, and db/db donor mice on the C57BL/6J background were purchased from The Jackson Laboratory. Mice were subjected to whole-body irradiation with a single 10-Gy dose from a 137Cs γ-source. Bone marrow cells were subsequently collected from WT and db/db mice by flushing long bones with sterile PBS containing 50 μg/mL gentamycin (Sigma-Aldrich). Recipient mice were each reconstituted with 2 × 107 WT (n = 8) or db/db (n = 8) bone marrow cells through retro-orbital iv injection. Four irradiated mice were not reconstituted and were used to confirm irradiation effectiveness by measuring circulating leukocytes. Reconstituted mice were housed in laminar-flow isolators and received acidified water ad lib supplemented with 100 mg/L neomycin and 10 mg/L polymyxin B sulfate 1 week before and 2 weeks after irradiation according to an established protocol (21). Reconstituted mice received an irradiated normal-fat diet (D12450B, 10% calories from fat; Research Diets Inc) for 6 weeks at the end of which reconstitution was confirmed by measuring circulating T and B cells by fluorescence analysis and comparing with nonirradiated controls. At that time, mice were moved to standard housing and were fed a HFD (D12492, 45% calories from fat; Research Diets Inc) for 16 weeks. Body weights and food intake were measured weekly. At study termination, mice were euthanized by isoflurane inhalation, and AT pads were dissected, weighed, and processed as described below. All animal procedures were performed with previous approval from the Institutional Animal Care and Use Committees at Kansas State University and the University of Kansas Medical Center.
Serum analysis
Insulin, leptin, monocyte chemoattractant protein (MCP)-1, IL-6, and TNF-α were measured in serum of fasted mice by fluorescent immunoassay using a Milliplex MAP kit (Millipore) at 0, 8, and 16 weeks of dietary treatment. Fasting blood glucose was measured at each blood withdrawal using a digital glucometer (Contour Blood Glucose Monitoring System; Bayer). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: fasting blood glucose (milligrams per deciliter) × fasting insulin (microunits per milliliter)/405 (22).
RNA isolation and gene expression quantification
AT RNA was extracted with the Trizol method. RNAs were reverse transcribed into cDNA with the RT2 First Strand cDNA Kit (SABiosciences). AT cDNA was used to measure transcripts of interest by real-time PCR using a customized SYBR Green real-time PCR detection superarray from SABiosciences. Sequences coding for Tnfa, Il6, Ccl2, Ccr2, Mgl1, and Lep were targeted by the assay. Actb (β-actin), used as the internal standard for normalization and reverse transcription, and genomic DNA were assessed for each sample as quality controls.
ATM sorting
Gonadal white AT (gWAT) was digested as previously described (23), and the stromal-vascular fraction (SVF) pellet was collected. Cells were blocked with 50% normal goat serum in PBS for 30 minutes at 4°C and incubated with F4/80 and CD11b antibodies for 1 hour at 4°C followed by 2 washes in PBS. ATMs were then sorted by coexpression of F4/80 and CD11b using a MoFLO XDP cell sorter (Becton Dickinson).
AT immunohistochemistry
Samples from gWAT were fixed in 10% formaldehyde for 24 hours before embedding in paraffin. Sections (10 μm thick) were cut and deparaffinized with xylene and decreasing concentrations of ethanol. Antigen retrieval was performed by immersing the slides in citrate buffer (10mM citric acid, 0.05% Tween 20 [pH 6.0]) for 30 minutes at 90°C to 95°C. Sections were stained overnight at 4°C with antimouse F4/80, MGL1, and C-C chemokine receptor type 2 (CCR2) antibodies (AbD Serotec). Sections were examined using a laser scanning confocal microscope (Zeiss LSM 5 PASCAL).
Statistical analysis
Data are displayed as mean ± SEM. Mean comparison analysis was performed using a simple t test. All analyses was performed using SPSS software. For all analyses, statistical significance was considered at P < .05.
