Abstract
Globally, an estimated 13 million preterm babies are born each year. These babies are at increased risk of infant mortality and life-long health complications. Interventions to prevent preterm birth (PTB) require an understanding of processes driving parturition. Prostaglandins (PGs) have diverse functions in parturition, including regulation of uterine contractility and tissue remodeling. Our studies on cervical remodeling in mice suggest that although local synthesis of PGs are not increased in term ripening, transcripts encoding PG-endoperoxide synthase 2 (Ptgs2) are induced in lipopolysaccharide (LPS)-mediated premature ripening. This study provides evidence for two distinct pathways of cervical ripening: one dependent on PGs derived from paracrine or endocrine sources and the other independent of PG actions. Cervical PG levels are increased in LPS-treated mice, a model of infection-mediated PTB, consistent with increases in PG synthesizing enzymes and reduction in PG-metabolizing enzymes. Administration of SC-236, a PTGS2 inhibitor, along with LPS attenuated cervical softening, consistent with the essential role of PGs in LPS-induced ripening. In contrast, during term and preterm ripening mediated by the antiprogestin, mifepristone, cervical PG levels, and expression of PG synthetic and catabolic enzymes did not change in a manner that supports a role for PGs. These findings in mice, supported by correlative studies in women, suggest PGs do not regulate all aspects of the parturition process. Additionally, it suggests a need to refocus current strategies toward developing therapies for the prevention of PTB that target early, pathway-specific processes rather than focusing on common late end point mediators of PTB.
Preterm births (PTBs) occur globally at an alarming rate of 13 million babies per year, resulting in increased infant mortality in the first year of life and increased risk of life-long health complications in infants that survive (1). Although some risk factors such as infection, a previous preterm birth, environmental stressors (eg, smoking), and a premature decline in cervical length have been defined as causal, approximately half of the PTBs are of unknown etiology. Improved understanding of the mechanisms that drive term and preterm birth is essential for better prevention of premature birth.
Prostaglandins (PGs) play important roles in female reproduction including ovulation, implantation, luteolysis, and parturition, and the process of labor. Prostaglandin-cyclooxygenase-endoperoxide synthase (PTGS) 1 and PTGS2 synthesize PGH2 from arachidonic acid liberated from membrane phospholipids. Specific synthases convert PGH2 to other PGs and thromboxane (TXB; PGE2, PGF2a, PGD2, 6-KPGF1, and TXB2), whereas 15-hydroxyprostaglandin dehydrogenase (PGDH) is responsible for PG catabolism. Although PTGS1 and PTGS2 have the same function, their cell-specific expression and regulation differs markedly. In general, PTGS1 is a constitutively expressed enzyme, whereas PTGS2 is induced with inflammation or injury (2).
Parturition requires the initiation of uterine contractions, transformation of the cervix from a rigid and closed to soft and open structure (termed cervical ripening), and rupture of the fetal membranes. Although the essential requirement for PGs has been conclusively demonstrated for some of these processes during normal labor, it is not known whether PGs are required for initiation of parturition or whether PG increases are a secondary sequelae of the inflammatory processes that occur during labor (3, 4). The efficacy and widespread administration of exogenous PG to women undergoing planned induction of labor support a physiological role of PGs in cervical ripening (5). In addition, PGs act directly in the uterus during labor to promote coordinated contractions, and PG levels are increased in the amniotic fluid of numerous species during labor (6, 7). Furthermore, PGs derived from uterine PTGS1 are critical for the initiation of luteolysis, the process by which ovarian synthesis of the pregnancy-supporting hormone, progesterone, is blocked to allow the onset of labor in mice (8, 9). Although PGs are clearly capable of inducing cervical ripening, the necessity of PGs in this process is unclear and confounded by conflicting data. In vitro studies report expression of PG synthesis enzymes and PG receptors in human cervical tissue and cervical cells (10–12). However, endogenous levels of PGs in human cervical mucus do not increase in the last trimester (13–15), and inhibition of PG synthesis does not prevent antiprogestin induced cervical ripening in women, guinea pigs, or rats (16–18). Cervical exposure to high levels of PGs in seminal fluid does not result in premature cervical ripening, suggesting that PGs alone are insufficient to initiate cervical ripening (19).
In contrast to term labor, PG synthesis by PTGS2 is a hallmark of infection-mediated preterm birth. The observation that administration of PTGS2 inhibitors blocks infection-mediated PTB in mice and nonhuman primates supports the role of PGs in the pathway of infection-mediated PTB (20–22). Although critical to infection-mediated preterm labor, studies have not addressed whether PGs are necessary in the cervix for either term or infection-mediated premature cervical ripening. Studies from our group have determined that the mechanisms of cervical ripening in intrauterine LPS-mediated preterm birth are distinct from term ripening and from premature ripening induced by the progesterone receptor antagonist, mifepristone (23). Although there are numerous differences between term and preterm ripening, one key difference is the expression pattern of genes encoding PG-cyclooxygenase-endoperoxide synthases. Specifically, lipopolysaccharide (LPS)-mediated cervical ripening is characterized by an increase in expression of Ptgs2 mRNA, with no appreciable change in Ptgs1 mRNA (23).
Given that infection is a leading cause of preterm birth, defining the mechanisms driving this specific pathway is critical. With this goal in mind, the focus of the current study was to elucidate the distinct pathways that mediate term and preterm cervical ripening in the mouse; in particular, we investigated the contribution of PGs to physiological and premature cervical ripening. We provide evidence that neither PG levels nor PG synthesizing enzymes are increased in term or mifepristone-mediated preterm cervical ripening. In contrast, LPS-mediated premature ripening is dependent on a PTGS2-driven increase in local PG synthesis that results in increased PG levels but is not dependent on a marked decline in the progesterone to estrogen ratio as occurs during term ripening.
Materials and Methods
Mice
Virgin Bl6/129 SvEv (University of Texas Southwestern Medical Center, Dallas, TX) and National Institutes of Health (NIH) Swiss (Harlan Laboratories) female mice (3–6 months old) were caged with fertile males of the same strain for 6 hours, and the presence of a vaginal plug at the end of the 6-hour period indicated day 0 of pregnancy. To ensure a sufficient number of animals for flow cytometry, NIH Swiss mice were used for studies. We have previously described similar changes in cervical immune cell populations during pregnancy in three strains of mice: Bl6/129SvEv, NIH Swiss, and ICR (CD-1) (24). All studies were conducted on approval by the University of Texas Southwestern Medical Center Institutional Animal Care and Research Advisory Committee.
Tissue collection
Cervices were isolated by dissection at the uterocervical junction (caudal to the uterine bifurcation), and all vaginal tissue was removed before freezing in liquid nitrogen. Cervices for immunohistochemical analysis were fixed in 4% paraformaldehyde (Sigma Aldrich) and paraffin embedded. Sections were cut in the longitudinal plane.
