Abstract
The paucity of clinical and preclinical studies investigating sex differences in sleep has resulted in mixed findings as to the exact nature of these differences. Although gonadal steroids are known to modulate sleep in females, less is known about males. Moreover, little evidence exists concerning the origin of these sex differences in sleep behavior. Thus, the goal of this study was to directly compare the sensitivity of sleep behavior in male and female Sprague Dawley rats to changes in the gonadal steroid milieu and to test whether the sex differences in sleep are the result of brain sexual differentiation or differences in circulating gonadal steroids. Here we report the magnitude of change in sleep behavior induced by either estradiol (E2) or testosterone (T) was greater in females compared with males, suggesting that sleep behavior in females is more sensitive to the suppressive effects of gonadal steroids. Furthermore, we demonstrated that the organizational effects of early gonadal steroid exposure result in male-like responsivity to gonadal steroids and directly alter the activity of the ventrolateral preoptic area (VLPO), an established sleep-promoting nucleus, in adult masculinized females. Moreover, the nonaromatizable androgen dihydrotestosterone did not suppress sleep in either males or females, suggesting that the T-mediated effect in females was due to the aromatization of T into E2. Together our data suggest that, like sex behavior, sex differences in sleep follow the classical organizational/activational effects of gonadal steroids.
Men and women sleep differently, with women reporting poorer quality and more disrupted sleep across various stages of life (for review see References 1–5). The reason for these sex differences in sleep is unknown; however, findings from clinical and basic research studies strongly implicate a role for gonadal steroids, like estradiol (E2) and progesterone (P4), in sleep modulation in females (for review see Reference 1). Sleep disruptions and complaints in women typically coincide with marked changes in the gonadal steroidal profile during puberty, the menstrual cycle, pregnancy, and menopause (for review see References 1–10). Likewise, fluctuations in the ovarian hormonal milieu across the estrous cycle correlate with changes in sleep and wakefulness in female rodents (11–17). On the night of proestrus, when the ovarian steroids E2 and P4 are elevated, both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep are significantly reduced compared with other phases of the estrous cycle. Ovariectomy (OVX) eliminates these fluctuations in time spent asleep or awake typically observed throughout the estrous cycle, and hormone replacement reinstates the suppression of sleep (both NREM and REM) seen on proestrus (18–25).
In our established treatment paradigm of acute injections that mimics the natural cyclic change in E2, sleep is markedly reduced by E2 alone (23). However, in one study using chronic exposure to E2 and P4, the addition of P4 to low levels of E2 moderately enhances E2-mediated suppression of REM sleep (18). Taken together, these studies strongly implicate E2 as the primary steroid eliciting changes in sleep-wake behavior. In male rodents, castration does not significantly alter sleep and wakefulness compared to gonadally intact males (26, 27). Curiously, in castrated mice T replacement results in a small but significant increase in the amount of NREM sleep during the dark phase (22). These studies suggest that sleep behavior in males may be less sensitive to the effects of gonadal steroids.
Previous work in our laboratory suggests that there are sex differences in the hormonal modulation of both the activity of known sleep nuclei and the expression of the enzymes responsible for producing known somnogens (16). In the ventrolateral preoptic area (VLPO), an established sleep-promoting nucleus, we have demonstrated sex differences in gonadal steroid-mediated suppression of sleep-active neurons and protein levels of the lipocalin-type prostaglandin D synthase (L-PGDS), the synthesizing enzyme for the somnogen prostaglandin D2. Female rats but not male rats respond to changes in the hormonal milieu (via E2 replacement after OVX or castration, respectively). Taken together with the behavioral data, these findings raise the possibility that there are sex differences in the activational effects of gonadal steroids on organized sleep circuitry.
Few comparative studies of sleep in males and females have been done (26, 28, 29); therefore, our understanding of the underlying cause of these sex differences in sleep is still unclear. Here we hypothesize that the sex differences observed in sleep and wakefulness are due to the activational effects of E2 on differentially organized brain circuitry. In this study, we set out to address the origin of the sex differences observed in sleep by systematically testing the basic principles defining sex differences in the brain as recently outlined by McCarthy et al (30). These sex differences may be due to activational effects (transient actions after exposure to gonadal steroids), organizational effects (permanent changes in the neural substrate induced by exposure to gonadal steroids during perinatal development or puberty that can alter how the brain responds), or both. To test these possible outcomes, we first gonadectomized (GDX) adult male and female rats and analyzed their sleep-wake behaviors after sex-specific steroidal manipulations. We also tested whether pubertal events in females sensitize their sleep behavior to E2's effects. Then, to test whether organization of the brain circuitry by early exposure to gonadal steroids contributes to sex differences in sleep, female pups were masculinized via neonatal exposure to T. These manipulations allowed for a direct comparison in the magnitude of change after steroid replacement in males and females as well as a test of whether sex differences in the sleep-wake cycle are due to organizational and/or activational effects of gonadal steroids, a significant gap in our knowledge.
Materials and Methods
Animals
All experiments were performed in adult Sprague Dawley female (250–300 g) and male (350–400 g) rats. Adult females were mated in our animal facility to allow for manipulations immediately after birth when necessary. Due to a limited number of transmitters, recording slots, and animals per litter, experiments were run in multiple cohorts, containing a minimum of two experimental groups per cohort. The animals were housed under a 12-hour light, 12-hour dark cycle with free access to food and water for the duration of the study. All procedures were performed in accordance with the National Institutes of Health guide for care and use of laboratory animals. All experiments were approved by and were in accordance with the guidelines of the University of Maryland Institutional Animal Care and Use Committee.