Results and Discussion
Effect of bone marrow leptin receptor deficiency on body weight and body composition
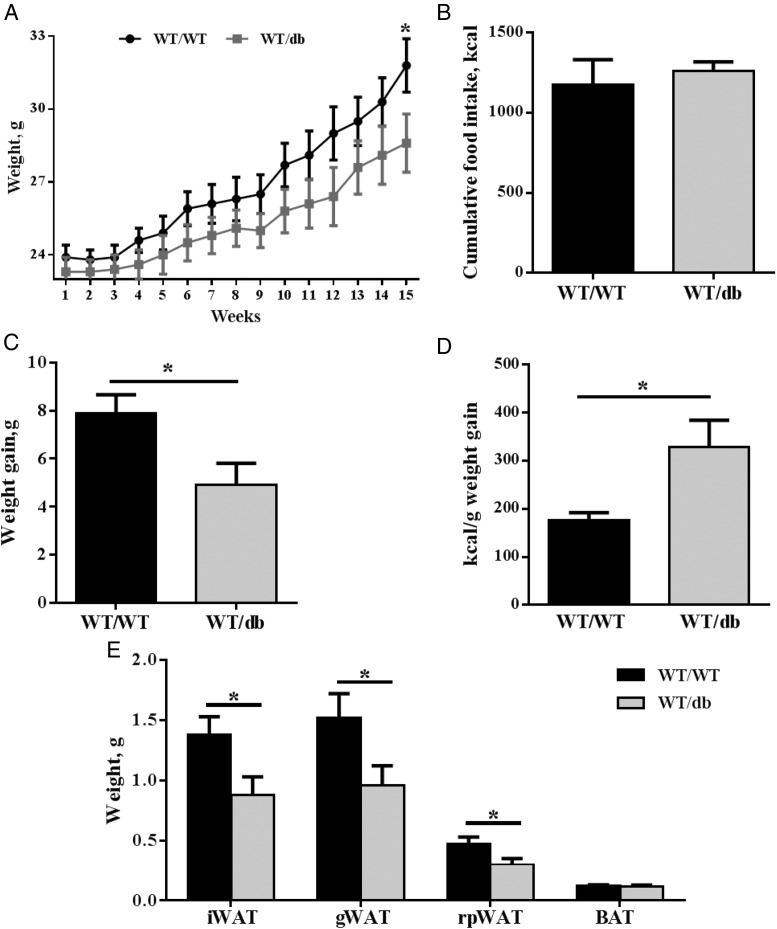
We generated bone marrow chimeric mice, reconstituted with either WT or db/db bone marrow, to investigate whether leptin signaling is involved in ATM recruitment and promotion of a proinflammatory condition in AT. We observed that WT/db mice experienced significantly less weight gain on HFD (Figure 1, A and B) and lower total adiposity (Figure 1C) compared with mice that received WT bone marrow. The lower adiposity in WT/db mice compared with WT/WT mice was driven by lower gWAT, inguinal WAT (iWAT), and retroperitoneal WAT mass in the former compared with the latter (Figure 1C). No difference was observed in brown AT. Both groups had equal food intake (Figure 1D). However, WT/db mice were considerably less energy-efficient than their WT/WT counterparts (Figure 1E). It is known that irradiation affects non–bone-marrow tissues, including AT (24, 25). Furthermore, our data show that WT mice reconstituted with db/db bone marrow have even greater resistance to HFD-induced weight gain and adiposity than irradiated WT mice (WT/WT) on HFD. Therefore, leptin signaling in bone marrow-derived cells must play a role in body weight regulation in response to HFD, independently of its known role in preventing weight gain and fat mass accumulation (20). It has been suggested that reducing inflammation in AT could prevent weight gain associated with HFD feeding (26, 27). However, attenuation of AT inflammation did not endow mice with the ability to resist weight gain and fatness in response to HFD (28–30). Therefore, reduced AT inflammation is unlikely to be the cause of the reduced body weight observed in WT/db mice. Although we did not investigate possible mechanisms underlying this surprising phenotype of HFD-fed WT/db mice, several factors might be involved. These include the inhibition of adipogenesis or differentiation of bone marrow-derived stem cells into adipocytes (31). In that regard, although WT/db mice had smaller gWAT, their average adipocyte size was larger than WT/WT (3.7 ± 1.2 vs 2.7 ± 0.6 μm2 [×103]) with fewer number of cells (25 ± 5 vs 32 ± 7 × 103 cells/cm2), suggesting a compromised adipogenesis. It is conceivable that leptin signaling may be involved in adipocyte recruitment into AT. Certainly, leptin and leptin receptor expression is vital for normal adipocyte commitment and maturation (32). It is also plausible that loss of bone density contributes to reduction in WT/db body weight gain compared with WT/WT mice. Our observation that WT/db mice experienced significantly less weight gain on HFD contrasts with the work of Gove et al (33). They found that chow-fed WT mice reconstituted with db/db or WT bone marrow exhibited similar body weight profiles 4 weeks after irradiation (33). In our study, WT/WT and WT/db mice on a normal-fat diet started showing a difference in body weight at 18 weeks after irradiation, although the difference was not significant (data not shown). At this point, we cannot rule out that the difference in body weight might have reached significance at later stages.