Preterm labor models
In the current study, three models of PTB were studied. These included intrauterine application of LPS (IU-LPS), ip application of LPS (IP-LPS), and sc injection of the antiprogestin, mifepristone. The rationale for timing of administration and dose in all three models was to achieve preterm birth between midday to evening on gestational day 15. Intrauterine infection (IU-LPS)-induced and noninfection (mifepristone)-induced preterm labor models were carried out as previously described (23). With the exception of Supplemental Figure 1, tissue collection was carried out after 6 hours in IU-LPS-treated mice and 12 hours in mifepristone-treated mice. In studies described in Supplemental Figure 1, tissues were collected at 3, 6, and 12 hours after the injection in the mifepristone-treated mice and at 1, 2, 3, and 6 hours in the IU-LPS treated mice. In the ip infection-induced preterm birth (IP-LPS) model, injections of 100 μg of LPS (O55:B5; Sigma) in sterile PBS were performed as described previously (20). For the PTGS2 inhibitor study, 300 μg of SC-236 (Sigma) with 1% Tween 80 in PBS was given via gavage an hour before IP-LPS injections and 4 hours after the injections. Tissues were collected 12 hours after the injection. In the SC-236 + IP-LPS studies in which mice were observed for preterm labor, another oral dose of SC-236 was administered 16 hours after the LPS injection to the animals that had not delivered by then. These animals were observed 48 hours after the injection for PTB. To evaluate the effect of SC-236 on term labor, mice were given SC-236 by oral gavage at 2:30 and 7:30 pm on gestational day 18 and observed for delivery at term on day 19. To evaluate the effect of SC-236 on mifepristone-induced preterm labor, mice were given SC-236 1 hour before the mifepristone injection and 4 and 10 hours after the injection. These animals were observed 24 hours after the injection for PTB.
Misoprostol administration
Ten micrograms of misoprostol (Teva) was inserted vaginally into mice anesthetized with Avertin. A 1.9-mm endoscope (Karl Storz) was used to ensure the tablet was up against the cervix. The mice were placed on a warmed surface until they were awake. The animals were killed after 6 hours and tissues collected.
Quantification of PGs in tissue samples
PGs were measured at the Eicosanoid Core Laboratory at Vanderbilt University by gas chromatography/mass spectrometry analysis as described in the Supplemental Methods. The interday variability for each assay was less than 10%. The precision for each assay was ± 5%, whereas the accuracy for each assay was 95%. Gas chromatography/mass spectrometry requires 100 mg of tissue for each measurement. Because a pregnant mouse cervix ranges in weight from 10 to 30 mg, for each sample, multiple cervices were pooled to have a sufficient material (n = 3 pooled samples per time point or treatment).
Flow cytometry
Cervical cells were dispersed and stained using methods described previously (10). Fluorescently conjugated monoclonal antibodies used included neutrophil (Neu) 7/4-PE (Serotec), CD45-PE-Cy7 (eBiosciences), and Gr-1 (Ly6C/Ly6G)-allophycocyanin-Cy7 (eBiosciences). Stained cells were run on a LSRII flow cytometer using BD FACSDiva (BD Biosciences) software and analyzed with FloJo 7.1 analysis software (Tree Star Inc).
Biomechanics
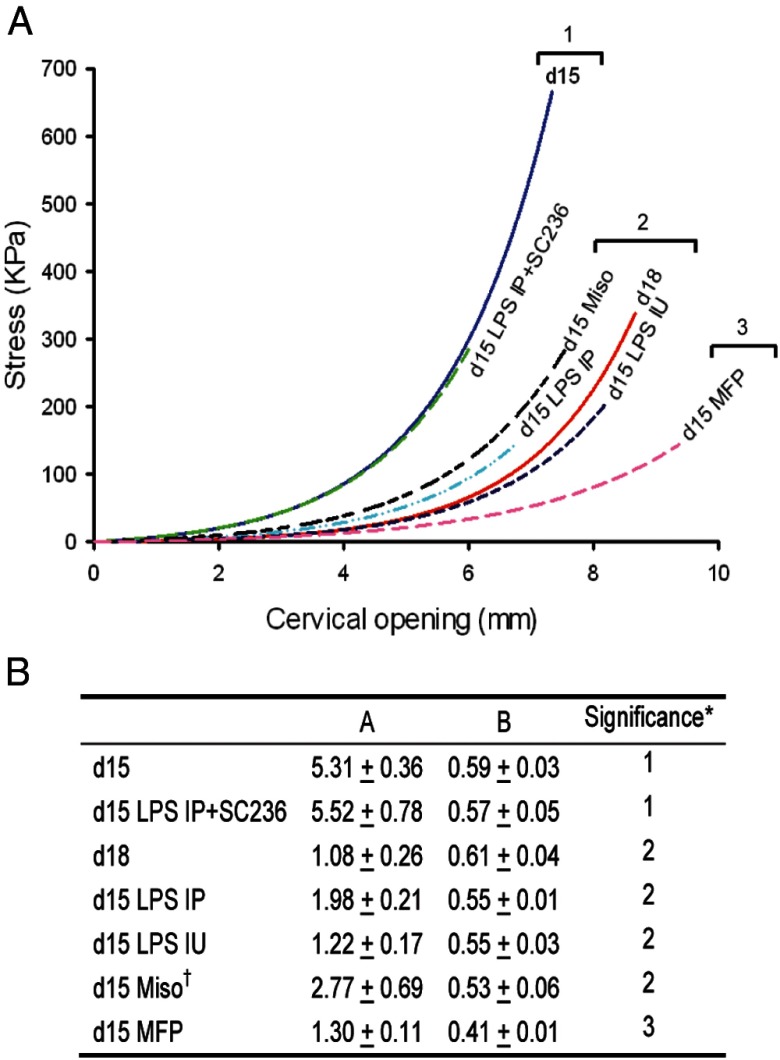
Biomechanical properties of cervical specimens were evaluated by extending the cervical canal along the circumferential direction, at a 0.1 mm/sec rate up to failure. The specimens were equilibrated in PBS at 37°C and mounted with braided silk sutures. A universal material testing machine (Zwick Z2.5/TS1S) with a 20-N load cell was used to collect load-displacement data. The cross-sectional area, measured using a stage micrometer in a dissecting microscope, was used to normalize the applied stretching force and obtain the nominal stress level in the tissue. These data are displayed as exponential curve fits to the measurements of nominal stress vs cervical opening for each study group with corresponding model parameters in the adjacent table (see Figure 7).
Figure 7.
Prostaglandins are sufficient to promote cervical ripening and are necessary for LPS-induced premature cervical ripening. A, Biomechanical testing of cervices obtained at gestational day 15 (d15), day 18 (d18), day 15 mifepristone (MFP), day 15 6-hour IU-LPS (IU LPS), day 15 12-hour IP-LPS (IP LPS), day 15 12-hour IP-LPS + SC-236, and day 15 6-hour misoprostol (Miso) [n = 17 (d15); n = 6 (d18); n = 5 (MFP); n = 9 (IU LPS); n = 3 (IP LPS); n = 5 (IP LPS+SC-236); and n = 5 (d15 misoprostol)]. Groups within a single bracket are not significantly different from each other, whereas each bracketed group is significantly different (P ≤ .05) from every other bracketed group. B, Table showing coefficient estimates (mean ± SEM) for all groups in panel A. *, Rows with the same numeric indicator are not judged statistically different.
For each specimen, load-displacement data were recorded for continued increases in cervical opening until complete tissue failure (tearing). Failure initiation for each data curve was identified as the first slope discontinuity or partial load drop. Data analysis was restricted to data collected before failure initiation. Because the cervical opening observations are not the same for each mouse (particularly the extent of cervical opening achievable before failure initiation), the cervical opening was treated as a random effect. The observations were repeated observations within a mouse with the mice being a random effect as well. The mouse time points/treatments were considered fixed effects. Because cervical opening directly relates to tissue strain, the curve for the nominal stress vs cervical opening for each specimen can be interpreted as an approximate stress-strain curve for the tissue. Similar to the cervical tissue compliance model of Myers et al (25), the stress to cervical opening curve for each mouse was assumed to follow an exponential curve:
where A and B are estimated coefficients. The parameter A is a measure of the tissue stiffness at small deformations, whereas the parameter B relates to the tissue nonlinear resistance to large extensions. Both parameters are inversely related to the tissue compliance. Estimates and variance components were prepared using nonlinear mixed effects ANOVA with observations repeated within mice. Time points/treatments are compared using a linear contrast of the bivariate estimated observation Ai and Bi. A Bonferroni correction is used to adjust the P value for the pairwise comparisons between mouse time points/treatments groups to obtain an overall significance level of P < .05.