Surgeries
In adulthood, all animals were GDX and simultaneously implanted with TL11M2-F40-EET transmitters (Data Sciences International) under isoflurane anesthesia as previously described (16, 23, 24, 31). Briefly, immediately after GDX, a bipotential-lead transmitter was implanted sc through a dorsal incision of the abdominal region. Another incision was made along the midline of the head and neck to expose the skull and neck muscle. Two burr holes were drilled asymmetrically and stainless steel screws (Plastics One) were implanted at 3 mm anterior/+1.5 mm lateral and 7 mm posterior/−1.5 mm lateral relative to the bregma. The four transmitter leads were threaded sc to the head incision. Electroencephalographic (EEG) leads were wrapped around the screws and secured with dental cement. Electromyogram (EMG) leads were implanted directly in the dorsal cervical neck muscle, approximately 1.0 mm apart, and sutured in place. The skin along the head was sutured closed, and the dorsal incision was stapled closed with wound clips. All animals were treated with antibiotic ointment and topical lidocaine as well as carpofen (5 mg/kg) postoperatively and then allowed 7 days to recover before the start of the experiments.
Experiment 1
Female (n = 8) and male (n = 4) rats were used to determine whether there are sex differences in the effects of sex-specific gonadal steroid replacement on sleep. A subset (n = 4) of control females was OVX on postnatal day 22, prior to puberty. Vaginal opening, a commonly used marker of puberty, did not occur in any of these females, based on visual inspection. Prepubertal OVX effectively eliminates the onset of adult ovarian hormones and estrus cyclicity and thus was used to determine whether females' responsivity to the modulatory effects of E2 on sleep and wakefulness in adulthood is set during development or whether further modification at the time of puberty is required.
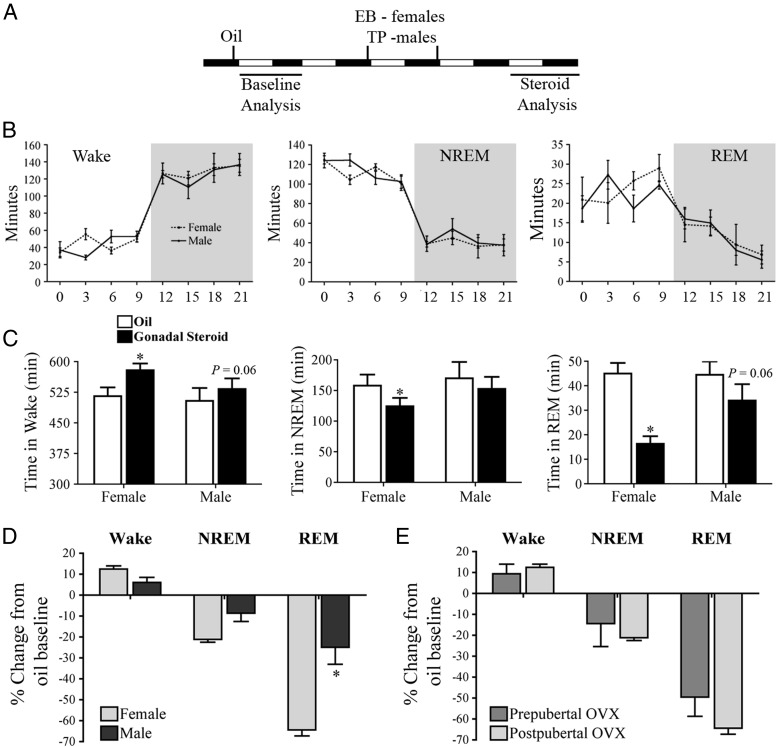
All animals received an oil injection for baseline data recordings (Figure 1A). Then females received two injections, 24 hours apart of estradiol benzoate (EB; 5 μg, then 10 μg; Sigma-Aldrich), whereas males received two injections 500 μg of testosterone propionate (TP) (Figure 1A). EB is a synthetic ester of E2, which nonspecific steroidal esterases deesterify to produce biologically active E2 in vivo (32, 33). Our EB replacement paradigm mimics the gradual rise in E2 that occurs during proestrus as we have previously shown (23). The dosage of TP has been reported to reinstate physiological levels of T in males within 24 hours (34).
Figure 1.
Sex differences in sleep and wakefulness are hormonally modulated. A, Gonadal steroid replacement and analysis paradigm-within animal design. B, There are no differences in sleep and wakefulness in females and males after GDX under baseline conditions in either the light or dark phase. C, E2 significantly increased wake and decreased both NREM and REM sleep in females, whereas T does not significantly alter sleep and wake in males. *, P < .05 vs oil. D, The magnitude of change by gonadal steroids was significantly different for REM sleep. E2 suppressed sleep by 64%, whereas T reduced it by 25%. *, P < .05 vs female. E, Sleep/wake modulation by E2 in females is determined during development and does not require further hormonal events during puberty. Prepubertal OVX did not significantly affect female's responsivity to E2's arousing and sleep suppressive effects. *, P < .05 vs female. Data are represented as mean ± SEM.
Experiment 2
A subset of female pups (n = 5) received sc injections of 100 μg of TP (Sigma-Aldrich) dissolved in 100 μL sesame oil on the day of birth and postnatal day 1 to masculinize the neural substrates, as previously described (35, 36). These females are referred to as masculinized females throughout the manuscript and represent genetic females with male-like brain organization. Control female (n = 6) and male littermates (n = 7) were treated with 100 μL sesame oil on the same treatment days. All animals were raised to adulthood (∼80 d old) and used for the sleep studies described below.