Figure 1.
Anthropometric and food intake of WT/WT and WT/db mice on an HFD. A–C, WT male mice reconstituted with leptin receptor-deficient (WT/db) bone marrow gained significantly less weight at the end of a 16-week HFD challenge (A), with a significantly different cumulative weight gain (B) and significantly lower ratio adipose tissue pads to body weight (C) compared with controls (WT/WT). D and E, This occurred with no difference in food intake (D) and significantly different energy efficiency between groups (E). *, P < .05. Abbreviations: BAT, brown AT; rpWAT, retroperitoneal WAT.
Proinflammatory macrophage recruitment to AT
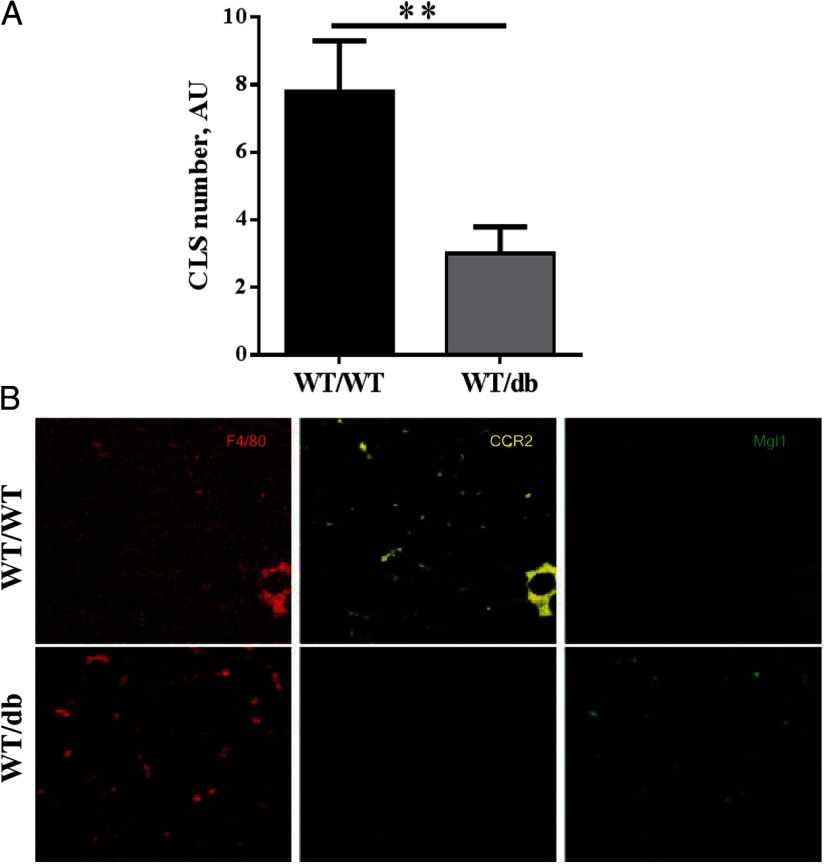
Obesity is associated with increased proinflammatory ATMs arranged in CLSs surrounding adipocytes. The high prevalence of CLS is highly correlated with AT inflammation and metabolic disorder (13). We observed significantly fewer CLSs in gWAT from WT/db HFD-fed mice compared with WT/WT HFD-fed mice (Figure 2A). Leptin induces mitogenesis (34), and to rule out that the decrease in CLS numbers was not secondary to an inhibition of mitosis, we measured the amount of macrophages in the SVF of both WT/WT and WT/db mice by fluorescence-activated cell sorting. Both WT/WT and WT/db groups had similar total macrophage infiltration in their gWAT as measured by the percentage F4/80+/CD11b+ cells in the SVF (33% and 10% of all SVF cells in gWAT and iWAT, respectively, in both groups, P > .05). However, although most ATMs in WT/WT mice were CCR2+, most ATMs in WT/db mice were MGL1+/CCR2− (Figure 2B). Anti-inflammatory macrophages secrete catecholamines inducing uncoupling protein 1 (UCP1) in AT (35). We report a prevalence of anti-inflammatory ATMs in WT/db mice, which might induce an increase in UCP1 and can explain the difference in body and fat mass weight. Although we did not measure UCP1, we believe that exploring this hypothesis is warranted in the future. Both the presence of CLSs and the expression of CCR2+ ATMs correlate with AT inflammation (8, 36). Moreover, leptin signaling deficiency in HFD-fed WT mice reconstituted with leptin receptor-null bone marrow resulted in greater than 2-fold reduction in the expression transcript levels for Il6 and Ccl2 in gonadal fat and almost 2-fold for Tnfa (Table 1), indicative of a proinflammatory environment. Conversely, the increased prevalence of MGL1+ cells in WT/db AT compared with WT/WT AT (Figure 2B) is indicative of an anti-inflammatory phenotype of infiltrated macrophages. Although more than 80% of AT macrophages in gonadal and sc fat from lean mice are MGL1+, this value falls to 50% in obese mice, thus serving as a key determinant of pro- vs anti-inflammatory macrophage properties (9). We know that leptin is a potent chemoattractant for monocytes/macrophages in vitro (37, 38) and induces a proinflammatory phenotype