Steroid hormone measurements
Blood was collected by cardiac puncture or by submandibular bleed and plasma collected. Estradiol levels were measured by ELISA (Calbiotech; catalog number ES180S-100). Progesterone levels were measured by liquid chromatography and mass spectrometry analysis as described in the Supplemental Methods.
Results
Prostaglandin metabolism is up-regulated in infection-mediated preterm ripening but not during term or during preterm ripening initiated by mifepristone
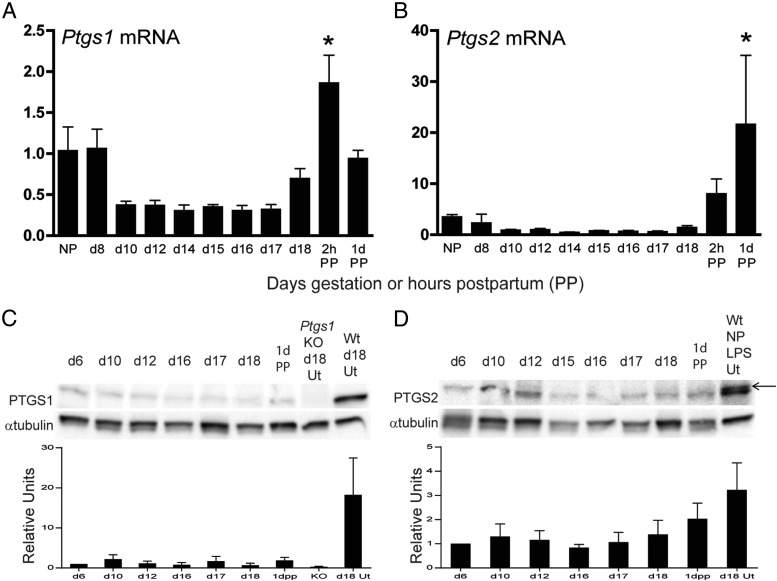
The expression of PTGS1 and PTGS2 at the transcriptional and protein level was evaluated in the mouse cervix through pregnancy and shortly after birth [postpartum (PP)]. Cervical Ptgs1 and Ptgs2 transcripts had similar patterns of expression during gestation and PP (Figure 1, A and B). Compared with the nonpregnant (NP) cervix, mRNA expression of both Ptgs1 and Ptgs2 remained low during most of pregnancy and increased PP. Similarly, protein expression was low to absent through gestation and postpartum (Figure 1, C and D). In contrast to term ripening, we have previously reported that preterm cervical ripening is associated with up-regulation of transcripts encoding Ptgs1 and Ptgs2 mRNA when induced on gestation day 15 by mifepristone (12 h treatment) or IU-LPS (6 h), respectively (23). As illustrated in Supplemental Figure 1, the increase in Ptgs2 transcripts occurred very rapidly in response to LPS treatment, whereas Ptgs1 gene induction occurred more slowly after mifepristone treatment. Ptgs1 expression was not significantly increased until 12 hours after the mifepristone injection while LPS-induced Ptgs2 expression was increased 30-fold by 2 hours after the LPS injection. In addition, the Ptgs1 expression is not induced with LPS treatment, whereas Ptgs2 expression is induced by after 12 hours of mifepristone treatment.
Figure 1.
Cervical expression for PTGS1 and PTGS2 mRNA and protein remains low to undetectable throughout pregnancy. The mRNA expression of Ptgs1 (A) and Ptgs2 (B) in the nonpregnant (NP), gestational days 8–18. and postpartum (PP) cervix. Data indicate average ± SEM (n = 4–6). *, Significance (P ≤ .05) compared with gestational day 18. C, Protein expression of PTGS1 at time points between gestational days 6–18 and 1 day postpartum. Gestational day 18 uterus from Ptgs1 knockout (KO) mice is a negative control, whereas the wild-type (WT) day 18 uterus serves as a positive control. D, Protein expression of PTGS2 at time points between gestational days 6–18 and 1 day postpartum. As a positive control, LPS-treated uterine tissue from a NP WT mouse was evaluated. Anti-PTGS2 recognizes two closely migrating bands. The larger-size band at 75 kDa is the specific band because it is not present in the Ptgs2 KO cervix (see Figure 2A). Anti-α-tubulin is the loading control. These Western blots are a representative image from one of three experiments. Densitometric quantitation of three protein blots is indicated in panel C and panel D. The positive control was not included in the statistical analysis. *, Significance (P ≤ .05) compared with gestational day 18.
Cervical PTGS1 and PTGS2 protein expression was also analyzed in the two preterm birth models (Figure 2A). Uterine tissues were used as positive controls because PTGS1 protein expression is induced in the uterus on gestational day 18 and PTGS2 expression is induced in the uteri of LPS-treated mice (20, 26). Cervical PTGS1 protein expression remained low to undetectable after either mifepristone or IU-LPS treatment but was readily detectable in gestational day 18 uterus as previously described (26). The lack of PTGS1 protein in the mifepristone-treated cervices is not in agreement with the observed increase in mRNA expression (23) (Supplemental Figure 1). In agreement with the pattern of transcripts, there was a significant increase in PTGS2 protein expression in the IU-LPS preterm ripening model (Figure 2A). PTGS2 was undetectable in cervices from mifepristone-treated mice.
Figure 2.
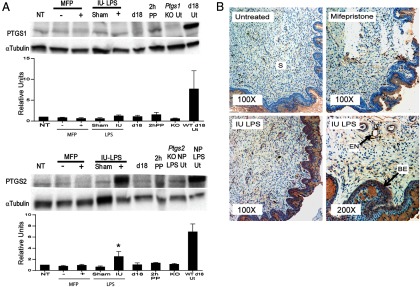
Cervical PTGS2 but not PTGS1 protein expression is induced in LPS-mediated cervical ripening. A, From left to right, cervical protein from gestational day 15 (NT), gestational day 15 ± mifepristone (MFP), day 15 ± IU-LPS, gestational day 18, and 2 hours PP. As a negative and positive control, respectively, in the upper panel, uterine protein (Ut) from nonpregnant (NP) Ptgs1 null (Ptgs1 KO Ut) and gestational day 18 wild-type (d18 Ut), and lower panel, uterine protein from Ptgs2 null mice treated with LPS (Ptgs2 KO NP LPS Ut) and LPS-treated wild-type mice (NP LPS Ut). α-Tubulin is the loading control. Blots are representative of three experiments each. Densitometric quantitation of three protein blots is indicated below each blot. *, Significance (P ≤ .05) compared with NT. The ± controls were not included in the statistical analysis. B, Immunohistochemical detection of PTGS2-expressing cells in cervical sections collected at gestational day 15 (untreated), day 15 mifepristone, day 15 IU-LPS at a ×100 magnification and day 15 IU-LPS at a ×200 magnification. Positive staining is indicated by a brownish red stain. Stroma (S), basal epithelia (BE), and endothelial cells surrounding the blood vessels (EN) are shown. Each image is representative of three animals for each treatment.