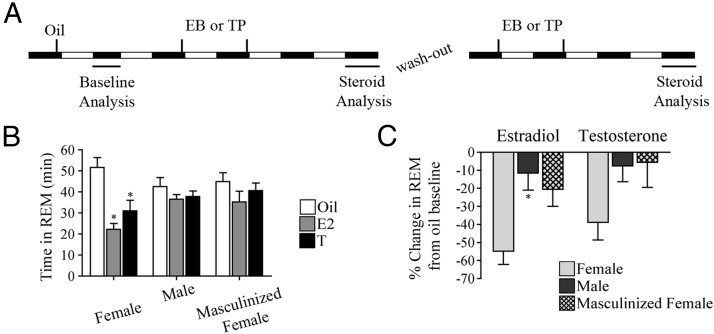
These cohorts of animals were used to test whether exposure to gonadal steroids neonatally organizes the sleep circuitry in such a way, which alters the effects of E2 and T on sleep behavior. The order of hormone replacement (either EB or TP) was randomized in each group to control for timing. Exogenous steroids were allowed 7 days to wash out before the next round of injections were given (Figure 2A).
Figure 2.
The responsivity of the sleep/wake circuitry to the modulatory effects of gonadal steroids is developmentally organized. A, Gonadal steroid replacement and analysis paradigm-within animal design. The order for EB or TP replacement was randomized to control for the order of exposure. B, E2-mediated suppression of REM sleep was dependent on brain organization. E2 significantly reduced REM sleep in females only. Similarly, T also suppressed REM sleep in females only. *, P < .05 vs oil. C, The magnitude of REM sleep suppression by E2 is significantly different based on brain organization. There is a significant difference between females and males. There are no differences between T's effects on REM sleep. *, P < .05 vs females. Data are represented as mean ± SEM.
Neonatal androgen treatment has been reported to result in an anovulatory condition characterized by lack of estrous cyclicity and reduced ovarian weight that has been attributed to a reduced number of ovulatory follicles (37–40), traits indicative of a masculinized hypothalamic-pituitary-gonadal axis. Paired ovarian weights were recorded at the time of adult ovariectomies, as an independent measure of the masculinizing effects of neonatal TP exposure in females. Neonatal TP exposure significantly reduced the paired ovarian weight by 31% compared with females treated with oil (two tailed t test; t10 = 3.96, P < .003; mean weights ± SEM in grams were: masculinized females, 0.142 ± 0.01; and females, 0.206 ± 0.01). These control females and masculinized females were used in the Fos immunoreactivity experiment described below.
Experiment 3
Dihydrotestosterone (DHT) benzoate (DHTB; Sigma-Aldrich), a nonaromatizable androgen, was administered to GDX male and female rats (n = 4 per group) to determine whether T exerts its effects through androgens or via aromatization to E2. Similarly to the EB and TP injection paradigm, two injections of 500 μg DHTB were given 24 hours apart. This dose of DHTB has been reported to effectively restore copulation in GDX males (41).
Data acquisition
EEG and EMG data were collected using Dataquest ART 4.0 software (DSI). For each animal, data collected during the baseline and hormone days were scored in 10-second epochs with Neuroscore 2.0 (DSI). Each epoch was scored as wake (low amplitude, high frequency EEG with high amplitude EMG), NREM sleep (high amplitude, low frequency EEG with low amplitude EMG), or REM sleep (low amplitude, high frequency EEG with muscle atonia or periodic muscle twitches). Transitions into a new vigilance state were considered only if two epochs (20 sec) of the new vigilance state was observed. The total duration (in minutes) for each vigilance state was analyzed for the light and dark phase for each scoring period (oil, EB, TP, or DHTB treatment). Table 1 includes data from both the light phase and dark phase. Only the dark phase data are represented in Table 2, Table 3, Figure 1E, and Figure 2, as it is when we see the most prominent effects of gonadal steroids on sleep.
Table 1.
Mean Time Spent in Each Vigilance State During the Light Phase After Oil and Sex-Specific Gonadal Steroid Replacement
| Sex | Wake, min |
NREM Sleep, min |
REM Sleep, min |
|||
|---|---|---|---|---|---|---|
| Oil | Sex-Steroid | Oil | Sex-Steroid | Oil | Sex-Steroid | |
| Females | 176.2 ± 9.7 | 159.1 ± 11.3a | 447.7 ± 16.4 | 456.0 ± 14.0 | 95.8 ± 13.6 | 104.9 ± 10.4 |
| Males | 170.8 ± 16.1 | 168.2 ± 8.5 | 457.6 ± 16.1 | 460.6 ± 12.8 | 89.3 ± 10.1 | 91.2 ± 8.1 |
Data are represented as mean ± SEM.
P < .05 vs oil.
Table 2.
Mean Time Spent in Each Vigilance State During the Dark Phase After Oil, E2, and T Replacement
| Wake, min |
NREM Sleep, min |
|||||
|---|---|---|---|---|---|---|
| Oil | E2 | T | Oil | E2 | T | |
| Females | 488.6 ± 14.7 | 551.1 ± 15.3a | 532.2 ± 16.8 | 178.7 ± 10.8 | 146.5 ± 12.6a | 156.7 ± 12.4 |
| Males | 517.3 ± 17.5 | 500.6 ± 10.5 | 515.4 ± 8.4 | 156.7 ± 13.5 | 179.7 ± 10.0 | 164.8 ± 8.1 |
| Masculinized Females | 486.7 ± 21.5 | 489.1 ± 12.0 | 479.5 ± 10.9 | 187.7 ± 17.2 | 195.6 ± 9.3 | 199.8 ± 9.8 |
Data are represented as mean ± SEM.
P < .05 vs oil.
Table 3.