in T cells (39) and primes macrophages to secrete inflammatory cytokines under septic conditions (40). However, at this point, we cannot rule out that the difference in AT inflammation is secondary to reduced weight gain in WT/db mice. Future studies examining more detailed kinetic changes are warranted to address this issue. Nevertheless, our findings for the first time implicate leptin in the phenotypic switch of ATMs from anti-inflammatory (M2) to proinflammatory (M1) in vivo.
Figure 2.
Adipose tissue inflammation. A, Mice with db/db bone marrow (WT/db) had a significantly lower prevalence of CLSs in their gonadal AT compared with WT/WT mice after a 16-week HFD challenge. B, Immunohistochemistry of gWAT labeled with F4/80, CCR2, and MGL1 antibodies shows a prevalence of MGL1+/CCR2− ATMs in WT/db mice, indicative of an anti-inflammatory phenotype as compared with a more CCR2+ ATMs in WT/WT mice. **, P < .01. Abbreviation: AU, arbitrary unit.
Table 1.
Relative mRNA Abundance of Select Adipokinesa
Results show the qRT-PCR–based differences in mRNA abundance of select adipokines vs control (Actb) in gonadal fat pad adipocytes in WT/db compared with WT/WT mice after 16 weeks of HFD feeding. Lep is the leptin gene, and Actb is the β-actin gene.
P < .05.
Effect of bone marrow leptin receptor deficiency on insulin sensitivity
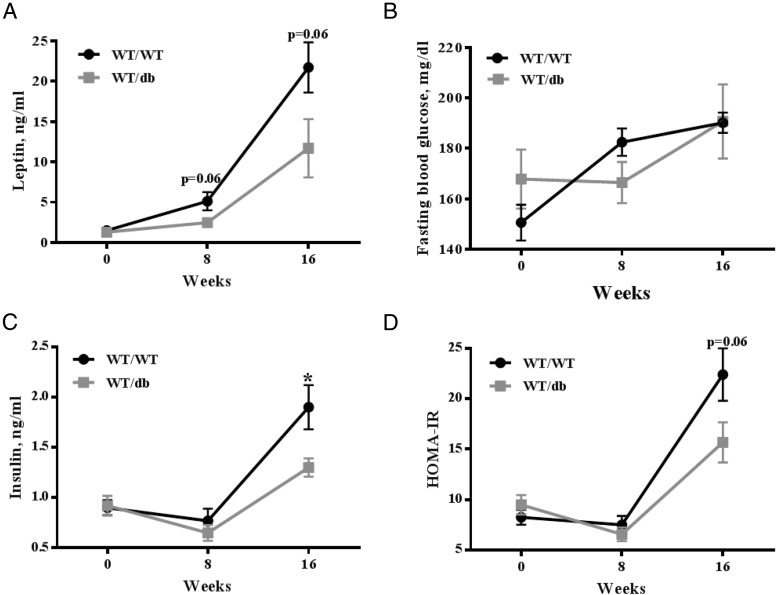
We measured fasting serum levels of TNF-α, IL-6, MCP-1, leptin, insulin, and glucose in all mice at 0, 8, and 16 weeks of the study. No differences were observed in IL-6, TNF-α, or MCP-1 serum levels between the 2 experimental groups (data not shown). Paralleling fat mass data, leptin serum levels were lower in the WT/db mice compared with WT/WT with a borderline statistically significant difference between groups (P = .06, Figure 3A). Fasting glucose, insulin, and HOMA-IR index were measured to assess glucose metabolism. HOMA-IR is a predictor for insulin resistance in rodents (22). Fasting blood glucose was not different between the WT/WT and WT/db mice (Figure 3B). Both insulin and HOMA were lower in WT/db mice compared with WT/WT with insulin levels being statistically lower (P < .05) and HOMA-IR approaching statistical significance (P = .06) (Figure 3, C and D). These data suggest that WT HFD-fed mice reconstituted with db/db bone marrow might be more insulin-sensitive compared with HFD-fed WT mice reconstituted with WT bone marrow. Although we used HOMA-IR to assess insulin resistance in this study, future studies measuring glucose tolerance and insulin tolerance are warranted to more conclusively assess glucose homeostasis in this model. It is probable that reduced AT inflammation in WT/db mice is the causal factor underlying the improved glucose homeostasis in these mice. The proinflammatory cytokine TNF-α, which in our study was close to 2-fold lower (mRNA) in