Immunohistochemistry was used to localize the site of PTGS2 induction in cervical tissue from the IU-LPS-treated mice as compared with day 15 untreated and day 15 mifepristone-treated mice (Figure 2B). Little to no PTGS2 expression was evident in the day 15 untreated cervices, whereas some PTGS2-positive cells were present in the stroma of mifepristone treated cervices, although the degree of PTGS2 induction was insufficient for detection by Western blot. PTGS2 staining was observed in the cervical stroma and epithelium of IU-LPS-treated mice. Higher magnification (×200) of IU-LPS-treated cervices identified PTGS2 expression along the endothelium lining blood vessels and in granulocytes within the blood vessels. Staining was also observed in stromal fibroblasts with strong staining in the basal epithelium. Thus, both cervical cells and infiltrating leukocytes contribute to the cervical PG pool in infection-mediated PTB.
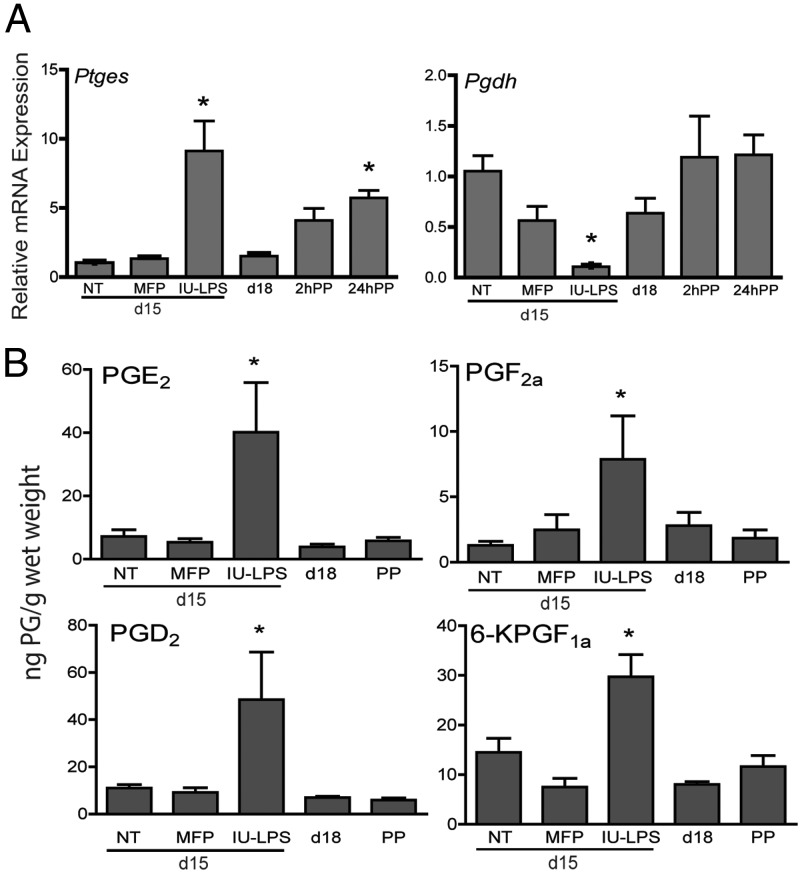
Prostaglandin E synthase (PTGES) catalyzes the generation of PGE2 from PGH2. PGDH converts most PGs into biologically inactive compounds. To assess changes in enzymes downstream of PTGS, transcripts encoding these two enzymes were evaluated using quantitative PCR (Figure 3A). Cervical Ptges expression was not increased during term ripening on day 18 or after mifepristone-mediated preterm ripening but was up-regulated 5-fold postpartum. Cervical Ptges2 was also induced 8-fold in mice treated with LPS as compared with gestation-matched untreated controls. Cervical Pgdh expression remained steady through pregnancy and postpartum; however, it was significantly decreased in LPS-treated mice. These data indicate that in LPS-mediated PTB, changes in both PG synthesis and degradative enzymes are coordinated to promote increased tissue PG levels.
Figure 3.
LPS treatment promotes prostaglandin synthesis. A, Cervical mRNA expression of prostaglandin E synthase (Ptges) is increased and 15-hydroxyprostaglandin dehydrogenase (Pgdh) is decreased in LPS-mediated ripening but not term or mifepristone-mediated ripening. Gestational day 15, no treatment (NT), day 15 mifepristone (MFP), day 15 IU-LPS, gestational day 18 (d18), 2 and 24 hours postpartum (PP) are shown. Data represent the average of four to six cervices for each time point or treatment ± SEM. *, Significance (P ≤ .05) compared with untreated day 15 cervices. B, Cervical tissue prostaglandin levels are elevated in LPS-mediated but not mifepristone-mediated preterm ripening or term ripening. Prostaglandin levels measured at gestational day 15 with no treatment (NT), day 15 mifepristone (MFP), day 15 IU-LPS, gestational day 18 (d18), and 24 hours PP are shown. Data represent an average of three pools of cervical tissue for each time point or treatment group ± SEM. Each pool consisted of 4–10 cervices. *, Significance (P ≤ .05) compared with untreated day 15 cervices. 6-KGF1a, 6-ketoprostaglandin F1a.
Cervical PG levels are increased in infection-mediated ripening
The observed changes in PTGS2, Ptges, and Pgdh in the IU-LPS-treated cervix but not term or mifepristone-mediated preterm ripening suggest that cervical PG levels might be elevated during LPS-mediated ripening. Thus, PGs were measured in cervices at gestational days 15 and 18, postpartum, and day 15 mifepristone-treated, and day 15-IU LPS-treated cervices (Figure 3B). A significant increase in the cervical tissue concentration of PGE2, PGF2a, PGD2, and 6-keto-PGF1a was observed in IU-LPS-treated cervices (Figure 3B). No significant change in the level of TBX was observed (data not shown). Low PGs were detectable but not found to change during normal gestation or postpartum. Similarly, PG levels were not increased with mifepristone treatment.
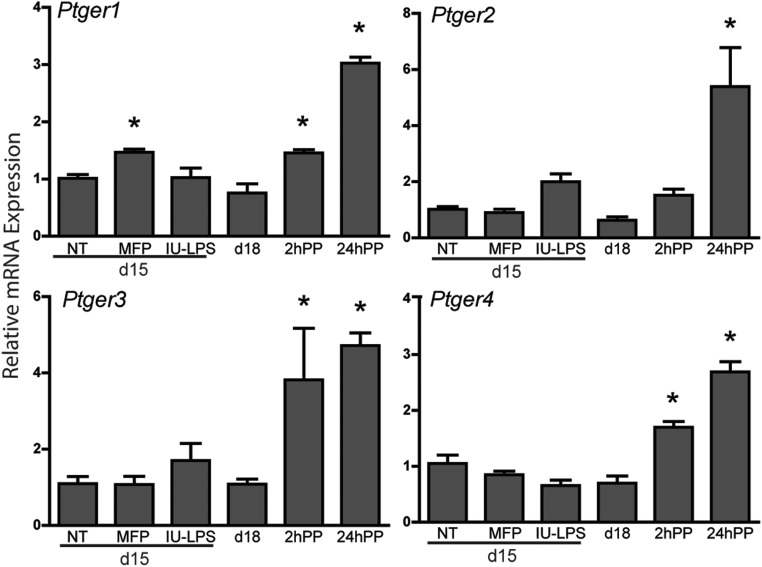
Although PG levels are low and unchanged during ripening and postpartum repair phases, changes in expression of specific PG receptors could modulate PG function in term or preterm ripening. Transcripts encoding the four PGE2 receptors were evaluated (Figure 4). All four PGE2 receptors (Ptger1–4) are expressed in the mouse cervix, and levels of all four significantly increased 1 day PP. Receptor expression did not change in the PTB models with the exception of Ptger1 in mice treated with mifepristone. Pgf2a receptor expression did not change significantly during gestation, PP, or in either PTB models (Supplemental Figure 2). These data do not support increased PG receptor expression as a regulatory step in either model of PTB.