Mean Time Spent in Each Vigilance State During the Dark Phase After Oil and DHT Replacement in Females and Males
| Sex | Wake, min |
NREM Sleep, min |
REM Sleep, min |
|||
|---|---|---|---|---|---|---|
| Oil | DHT | Oil | DHT | Oil | DHT | |
| Females | 510.8 ± 43.3 | 523.4 ± 36.5 | 173.9 ± 35.3 | 165.3 ± 28.7 | 35.3 ± 8.1 | 30.8 ± 8.2 |
| Males | 541.8 ± 13.8 | 506.2 ± 17.1 | 146.4 ± 6.4 | 174.0 ± 16.0 | 31.4 ± 7.9 | 39.8 ± 5.6 |
Data are represented as mean ± SEM.
Fos immunoreactivity (Fos-ir)
In a separate cohort of masculinized females (n = 5), control females (n = 6), and males (n = 6), animals were overdosed with pentobarbital before being transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde in PBS. The brains were removed, postfixed in 2.5% acrolein/4% paraformaldehyde in PBS, cryoprotected in 30% sucrose in PBS, frozen on dry ice, and stored at −80°C before being sectioned in a cryostat into 30-μm-thick sections along the coronal plane. The sections were placed into an ethylene glycol-based storage solution at −20°C until processed for Fos-ir. Cohorts containing animals from all treatment groups were immunocytochemically processed in the same tray. Briefly, sections were rinsed free of the cryoprotectant in 0.1 M PBS, reacted with 1% sodium borohydride in PBS, and rinsed. Sections were then incubated in 0.015% phenylhydrazine to release endogenous peroxidase activity, rinsed, and incubated for 48 hours at 4°C with a rabbit polyclonal Fos antibody (Ab 4191; Oncogene Science) at a dilution of 1:250 000 in 10% normal goat serum and 0.3% Triton X-100 in PBS. After primary incubation, the sections were rinsed in PBS and incubated in biotinylated secondary antibody (goat antirabbit; Vector Laboratories) for 1 hour, followed by washes in PBS. Finally, the samples were incubated with an avidin-biotin horseradish-peroxidase complex (Vectastain ABC Elite kit; Vector Laboratories) for 1 hour at room temperature and washed in PBS and then in 0.175 M sodium acetate solution. The sections were visualized with a nickel sulfate (25 mg/mL) and 3,3′-diaminobenzidine tetrahydrochloride (0.2 mg/mL; Polysciences) in sodium acetate solution containing 0.005% H2O2, rinsed in acetate solution, and transferred to PBS. After visualization, the sections were mounted serially on 2% gelatin-coated glass slides and coverslipped.
Fos-ir quantification and analysis
We systematically counted the number of Fos-ir cells in the VLPO with the aid of the Neurolucida software, an image-combining computer microscopy program (MicroBrightField) as previously described (16). Briefly, slides were anatomically matched and numerically coded so that the investigator conducting the analysis was blind to the experimental group. Sections analyzed were from one in three series (adjacent sections were separated by 90 μm). Three brain sections corresponding to plates 19 and 20 of the rat brain atlas of Paxinos and Watson (42) were used in the analysis. The placement and size of the contours were in accordance with previously defined parameters (43). The contour began approximately 1 mm from the ipsilateral ventricular wall and extended 0.7 mm laterally and 0.3 mm dorsally. Six contours per bilateral section of the VLPO were counted and an average count of Fos-ir was derived.
Statistical Analysis
All data are represented as mean ± SEM, and all statistical tests were conducted using GraphPad Prism 4 on a Macintosh computer.
Two-way, repeated-measures ANOVAs were run on the mean times spent in each vigilance state between GDX, oil treated males, and females during the light and dark phase of the 24-hour baseline day. Data were binned into 3-hour blocks across the light-dark cycle. Paired t tests were used to determine whether E2 in females and T in males alter the mean time in wake or NREM or REM sleep in both phases.
The mean times in each vigilance state were analyzed using two-way, repeated-measures ANOVAs followed by Bonferroni post hoc tests with the adult hormone status (oil baseline vs gonadal steroid replacement) and brain organization (feminization vs masculinization) as independent variables. To compare the magnitude of change induced by either E2 or T between females, males, and masculinized females, the percent change from oil baseline for each gonadal steroid was calculated [((hormone-oil)/oil) 100]. Mann-Whitney U nonparametric tests were used to compare the percent change of a vigilance state induced by either sex-specific gonadal steroids between males and females. Mann-Whitney U nonparametric tests were also used to determine whether undergoing puberty is required for E2's effects on sleep in adulthood. A Kruskal-Wallis test was used to compare the percent change between females, males, and masculinized females for each steroid treatment.
One-way ANOVA was performed on the mean number of Fos-ir cells in the VLPO and a Tukey's multiple comparison post hoc test was used to determine significance between groups.
Results
Steroid replacement results in sexually differentiated sleep behavior in gonadectomized adult male and female rats
Quantitative analysis of the EEG/EMG traces demonstrated no significant differences in the total time spent in wake (F1,42 = 0.11, P > .05), NREM sleep (F1,42 = 0.18, P > .05) and REM sleep (F1,42 = 0.31, P > .05) between GDX males and females in either the dark or light phase (Figure 1B). For each vigilance state, there was a main effect of time (wake: F7,42 = 61.42 P < .0001; NREM: F7,42 = 87.53 P < .0001; REM: F7,42 = 8.34 P < .0001). Both males and females showed a circadian rhythm in sleep and wake behaviors. During the dark (or active) phase, both sexes are awake more, whereas they acquire more NREM and REM sleep during the light (or quiescent) phase. From this point, sleep in the absence of gonadal steroids is referred to as baseline sleep.