WT/db AT compared with that of WT/WT mice (Table 1), directly contributes to insulin resistance through suppression of glucose transporter GLUT4 gene expression (41) and induction of serine phosphorylation of insulin receptor substrate 1 (42). The elevation of proinflammatory cytokines such as TNF-α can also inhibit insulin signaling by activating Jun N-terminal kinase 1 (JNK1) (43). CCL2 mRNA, which was reduced in AT of WT/db mice compared with WT/WT mice, is also directly involved in insulin function. CCL2 induces insulin resistance on adipocytes in an autocrine fashion by decreasing insulin-stimulated glucose uptake (28, 44). Our findings of reduced proinflammatory cytokine transcripts (Table 1) and reduced insulin levels (Figure 3B) in WT/db mice on an HFD support a role for leptin signaling in bone marrow-derived macrophage infiltration in obesity-associated insulin resistance. Many studies investigating ATM involvement in obesity-associated insulin resistance have used HFDs with a higher fat content (60%) (28, 45) than the diet used (45% calories from fat) in this study. Perhaps the use of a more aggressive dietary approach would reveal more pronounced differences in the proinflammatory response to HFD between WT/WT and WT/db mice.
Figure 3.
Fasting serum levels of leptin and insulin and measures of HOMA-IR at 0, 8, and 16 weeks of HFD treatment. A–D, Mice reconstituted with leptin receptor-deficient bone marrow (WT/db) had lower serum leptin (A) (P = .06), fasting blood glucose (B) (P > .05), serum insulin (C) (P < .05), and HOMA-IR (D) (P = .06) compared with WT/WT mice.
In summary, the data presented support the hypothesis that leptin signaling in bone marrow-derived cells is important for the development of the AT inflammation that accompanies obesity and possibly the pathogenesis of insulin resistance.
Acknowledgments
We thank Dr Bruce Kimler at Kansas University Medical Center for generously performing the irradiation procedures.
This project was supported by American Heart Association Grant 0950036G; National Institutes of Health Grants AI061691, AI088070, RR17708, and P20 RR017686; NASA Grant NNX08BA91G; and The Kansas State Agricultural Experiment Station (Publication 11-359-J).
Disclosure Summary: The authors report no conflict of interest.
For article see page 12
- AT
- adipose tissue
- ATM
- AT macrophage
- CLS
- crown-like structure
- CCR2
- C-C chemokine receptor type 2
- CD11c
- cluster of differentiation molecule 11c
- F4/80
- mouse EGF-like module-containing mucin-like hormone receptor-like 1
- gWAT
- gonadal white AT
- HFD
- high-fat diet
- HOMA-IR
- homeostasis model assessment of insulin resistance
- iWAT
- inguinal WAT
- MCP
- monocyte chemoattractant protein
- Mgl1
- macrophage galactose-type-C-type lectin 1
- SVF
- stromal-vascular fraction
- UCP1
- uncoupling protein 1
- WT
- wild-type.