Figure 4.
The level of cervical transcripts encoding PGE2 receptors (Ptger1–4) is not influenced by LPS treatment. Gestational day 15 (NT), day 15 mifepristone (MFP), day 15 IU-LPS, gestational day 18 (d18), 2 and 24 hours PP are shown. Data represent the average of four to six cervices for each time point or treatment ± SEM. *, Significance (P ≤ .05) when compared with untreated day 15 cervices.
Administration of the PTGS2 inhibitor, SC-236, modulates cervical ripening and PTB rates
The ability of nonselective PTGS inhibitors or PTGS2-specific blockade to reduce rates of ip LPS-induced PTB demonstrates the importance of PTGS2-driven PG synthesis in this model (20–22, 27). In the current study, we sought to determine the mechanism by which the PTGS2-selective inhibitor, SC-236, blocks key steps in the pathway of LPS-mediated cervical ripening. To this end we assessed PTB rates in LPS treated mice ± SC-236 administered via gavage on gestational day 15 prior to and after intrauterine (IU) or ip injection of LPS (Table 1). Interestingly, the PTGS2 inhibitor did not reduce PTB rates when LPS was applied directly into the uterine horn (IU-LPS). In contrast, SC-236 given 1 hour before and 4 and 16 hours after the IP-LPS injection reduced the PTB rate from 76.4% to 14.3% (Table 1). SC-236 treatment in gestational day 15 mifepristone-treated mice or on the last day of pregnancy does not affect the timing of parturition, confirming that PTGS2 is not required for mifepristone-induced premature ripening or term cervical ripening. Consistent with the inhibition of PTGS2, PGE2 levels were significantly lower in IP-LPS + SC-236 as compared with IP-LPS alone (Supplemental Figure 3A). To confirm that the route of LPS administration (ip vs intrauterine) does not differentially affect PG metabolism, the mRNA expression of Ptgs1, Ptgs2, Pges, Pgdh, and PGE and PGf2a receptors were evaluated in cervices from IP-LPS ± SC-236-treated mice (Supplemental Figure 3B). IP-LPS results in increased Ptgs2 and Pges and decreased Pgdh as observed with IU-LPS. In addition, PTGS2 protein expression is induced in the cervix from mice treated with IP-LPS (Supplemental Figure 3C) as was observed in IU-LPS-treated mice (Figure 2).
Table 1.
LPS-Induced PTB Rates ± SC-236
| Treatment, Gestation Day | Number of Animals | PTB < 24 Hours | PTB < 48 Hours | No PTB | PTB Within 24 Hours, % |
|---|---|---|---|---|---|
| 150 μg IU-LPS + SC-236, day 15 | 3 | 3 | 0 | 0 | 100.0 |
| 100 μg IP-LPS, day 15 | 17 | 13 | 1 | 3 | 76.4 |
| 100 μg IP-LPS + SC-236, day 15 | 7 | 1 | 1 | 5 | 14.3 |
| 50 μg MFP + SC-236, day 15 | 7 | 7 | 0 | 0 | 100.0 |
| SC-236, day 18 | 6 | 0 | 0 | 6 | 0 |
The ability of PTGS2 inhibition to reduce IU or IP LPS-mediated PTB rates within 24 or 48 hours was observed as well as the effect of SC236 treatment on mifepristone-treated (MFP+SC-236) or term birth (SC236, day 18). The number of PTBs occurring within 24 hours was expressed as a percentage. The timing and dosing regimen for LPS and SC-236 is described in Materials and Methods.
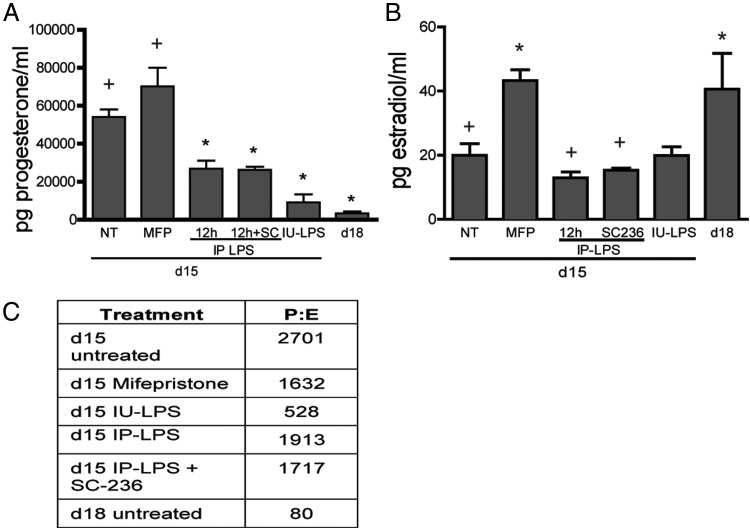
The effect of the PTGS2-selective inhibitor on steroid hormone levels, proinflammatory gene expression, immune cell populations, and tissue biomechanics was evaluated to establish which aspects of infection-mediated ripening are regulated by PGs. Studies have reported that progesterone (P) levels decline in PTB induced by intrauterine bacterial inoculation or LPS administration but not to the levels observed at term (28, 29). In the current study, progesterone and estrogen levels were measured in IP-LPS ± SC-236 and in IU-LPS-treated mice (Figure 5A). Both IU and ip routes of administration caused a significant decline in P levels compared with day 15 untreated controls. In addition, progesterone levels declined in IP-LPS + SC-236-treated mice to a similar level as IP-LPS alone. Progesterone levels in mifepristone-treated mice remained similar to the untreated animals on day 15. Estradiol (E) levels were not significantly altered with IU-LPS or IP-LPS ± SC-236 relative to gestational day 15 (Figure 5B); however, serum estradiol levels were increased with mifepristone treatment to a similar level as observed during term ripening on gestational day 18. The resulting plasma P to E ratios are summarized in Figure 5C. Although the P to E ratio declined by 34-fold from day 15 to day 18, there was only a 5-fold decline in the ratio in the 6-hour IU-LPS group and a 1.4- to 1.5-fold decline in the 12-hour IP-LPS ± SC-236 compared with untreated day 15. Although P levels do not decline in the mifepristone-treated mice, estradiol levels are elevated, resulting in a small decline in the P to E ratio. Thus, dramatic falls in the P to E ratio occur at term but not during LPS-induced PTB.
Figure 5.
Plasma progesterone levels decline yet estrogen levels do not increase in mice treated with LPS ± prostaglandin synthesis inhibitor to the same degree as observed in physiological cervical ripening at term. A, Plasma concentrations of progesterone at gestational day 15 (NT), day 15 mifepristone (MFP), day 15 12-hour IP-LPS, day 15 12-hour IP-LPS + SC-236, day 15 6-hour IU-LPS, and gestational day 18 (d18). B, Plasma estradiol levels as described in panel A. Steroid data represent the average of 6–10 animals ± SEM. *, Significance (P ≤ .05) compared with day 15 untreated; +, significance (P ≤ .05) compared with day 18. C, Progesterone to estrogen (P:E) ratios in preterm models of infection (LPS) and hormone withdrawal (mifepristone).