E2 and T replacement in females and males, respectively, was used to examine the effects of gonadal steroids on time spent in each vigilance state. Similar to our previous findings (16, 23, 24), E2 in females increased wake at the expense of sleep, during the 12-hour dark phase. On average, E2 significantly increased wakefulness (t3 = 11.51, P < .001; Figure 1C) by about 12.5% or 63.5 minutes. NREM sleep (t3 = 6.698, P < .01; Figure 1C) and REM sleep (t3 = 20.86, P < .001; Figure 2C) are both significantly suppressed by E2 by about 21% (33.7 min) and 64% (28.6 min), respectively. In males, T did not significantly change any vigilance state (Figure 1C); however, there were trends for T-mediated increased wakefulness (t3 = 2.925, P = .06; Figure 1C) and decreased REM sleep (t3 = 3.039, P = .06; Figure 1C). In the light phase, gonadal steroid replacement had fewer effects on behavior (Table 1). In females, there was a small but significant suppression of wake by E2 (∼17 min; t3 = 3.189, P < .05; Table 1). There were no additional effects on either sleep phase or any significant effects of T in males during the light phase; therefore, only dark-phase data will be represented in subsequent results.
Analysis of the percent change from baseline for each vigilance state demonstrates a sex difference in the magnitude of change induced by the steroid replacement for REM sleep only (U = 0.00, P < .05, Figure 1D). E2 significantly suppresses REM sleep in females by about 64%, whereas T suppresses REM sleep in males by about 25%.
Furthermore, neither the timing of the OVX (before or after puberty) nor the timing of the first exposure to E2 significantly affected the magnitude of E2's increase in wake or suppression of sleep (Figure 1E).
E2 and T affect sleep and wake in females but not males or masculinized females
Sleep and wake analysis after E2 and T replacement (randomized) in females, males, and masculinized females was used to determine whether brain organization (eg, masculinized vs feminized) underlies the responsivity of the sleep circuitry to the modulatory effects of gonadal steroids. All groups were compared back to their oil baseline after E2 replacement. There was a significant interaction between E2 and brain organization for wake (F2,15 = 5.277, P < .05, Table 2) and REM sleep (F2,15 = 5.708, P < .05; Figure 2B). E2 in females significantly increased wakefulness (t = 3.406, P < .05) and decreased REM sleep (t = 5.507, P < .001); however, E2 was ineffective at modulating sleep and wakefulness in animals with masculinized brain circuitry. There was a significant interaction between E2 and brain organization for NREM sleep (F2,15 = 3.967, P < .05); however, due to the stringency of the Bonferroni post hoc analysis and the increase in comparisons, no significant effects were found. Because E2 has been previously shown to suppress NREM sleep in females in our laboratory (16, 23, 24), an uncorrected Fisher's least significant differences test was applied and demonstrated that E2 significantly suppressed NREM sleep (t = 2.196, P < .05; Table 2). Next, all groups were compared back to their oil baseline after T replacement. T also had a significant main effect on REM sleep (F1,15 = 10.60, P < .01; Figure 2B). T suppressed REM in females (t = 3.819, P < .01) but not masculinized females or males. There were no significant effects of T or brain organization on wake (Table 2) or NREM sleep (Table 2). The significant interactions between E2 and brain organization for wake (Table 2) and REM sleep (Figure 2B) as well as the significant effect of T on REM sleep in females only (Figure 2B) indicate the importance of brain organization for gonadal steroid mediated effects on wake and REM sleep. Masculinization of the brain during early development renders males and masculinized females unresponsive to E2.
Analysis of the percentage change in REM sleep induced by gonadal steroid replacement further supports the notion that brain organization underlies the responsivity to the modulatory effects of gonadal steroids on sleep. The magnitude of E2's suppression of REM sleep is significantly different (H2 = 7.630, P < .05; Figure 2C). Dunn's multiple comparison test revealed a significant difference between females and males (P < .05) but not between females and masculinized females or males and masculinized females. There was not a significant difference in the percent change in REM sleep induced by T replacement among the three groups (H2 = 4.841, P > .05; Figure 2C).
Fos expression in the VLPO is sensitive to the organizing effects of gonadal steroids
We have previously shown that there is a sex difference in the number of sleep-active cells (via the expression of c-Fos) in the VLPO. Females have more Fos-ir cells at baseline than males (16). Here Fos-ir cells in the VLPO of females, males, and masculinized females collected 8 hours into their sleep (light) phase were quantified to examine whether this sleep-associated brain nucleus is sensitive to the organizing effects of gonadal steroids. Males and masculinized females possessed 58% and 46% less Fos-ir cells than control females, respectively (F2,14 = 7.176, P < .01; Figure 3).
Figure 3.
Fos expression in the VLPO is developmentally organized. A, Photomicrographs of Fos-ir in females, males, and masculinized females in the VLPO. B, Males and masculinized females have 58% and 46% less Fos-ir cells, respectively, compared with females. *, P < .05 vs male and masculinized female. Data are represented as mean ± SEM.
Estradiol, not androgens, mediate changes in sleep and wakefulness
DHT, a nonaromatizable T, was used to determine whether T-mediated effects on sleep are mediated by androgens or through the aromatization of T to E2. DHT does not significantly alter female or male wake, NREM sleep, or REM sleep (Table 3), suggesting androgens do not play a role in sleep/wake modulation.
Discussion
In this study, the role of gonadal steroids was tested in the organization and the modulation of sleep and wakefulness in male and female rodents. Our first major finding is that the magnitude of E2-mediated changes in sleep differs between males and females. Such a finding indicates a sex difference in the sensitivity of the circuitry to the modulatory effects of E2. Our data strongly suggest that our T-mediated effects are due to its aromatization to E2 because DHT did not affect sleep and wakefulness. Our second novel finding is that a component of the hypothalamic neural circuitry involved in sleep is organized by early gonadal steroid exposure. These experiments have systematically shown that the sex differences observed in sleep and wakefulness are due to the activational effects of E2 on the brain circuitry organized in early life.