References
- 1. Falagas ME, Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–446 [DOI] [PubMed] [Google Scholar]
- 2. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7 [DOI] [PubMed] [Google Scholar]
- 4. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S [DOI] [PubMed] [Google Scholar]
- 5. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783 [DOI] [PubMed] [Google Scholar]
- 6. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 11. Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23 [DOI] [PubMed] [Google Scholar]
- 12. Westcott DJ, Delproposto JB, Geletka LM, et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206:3143–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aouadi M, Tencerova M, Vangala P, et al. Gene silencing in adipose tissue macrophages regulates whole-body metabolism in obese mice. Proc Natl Acad Sci U S A. 2013;110:8278–8283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379 [DOI] [PubMed] [Google Scholar]
- 15. Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271 [DOI] [PubMed] [Google Scholar]
- 16. Batra A, Okur B, Glauben R, et al. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology. 2010;151:56–62 [DOI] [PubMed] [Google Scholar]
- 17. Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J Immunol. 2006;176:7745–7752 [DOI] [PubMed] [Google Scholar]
- 18. Raso GM, Pacilio M, Esposito E, Coppola A, Di Carlo R, Meli R. Leptin potentiates IFN-γ-induced expression of nitric oxide synthase and cyclo-oxygenase-2 in murine macrophage J774A.1. Br J Pharmacol. 2002;137:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828 [DOI] [PubMed] [Google Scholar]
- 20. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295 [DOI] [PubMed] [Google Scholar]
- 21. Lim WS, Timmins JM, Seimon TA, et al. Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee S, Muniyappa R, Yan X, et al. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab. 2008;294:E261–E270 [DOI] [PubMed] [Google Scholar]
- 23. Ortega MT, Xie L, Mora S, Chapes SK. Evaluation of macrophage plasticity in brown and white adipose tissue. Cell Immunol. 2011;271:124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ablamunits V, Weisberg SP, Lemieux JE, Combs TP, Klebanov S. Reduced adiposity in ob/ob mice following total body irradiation and bone marrow transplantation. Obesity. 2007;15:1419–1429 [DOI] [PubMed] [Google Scholar]
- 25. Poglio S, Galvani S, Bour S, et al. Adipose tissue sensitivity to radiation exposure. Am J Pathol. 2009;174:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chiang SH, Bazuine M, Lumeng CN, et al. The protein kinase IKKϵ regulates energy balance in obese mice. Cell. 2009;138:961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity. 2008;16:1248–1255 [DOI] [PubMed] [Google Scholar]
- 28. Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lesniewski LA, Hosch SE, Neels JG, et al. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat Med. 2007;13:455–462 [DOI] [PubMed] [Google Scholar]
- 31. Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hwang CS, Loftus TM, Mandrup S, Lane MD. Adipocyte differentiation and leptin expression. Annu Rev Cell Dev Biol. 1997;13:231–259 [DOI] [PubMed] [Google Scholar]
- 33. Gove ME, Sherry CL, Pini M, Fantuzzi G. Generation of leptin receptor bone marrow chimeras: recovery from irradiation, immune cellularity, cytokine expression, and metabolic parameters. Obesity. 2010;18:2274–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takahashi Y, Okimura Y, Mizuno I, et al. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem. 1997;272:12897–12900 [DOI] [PubMed] [Google Scholar]
- 35. Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–1568 [DOI] [PubMed] [Google Scholar]
- 37. Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am J Physiol Cell Physiol. 2007;293:C1481–C1488 [DOI] [PubMed] [Google Scholar]
- 38. Curat CA, Miranville A, Sengenès C, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292 [DOI] [PubMed] [Google Scholar]
- 39. Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901 [DOI] [PubMed] [Google Scholar]
- 40. Shapiro NI, Khankin EV, Van Meurs M, et al. Leptin exacerbates sepsis-mediated morbidity and mortality. J Immunol. 2010;185:517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie L, Ortega MT, Mora S, Chapes SK. Interactive changes between macrophages and adipocytes. Clin Vaccine Immunol. 2010;17:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance. Science. 1996;271:665–668 [DOI] [PubMed] [Google Scholar]
- 43. Han MS, Jung DY, Morel C, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A. 2003;100:7265–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang H, Youm YH, Vandanmagsar B, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]