The necessity of PGs for the expression of several proinflammatory genes was analyzed via quantitative PCR (Figure 6A). The expression of Il6, Tnf, Cxcl2, and Mmp8 was increased with IP-LPS but not IP-LPS + SC-236. Although the inhibitor was effective in reducing gene expression, the levels were elevated compared with the gestational day 15 untreated controls. Our previous studies report no increase in the expression of these genes from gestational day 15 to term ripening (gestational day 18) (23).
Figure 6.
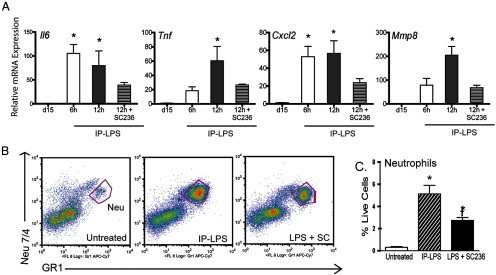
Blocking PTGS2 results in a decreased expression of proinflammatory molecules and a decrease in neutrophil recruitment. A, The mRNA expression of Il6, Tnf, Cxcl2, and Mmp8 in cervices of mice 6 and/or 12 hours after ip LPS injection. Data represent the average of six to seven cervices ± SEM. *, Significance (P ≤ .05) compared with untreated day 15 cervices. B, Flow cytometry was used to determine how SC-236 affects the recruitment of neutrophils to the cervix. The pan leukocyte marker CD45 was used to sort cells, and the neutrophil population was defined as GR1 positive and Neu 7/4 high expressing cells. C, SC-236 treatment results in a significant decline in the recruitment of neutrophils, although not to the level observed in the untreated day 15 cervices. The data represent four to eight animals ± SEM. Neu, neutrophils. *, Significance when compared with untreated day 15 cervices; †, significance compared with IP-LPS-treated cervices (P ≤ .05).
Leukocyte subsets that populate the cervix in premature ripening mediated by IU-LPS differs significantly from term ripening, in particular the robust increase in neutrophils (23). Flow cytometry was used to compare the cervical neutrophil recruitment of IP-LPS injected mice with and without PTGS2 inhibition (Figure 6B). IP-LPS treatment resulted in a robust recruitment of tissue neutrophils (Figure 6C). Administration of SC-236 along with LPS led to a significant decrease in neutrophil numbers compared with the LPS-treated group. However, PTGS2 blockade did not reduce granulocyte numbers to those observed in untreated mice cervices. Consequently, PTGS2-driven PG synthesis does in part modulate immune cell populations and the extent of the proinflammatory response.
PGs are necessary for LPS-mediated preterm ripening
Over the course of normal pregnancy, cervical compliance is progressively increased. A significant increase in compliance is evident by gestational day 12 as compared with the nonpregnant cervix, with maximal compliance at the end of pregnancy (Supplemental Figure 4). To directly assess the participation of PGs in cervical remodeling, we measured the stress-strain mechanical properties of cervical tissue in the IP-LPS ± SC-236-treated mice (Figure 7). Compared with gestational day 15 controls, the cervices of day 15 mice treated with IP-LPS for 12 hours were significantly more compliant and similar to term ripening on gestational day 18. Administration of SC-236 abrogated the LPS-induced increase in tissue compliance, confirming the dependence of PGs to this pathway of remodeling. To confirm that PGs are sufficient to induce changes in cervical distensibility in the mouse similar to what occurs in women, gestational day 15 mice were treated in vivo with misoprostol (synthetic prostaglandin E1 analog) for 6 hours. As indicated in Figure 7, compliance was significantly increased as compared with day 15 controls and reached a degree of distensibility that was similar to the gestational day 18 cervices. Similar to the IP-LPS-exposed mice, the tissue compliance of cervices from mice treated for 6 hours with IU-LPS increased to a level that was comparable with the day 18 cervices. The cervical distensibility of the mifepristone-treated group was significantly greater than what was observed at day 18.
Discussion
The detection and prevention of premature birth is hampered in part by our poor understanding of the distinct molecular mechanisms driving term and preterm birth. Irrespective of etiology of preterm labor, cervical changes precede the onset of uterine contractions. Thus, an understanding of the processes by which the cervix is remodeled in term and preterm labor of defined etiology is critical to understanding the mechanisms of PTB. The findings in the current study delineate PG-independent and PG-dependent cervical ripening pathways in the mouse. Term and mifepristone-mediated premature ripening do not require increased local PG synthesis or increased PGs from other tissue sources such as the uterus and fetal membranes. Gene and protein expression studies, cervical PG measurements, and the inability of PTGS2-specific inhibitors to block term parturition or mifepristone-induced preterm parturition support this interpretation. In contrast, premature cervical ripening with LPS is dependent on increased PTGS2-driven PG synthesis, likely derived from cervical expression and other sources. A concerted series of changes in gene expression with LPS-mediated ripening results in a net increase in tissue PG levels. This process includes increased PTGS2 and PGE synthase expression and decreased PG catabolizing enzyme (15Pgdh) expression with little change in PGE and PGF2a receptor expression. Similar findings have been observed in the myometrium and fetal membranes of mice treated with heat-killed Escherichia coli to induce preterm labor (30).
Vaginal application of prostaglandin E2 or E1 induces cervical softening in both nonpregnant and pregnant women (5, 31). We report a similar finding in the mouse because the exogenous insertion of misoprostol (E1) into the mouse cervix will recapitulate ripening as evidenced by a decline in tissue stiffness that is indistinguishable from term ripening (Figure 7). In vitro studies in human cervical fibroblasts or tissue have demonstrated expression of PG receptors and the ability of PGE2 to modulate progesterone receptor levels, glycosaminoglycan synthesis, and collagen metabolism (10, 12, 32–34). In addition, the expression of enzymes required for bioconversion of arachidonic acid to PGs and their cognate receptors are present in human cervical tissue (11). Despite the demonstrated ability of the cervix to respond to local or exogenous sources of PGs, numerous studies report no change in steady-state levels of PGE and PGF2a in cervical mucus from mid- to late pregnancy or after premature cervical ripening induced by mifepristone treatment in women (13–16). The evidence described in these studies of the human pregnant cervix corroborates our studies in the mouse to suggest that PGs might not be essential for cervical ripening at term. In contrast, PGs are critical mediators of other facets of the parturition process such as uterine contractility and luteolysis (9, 35) .
The importance of PG function in infection-mediated preterm birth has been previously established. In the current report, we build on this understanding to demonstrate that cervical PGs are increased in both a model of systemic infection (IP-LPS) and in infection localized to the reproductive tract (IU-LPS). Increased PTGS2 but not PTGS1 expression is observed in this model, and subsequent eicosinoid synthesis is a key step in this process because a specific PTGS2 inhibitor (SC-236) decreases IP-LPS-induced PTB rates by 62%. When PG synthesis is blocked via the administration of SC-236 during systemic infection (IP-LPS), the activation of proinflammatory genes (eg, Il6, Tnf, Cxcl2, Mmp8) is inhibited and neutrophil recruitment to the cervix is significantly reduced. Although PG inhibition reduces these indices of proinflammatory response, PGs are not the sole regulatory factor because the proinflammatory gene expression remained elevated in the IP-LPS + SC-236 group as compared with the untreated controls. Because the decline in the P to E ratio is modest, in the IP-LPS ± SC-236 groups, it is possible that neutrophil function remains compromised in the progesterone dominant, antiinflammatory environment.