Developmental influences
In rodents, sexual differentiation of the brain begins around day 18 of embryonic development and continues until postnatal day 10 (for review see References 44 and 45). During this critical period, the processes of masculinization, defeminization, or feminization take place so that each animal displays the appropriate behavior for its sex when exposed to gonadal steroids in adulthood, which is referred to as the organization/activational hypothesis (for review see Reference 46). Prior to the current study, few studies provided a thorough investigation into the organization of the sleep circadian rhythm and behavior. Branchey et al (47) reported that sleep behavior is suppressed by E2 and P4 in adult males that were neonatally castrated compared with males castrated in adulthood. Yamaoka (21) showed that the circadian NREM and REM sleep rhythms in females masculinized at birth did not change after a single injection of 20 μg EB. Together these studies suggest a role for sexual differentiation of the brain mediating E2's effects on sleep rhythm and behavior; however, neither study directly compared the effects of E2 with control females nor did they investigate which brain areas may underlie the sex difference in the sensitivity of sleep to E2. Our present study demonstrates that the critical period for brain sexual differentiation contributes to the foundations of sex differences in the physiological processes mediating sleep and wake. For the first time, clear evidence is provided in support of the hypothesis that sex differences in sleep are established by the organizational actions of gonadal steroids early in life. Furthermore, we clearly show that the VLPO is sexually differentiated during development.
Both the circadian and sleep systems undergo significant alterations during adolescence (for review see References 48 and 49), a transitional developmental period characterized by the maturation of adult social and cognitive behaviors (for review see Reference 50). Puberty demarks the activation of the hypothalamic-pituitary-gonadal axis and gonadal maturation (for review see Reference 50) and typically occurs during adolescence. It is unclear whether the development of adult sleep-wake patterns during adolescence is due to age-related maturation or hormone-dependent events that occur during puberty. Hagenauer et al showed in both the rat (51) and the degu (52) that a shift in chronotype (timing of activity) occurs during puberty, and this phase shift depends on gonadal steroids. By adulthood, most activity is consolidated to the beginning of the active phase (51, 52). This shift in chronotype during the adolescent transition is similar across multiple species, including humans (for review see References 49 and 53). The role of gonadal steroids in adolescent sleep changes is less clear. Few studies have investigated the role of ovarian steroids on sleep during puberty. Sieck et al (54, 55) assessed sleep and wakefulness before and after either natural or precociously induced puberty. After their designated pubertal event, vaginal opening, there is an increase in wake at the expense of both NREM and REM sleep, suggesting that these changes in sleep and wake are mediated by the hormonal events occurring during puberty, not age-related changes (54, 55). What remains unclear, however, is whether the hormone-dependent events during puberty are necessary for the adult-like suppression of sleep induced by E2. Our data suggest that the responsivity of the circuitry underlying E2's effects on sleep in females is set early during development and that hormone-dependent changes that occur during puberty have no further effects on adult sleep organization.
Sexual differentiation of sleep circuitry
The neural circuitry and mechanisms underlying sleep and wakefulness have been extensively studied (for review see Reference 56). However, the precise targets of E2 action in the sleep circuitry have not been fully elucidated. Our previous work suggests that the VLPO, a major player in sleep initiation and maintenance (for review see Reference 57), is one likely target. In female rodents, E2 down-regulates the mRNA expression and protein levels of L-PGDS (16, 58), the enzyme responsible for the production of prostaglandin D2, which potently promotes sleep (for review see Reference 59). In conjunction with the decrease in L-PGDS, E2 decreases the activation of sleep-active VLPO neurons while increasing wake-associated Fos expression in the histaminergic tuberomammillary nuclei (16). Similarly, the wake-promoting hypocretin system is highly sensitive to fluctuations in endogenous and exogenous ovarian steroids (60–63). Taken together, these findings suggest that E2 may influence sleep-wake states via coordinated actions in arousal- and sleep-active cells via distinct mechanisms.
Unlike the females, fluctuations of gonadal steroids in adult males do not influence L-PGDS protein levels in the VLPO or the activation states of sleep-wake neurons, suggesting that the male sleep-wake circuitry is less responsive to gonadal steroids (16). Indeed, our current behavioral findings that neither T nor E2 induces significant changes in the sleep-wake behaviors in male rats correlate with our previous anatomical and biochemical findings. Moreover, the current findings demonstrate that the sleep circuitry involving the VLPO as well as the sleep-wake behaviors are organized and established by early exposure to gonadal steroids.
Hormonal modulation of sleep
It is quite clear that sex differences in sleep and wakefulness in rodents are hormonally modulated. As demonstrated here and previously reported by others, exogenous E2 in females significantly increases wakefulness at the expense of both NREM and REM sleep (18–25). Males, on the other hand, seem insensitive to changes in gonadal steroids. Castration does not significantly change sleep or wakefulness in males (26, 27), suggesting a resilience of the male sleep circuitry to changes in T levels. T replacement, however, increases NREM sleep during the dark phase in mice (22). In the current findings, acute administration of T in males resulted in a minor increase in wake and concomitant suppression of REM that did not reach statistical significance, and unlike the robust and reproducible changes in female sleep-wake patterns induced by E2, the effects of gonadal steroids in males were not reproducible (compare Figures 1 and 2). In contrast, a recent finding in adult male rats suggests that chronic E2 replacement [via SILASTIC brand (Dow Corning Corp) capsule implants] induces arousal at the expense of sleep (27). Comparatively, long-term vs short-term exposure to E2 may account for the differences. However, the magnitude of change in males induced by chronic E2 exposure compared with females is uncertain because only males were examined in that particular study.