In term ripening, increased PG synthesis initiates luteolysis in the ovary with subsequent progesterone withdrawal and parturition. Therefore, the increased PTGS2-mediated PG synthesis in LPS-treated mice was predicted to instigate premature luteolysis. The decline in plasma progesterone levels in LPS treated mice does suggest that luteolysis is ongoing. The fact that progesterone declines to a similar level in the IP-LPS-treated animals exposed to the PTGS2 inhibitor suggests that additional routes of progesterone inactivation may also be in place. In addition, although plasma P levels declined compared with day 15, the levels are 7.5- and 3-fold elevated in IP- and IU-LPS models, respectively, as compared with plasma progesterone levels at gestational day 18. Furthermore, estrogen levels are not increased as normally occurs in term ripening. These observations suggest that the infection/PG-mediated pathway of remodeling can activate alternative mechanisms to achieve loss of progesterone function. These mechanisms may include increased metabolism of tissue progesterone, which has been described in both the mouse and human cervix at term as well as changes in progesterone receptor isoforms and progesterone receptor coactivators as described in human and mouse parturition (36–40). The proinflammatory cytokine, IL-1B, has been recently reported to increase expression of the progesterone-metabolizing enzyme, 20-α hydroxysteroid dehydrogenase, in human cervical fibroblasts (41). This observation suggests that local P metabolism might be a key facilitator for local withdrawal of P function with infection/inflammation. Further studies are required to address this important question.
It is of interest to note that the degree of cervical proinflammatory responses varied between the systemic LPS model (IP) vs LPS localized to the reproductive tract (IU). Compared with the systemic infection, cervical PGE2 levels were higher, the expression of proinflammatory cytokines (eg, Tnfa, Il6) was higher, and the decline in the P to E ratio was greater when LPS was administered directly into the reproductive tract. Consistent with the level of the inflammatory response and plasma P and E levels, there was a trend for a greater increase in tissue compliance in the IU-LPS group, and the time from LPS treatment to PTB also varied (∼12–24 h with IP-LPS vs 7–12 h with IU-LPS). Although further studies are warranted, the present data suggest that both routes of LPS administration elicit a similar response, although the robustness of the response differs based on the route of LPS administration. Consistent with this interpretation, the effectiveness of the PTGS2 inhibitor, the rate of decline in serum P and the time to preterm birth differed between systemic vs localized administration of LPS. In contrast to both models of infection-mediated PTB, PGs were not increased when PTB was induced by mifepristone. Similar findings have been reported in the human cervix (42). Despite the absence of PGs, there was a marked increase in tissue compliance that was greater than term cervix on gestational day 18. The observed increase in Ptgs1 transcripts with no increase in PTGS1 protein in the mifepristone preterm birth model suggests a posttranscriptional regulation of Ptgs1. MicroRNAs have been reported to fine-tune regulation of PTGS2 expression (43–45) and are a candidate for PTGS1 regulation as well.
In contrast to term cervical ripening, PGs may have functions in postpartum tissue repair, and future studies will explore this question. Appropriate repair after birth is required for the cervix to regain tissue stiffness, thus allowing appropriate protection of the reproductive tract. The modest but significant increase in the PGE receptors postpartum would indicate that even though there is no change in the production of PGs, the tissue might be more sensitive to the PGs present. Similarly, the increase in Ptger1 observed with mifepristone treatment may have specific functions in this pathway or ripening. Our previous studies have established that there is a significant immune response (both proinflammatory and antiinflammatory) in the postpartum cervix (24, 46). Modulation of PG receptor levels could titrate PG responses to facilitate tissue repair and remodeling. PGs are known to promote inflammation and cytokine release and alter extracellular matrix deposition (47). PGs also promote wound healing by inducing fibroblast proliferation and collagen fibrillogenesis and down-regulating inflammation. Further studies are required to understand which of the four PGE receptors may be important to postpartum cervical repair and to understand their mechanism of action.
The findings of the current study in mouse models provide evidence to suggest that PGs are not part of the obligatory pathway of physiological term cervical ripening. In addition, these studies clarify distinctions between a PG-dependent pathway in LPS-mediated premature ripening as compared with a PG-independent pathway at term or with mifepristone-induced premature ripening. These insights have significant implications with respect to development of accurate clinical tools for the assessment of PTB risk and specific therapies for PTB prevention. A clear understanding of how different etiologies lead to different pathways of cervical extracellular matrix remodeling would lead to more personalized and effective therapies. Although corroborative studies in women suggest a similar role of PGs in cervical remodeling (13–16, 19), further studies are warranted to define potentially diverse pathways of remodeling. We speculate that the application of PGs, as is routinely done to induce cervical ripening prior to labor induction in women, activates processes unique to the infection/inflammation-dependent pathway of ripening. Because PGs and the cytokines they activate are reported to alter the deposition of extracellular matrix molecules, further studies are required to delineate key PG-regulated processes that drive changes in the extracellular matrix in infection-mediated cervical ripening.
Acknowledgments
We thank Dr Jean Wilson for the reading and editing of the manuscript and Dr Yucel Akgul with assistance in the data analysis.
This work was supported by funding from the Commercial Real Estate Women Foundation (to M.M.) and National Institutes of Health Grant R01 HD043154 (to M.M.). Mass spectrometry for steroid measurements used the core services supported by Grant DK089503 (to R.J.A.) from the National Institutes of Health to the University of Michigan under the Michigan Nutrition Obesity Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- E
- estradiol
- IP-LPS
- ip application of LPS
- IU
- intrauterine
- IU-LPS
- IU application of LPS
- LPS
- lipopolysaccharide
- NP
- nonpregnant
- P
- progesterone
- PG
- prostaglandin
- PGDH
- 15-hydroxyprostaglandin dehydrogenase
- PP
- postpartum
- PTB
- preterm birth
- PTGS
- PG-cyclooxygenase-endoperoxide synthase
- PTGES
- PGE synthase
- TXB
- thromboxane.
References
- 1. Li R, Lam T-W, Kristiansen K, et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics (Oxford, England). 2009;25:1966–1967 [DOI] [PubMed] [Google Scholar]
- 2. Smith WL, Dewitt DL. Prostaglandin endoperoxide H synthases-1 and -2. Adv Immunol. 1996;62:167–215 [DOI] [PubMed] [Google Scholar]
- 3. Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids. 1996;54:187–191 [DOI] [PubMed] [Google Scholar]
- 4. MacDonald PC, Casey ML. The accumulation of prostaglandins (PG) in amniotic fluid is an aftereffect of labor and not indicative of a role for PGE2 or PGF2 α in the initiation of human parturition. J Clin Endocrinol Metab. 1993;76:1332–1339 [DOI] [PubMed] [Google Scholar]
- 5. Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong Cy. Labor Induction. In: Williams Obstetrics. 23rd ed New York: McGraw-Hill; 2010:chap 22 [Google Scholar]
- 6. Haluska GJ, Kaler CA, Cook MJ, Novy MJ. Prostaglandin production during spontaneous labor and after treatment with RU486 in pregnant rhesus macaques. Biol Reprod. 1994;51:760–765 [DOI] [PubMed] [Google Scholar]
- 7. Sadovsky Y, Nelson DM, Muglia LJ, et al. Effective diminution of amniotic prostaglandin production by selective inhibitors of cyclooxygenase type 2. Am J Obstet Gynecol. 2000;182:370–376 [DOI] [PubMed] [Google Scholar]
- 8. Gross GA, Imamura T, Luedke C, et al. Opposing actions of prostaglandins and oxytocin determine the onset of murine labor. Proc Natl Acad Sci USA. 1998;95:11875–11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci USA. 2000;97:9759–9764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmitz T, Dallot E, Leroy MJ, Breuiller-Fouche M, Ferre F, Cabrol D. EP(4) receptors mediate prostaglandin E(2)-stimulated glycosaminoglycan synthesis in human cervical fibroblasts in culture. Mol Hum Reprod. 2001;7:397–402 [DOI] [PubMed] [Google Scholar]
- 11. Tornblom SA, Patel FA, Bystrom B, et al. 15-Hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab. 2004;89:2909–2915 [DOI] [PubMed] [Google Scholar]
- 12. Schmitz T, Leroy MJ, Dallot E, Breuiller-Fouche M, Ferre F, Cabrol D. Interleukin-1β induces glycosaminoglycan synthesis via the prostaglandin E2 pathway in cultured human cervical fibroblasts. Mol Hum Reprod. 2003;9:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Platz-Christensen JJ, Pernevi P, Bokstrom H, Wiqvist N. Prostaglandin E and F2 α concentration in the cervical mucus and mechanism of cervical ripening. Prostaglandins. 1997;53:253–261 [DOI] [PubMed] [Google Scholar]
- 14. Toth M, Rehnstrom J, Fuchs AR. Prostaglandins E and F in cervical mucus of pregnant women. Am J Perinatol. 1989;6:142–144 [DOI] [PubMed] [Google Scholar]
- 15. Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1β, -1α, and -6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab. 1993;77:805–815 [DOI] [PubMed] [Google Scholar]
- 16. Radestad A, Bygdeman M. Cervical softening with mifepristone (RU 486) after pretreatment with naproxen. A double-blind randomized study. Contraception. 1992;45:221–227 [DOI] [PubMed] [Google Scholar]
- 17. Cabrol D, Carbonne B, Bienkiewicz A, Dallot E, Alj AE, Cedard L. Induction of labor and cervical maturation using mifepristone (RU 486) in the late pregnant rat. Influence of a cyclooxygenase inhibitor (Diclofenac). Prostaglandins. 1991;42:71–79 [DOI] [PubMed] [Google Scholar]
- 18. Chwalisz K, Hegele-Hartung C, Schulz R, Shoa-Qing S, Louton PT, Elger W. Progesterone control of cervical ripening—experimental studies with the progesterone antagonists onapristine, lilopristone and mifepristone. In: Leppert P, Woessner F, eds. The Extracellular Matrix of the Uterus, Cervix and Fetal Membranes: Synthesis, Degradation and Hormonal Regulation. Ithaca, NY: Perinatology Press; 1991:119–131 [Google Scholar]
- 19. Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2001:CD003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gross G, Imamura T, Vogt SK, et al. Inhibition of cyclooxygenase-2 prevents inflammation-mediated preterm labor in the mouse. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1415–R1423 [DOI] [PubMed] [Google Scholar]
- 21. Gravett MG, Adams KM, Sadowsky DW, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol. 2007;197:518.e511–e518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sakai M, Tanebe K, Sasaki Y, Momma K, Yoneda S, Saito S. Evaluation of the tocolytic effect of a selective cyclooxygenase-2 inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Mol Hum Reprod. 2001;7:595–602 [DOI] [PubMed] [Google Scholar]
- 23. Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology. 2011;152:1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol. 2009;182:2700–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myers KM, Socrate S, Paskaleva A, House M. A study of the anisotropy and tension/compression behavior of human cervical tissue. J Biomech Eng 2010;132:0210031–02100315 [DOI] [PubMed] [Google Scholar]
- 26. Winchester SK, Imamura T, Gross GA, et al. Coordinate regulation of prostaglandin metabolism for induction of parturition in mice. Endocrinology. 2002;143:2593–2598 [DOI] [PubMed] [Google Scholar]
- 27. Silver RM, Edwin SS, Trautman MS, et al. Bacterial lipopolysaccharide-mediated fetal death. Production of a newly recognized form of inducible cyclooxygenase (COX-2) in murine decidua in response to lipopolysaccharide. J Clin Invest. 1995;95:725–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod. 2002;67:1337–1341 [DOI] [PubMed] [Google Scholar]
- 29. Fidel PL, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Neonat Med. 1998;7:222–226 [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Hirsch E. Bacterially induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod. 2003;69:1957–1963 [DOI] [PubMed] [Google Scholar]
- 31. Choksuchat C. Clinical use of misoprostol in nonpregnant women: review article. J Minim Invasive Gynecol. 2010;17:449–455 [DOI] [PubMed] [Google Scholar]
- 32. Rath W, Osmers R, Adelmann-Grill BC, Stuhlsatz HW, Szevereny M, Kuhn W. Biochemical changes in human cervical connective tissue after intracervical application of prostaglandin E2. Prostaglandins. 1993;45:375–384 [DOI] [PubMed] [Google Scholar]
- 33. Uldbjerg N, Ekman G, Malmstrom A, Ulmsten U, Wingerup L. Biochemical changes in human cervical connective tissue after local application of prostaglandin E2. Gynecol Obstet Invest. 1983;15:291–299 [DOI] [PubMed] [Google Scholar]
- 34. Fittkow CT, Maul H, Olson G, et al. Light-induced fluorescence of the human cervix decreases after prostaglandin application for induction of labor at term. Eur J Obstet Gynecol Reprod Biol. 2005;123:62–66 [DOI] [PubMed] [Google Scholar]
- 35. Brown N, Morrow JD, Slaughter JC, Paria BC, Reese J. Restoration of on-time embryo implantation corrects the timing of parturition in cytosolic phospholipase A2 group IVA deficient mice. Biol Reprod. 2009;81:1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5α-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–992 [DOI] [PubMed] [Google Scholar]
- 37. Andersson S, Minjarez D, Yost NP, Word RA. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab. 2008;93:2366–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100:9518–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23:947–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933 [DOI] [PubMed] [Google Scholar]
- 41. Roberson AE, Hyatt K, Kenkel C, Hanson K, Myers DA. Interleukin 1β regulates progesterone metabolism in human cervical fibroblasts. Reprod Sci. 2012;19:271–281 [DOI] [PubMed] [Google Scholar]
- 42. Radestad A, Bygdeman M, Green K. Induced cervical ripening with mifepristone (RU 486) and bioconversion of arachidonic acid in human pregnant uterine cervix in the first trimester. A double-blind, randomized, biomechanical and biochemical study. Contraception. 1990;41:283–292 [DOI] [PubMed] [Google Scholar]
- 43. Akhtar N, Haqqi TM. MicroRNA-199a* regulates the expression of cyclooxygenase-2 in human chondrocytes. Ann Rheum Dis. 2012;71:1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim SY, Romero R, Tarca AL, et al. miR-143 regulation of prostaglandin-endoperoxidase synthase 2 in the amnion: implications for human parturition at term. PloS One. 2011;6:e24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England). 2010;26:139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod. 2006;74:236–245 [DOI] [PubMed] [Google Scholar]
- 47. Van Ly D, Burgess JK, Brock TG, Lee TH, Black JL, Oliver BG. Prostaglandins but not leukotrienes alter extracellular matrix protein deposition and cytokine release in primary human airway smooth muscle cells and fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2012;303:L239–L250 [DOI] [PubMed] [Google Scholar]