Although it is clear that E2 in female rats induces robust and reproducible changes in sleep and wakefulness, the mechanism underlying E2 action is unclear and unaddressed by current studies. Our current finding of sex differences in the sensitivity of sleep behavior to E2 and circuitry can help direct more mechanistic research to understand how E2 is suppressing sleep in females. Rodent studies investigating how and why E2 can modulate sleep are crucial for furthering our understanding of sex differences in sleep in the clinical population.
It has been well documented that women report poorer sleep quality and more sleep complaints than men (64) (for review see References 1, 2, 4, and 5). Typically these sleep complaints are reported during the luteal phase, when E2 and P4 are elevated. Some polysomnographic studies also show that REM sleep is reduced during this phase (65–70). Additionally, women are at a 2-fold greater risk for developing insomnia throughout their lifetime compared with men (71–73). This difference in risk emerges at puberty (74) and increases with age (71–73), further implicating a role of gonadal steroidal modulation of sleep in women. Furthermore, women suffering from endocrine disorders like polycystic ovarian syndrome (75, 76) and premenstrual dysphoric disorder (77, 78) experience significant sleep disruptions as well. Because female rodents are sensitive to the sleep-suppressive effects of E2, they provide a good model for furthering mechanistic investigation into E2's actions on sleep.
The nature of the relationship between T and sleep in men remains unclear. T levels closely correspond to the sleep cycle (79, 80), and disruptions in sleep decrease the amount of circulating T (81, 82). Lower levels of T, which decline naturally with age, are correlated with less consolidated sleep (83). High-dose T replacement in older men decreases sleep efficiency and total sleep time and worsens sleep apnea (84). Others have also shown that T administration is associated with sleep apnea (84–87); however, blocking androgen action, via flutamide administration, does not affect sleep architecture or breathing parameters in men with sleep apnea (88). Interestingly, total sleep times in males undergoing male-to-female transgender therapy, which consists of high levels of estrogens and antiandrogens remain relatively unchanged (89). Stage 1 sleep and β-activity during NREM sleep significantly increased in individuals during the hormone replacement period, but all other sleep parameters remained the same (89). Together these data suggest that the male brain in humans, as in rodents, is relatively resilient to changes in gonadal steroids.
Conclusion
Modulation of sleep and wakefulness by E2 in females but not males is a classic example of the organizational/activational model for sex differences. Early exposure of gonadal steroids during development renders the male sleep/wake circuitry insensitive to the suppressive effects of E2. This finding in rodents may potentially translate in some way to the human population. Further investigation into inherent sex differences, like Fos-ir in the VLPO, and the mechanisms underlying E2 modulation throughout the sleep circuitry may aid in our understanding of why women are at a greater risk for sleep disruptions and insomnia. Because most sleep studies are done in men or male rodents, treatment generalized to the male physiology may not effectively alleviate sleep disruptions in women. Advancing our understanding of the mechanisms underlying hormonal modulation of sleep is imperative for uncovering novel drug targets for the treatment of sleep disruption in women.
Acknowledgments
We thank Shaun Viechweg for his technical assistance throughout this study.
This work was supported by Grant F31AG043329 (to D.M.C.) and Grant R01HL85037 (to J.A.M.). The authors are responsible for this work; it does not necessarily represent the official views of the NIA, NHLBI, or NIH.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DHT
- dihydrotestosterone
- DHTB
- DHT benzoate
- E2
- estradiol
- EB
- estradiol benzoate
- EEG
- electroencephalographic
- EMG
- electromyogram
- Fos-ir
- Fos immunoreactivity
- GDX
- gonadectomized
- L-PGDS
- lipocalin-type prostaglandin D synthase
- NREM
- non-REM
- OVX
- ovariectomy
- P4
- progesterone
- REM
- rapid eye movement
- TP
- testosterone propionate
- VLPO
- ventrolateral preoptic area.
References
- 1. Mong JA, Baker FC, Mahoney MM, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–555 [PubMed] [Google Scholar]
- 3. Manber R, Baker FC, Gress JL. Sex differences in sleep and sleep disorders: a focus on women's sleep. Int J Sleep Wakefulness Prim Care. 2008;1:125–133 [Google Scholar]
- 4. Moline ML, Broch L, Zak R. Sleep in women across the life cycle from adulthood through menopause. Med Clin North Am. 2004;88:705–736 [DOI] [PubMed] [Google Scholar]
- 5. Driver HS, Werth E, Dijk D-J, Borbély AA. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3:1–11 [Google Scholar]
- 6. Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115:77–83 [DOI] [PubMed] [Google Scholar]
- 7. Ibrahim S, Foldvary-Schaefer N. Sleep disorders in pregnancy: implications, evaluation, and treatment. Neurol Clin. 2012;30:925–936 [DOI] [PubMed] [Google Scholar]
- 8. Parry BL, Fernando Martinez L, Maurer EL, Lopez AM, Sorenson D, Meliska CJ. Sleep, rhythms and women's mood. Part II. Menopause. Sleep Med Rev. 2006;10:197–208 [DOI] [PubMed] [Google Scholar]
- 9. Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–1417 [DOI] [PubMed] [Google Scholar]
- 10. Polo-Kantola P. Sleep problems in midlife and beyond. Maturitas. 2011;68:224–232 [DOI] [PubMed] [Google Scholar]
- 11. Colvin GB, Whitmoyer DI, Lisk RD, Walter DO, Sawyer CH. Changes in sleep-wakefulness in female rats during circadian and estrous cycles. Brain Res. 1968;7:173–181 [DOI] [PubMed] [Google Scholar]
- 12. Sawyer CH. Some effects of hormones on sleep. Exp Med Surg. 1969;27:177–186 [PubMed] [Google Scholar]
- 13. Kleinlogel H. The female rat's sleep during oestrous cycle. Neuropsychobiology. 1983;10:228–237 [DOI] [PubMed] [Google Scholar]
- 14. Fang J, Fishbein W. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 1996;734:275–285 [PubMed] [Google Scholar]
- 15. Schwierin B, Borbely AA, Tobler I. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 1998;811:96–104 [DOI] [PubMed] [Google Scholar]
- 16. Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur J Neurosci. 2008;27:1780–1792 [DOI] [PubMed] [Google Scholar]
- 17. Koehl M, Battle SE, Turek FW. Sleep in female mice: a strain comparison across the estrous cycle. Sleep. 2003;26:267–272 [DOI] [PubMed] [Google Scholar]
- 18. Deurveilher S, Rusak B, Semba K. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep. 2009;32:865–877 [PMC free article] [PubMed] [Google Scholar]
- 19. Branchey M, Branchey L, Nadler RD. Effects of estrogen and progesterone on sleep patterns of female rats. Physiol Behav. 1971;6:743–746 [DOI] [PubMed] [Google Scholar]
- 20. Colvin GB, Whitmoyer DI, Sawyer CH. Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol. 1969;25:616–625 [DOI] [PubMed] [Google Scholar]
- 21. Yamaoka S. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res. 1980;185:385–398 [DOI] [PubMed] [Google Scholar]
- 22. Paul KN, Laposky AD, Turek FW. Reproductive hormone replacement alters sleep in mice. Neurosci Lett. 2009;463:239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz MD, Mong JA. Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R271–R280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwartz MD, Mong JA. Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats. Physiol Behav. 2011;104:962–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deurveilher S, Rusak B, Semba K. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep. 2011;34:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paul KN, Dugovic C, Turek FW, Laposky AD. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep. 2006;29:1211–1223 [DOI] [PubMed] [Google Scholar]
- 27. Wibowo E, Deurveilher S, Wassersug RJ, Semba K. Estradiol treatment modulates spontaneous sleep and recovery after sleep deprivation in castrated male rats. Behav Brain Res. 2012;226:456–464 [DOI] [PubMed] [Google Scholar]
- 28. Koehl M, Battle S, Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29:1224–1231 [DOI] [PubMed] [Google Scholar]
- 29. Franken P, Dudley CA, Estill SJ, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: Genotype and sex interactions. Proc Natl Acad Sci USA. 2006;103:7118–7123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32:2241–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDowell Ka, Hadjimarkou MM, Viechweg S, et al. Sleep alterations in an environmental neurotoxin-induced model of parkinsonism. Exp Neurol. 2010;226:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lund-Pero M, Jeppson B, Arneklo-Nobin B, Sjogren HO, Holmgren K, Pero RW. Non-specific steroidal esterase activity and distribution in human and other mammalian tissues. Clin Chim Acta. 1994;224:9–20 [DOI] [PubMed] [Google Scholar]
- 33. Oriowo MA, Landgren BM, Stenstrom B, Diczfalusy E. A comparison of the pharmacokinetic properties of three estradiol esters. Contraception. 1980;21:415–424 [DOI] [PubMed] [Google Scholar]
- 34. Davidson JM, Smith ER, Damassa DA. An effect of castration on the testosterone-LH relationship in male rats. J Reprod Fertil. 1974;41:197–200 [DOI] [PubMed] [Google Scholar]
- 35. Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mong JA, Roberts RC, Kelly JJ, McCarthy MM. Gonadal steroids reduce the density of axospinous synapses in the developing rat arcuate nucleus: an electron microscopy analysis. J Comp Neurol. 2001;432:259–267 [DOI] [PubMed] [Google Scholar]
- 37. Wolf CJ, Hotchkiss A, Ostby JS, LeBlanc GA, Gray LE., Jr Effects of prenatal testosterone propionate on the sexual development of male and female rats: a dose-response study. Toxicol Sci. 2002;65:71–86 [DOI] [PubMed] [Google Scholar]
- 38. McCoy SJ, Shirley BA. Effects of prenatal administration of testosterone and cortisone on the reproductive system of the female rat. Life Sci. 1992;50:621–628 [DOI] [PubMed] [Google Scholar]
- 39. Slob AK, den Hamer R, Woutersen PJ, van der Werff ten Bosch JJ. Prenatal testosterone propionate and postnatal ovarian activity in the rat. Acta Endocrinol (Copenh). 1983;103:420–427 [DOI] [PubMed] [Google Scholar]
- 40. Hendricks SE, McArthur DA, Pickett S. The delayed anovulation syndrome: influence of hormones and correlation with behaviour. J Endocrinol. 1977;75:15–22 [DOI] [PubMed] [Google Scholar]
- 41. Putnam SK, Sato S, Hull EM. Effects of testosterone metabolites on copulation and medial preoptic dopamine release in castrated male rats. Horm Behav. 2003;44:419–426 [DOI] [PubMed] [Google Scholar]
- 42. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998 [Google Scholar]
- 43. Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci. 2002;22:4568–4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442 [DOI] [PubMed] [Google Scholar]
- 45. MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302 [DOI] [PubMed] [Google Scholar]
- 46. Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Branchey L, Branchey M, Nadler RD. Effects of sex hormones on sleep patterns of male rats gonadectomized in adulthood and in the neonatal period. Physiol Behav. 1973;11:609–611 [DOI] [PubMed] [Google Scholar]
- 48. Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011;21:5–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hagenauer MH, Lee TM. The neuroendocrine control of the circadian system: adolescent chronotype. Front Neuroendocrinol. 2012;33:211–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047 [DOI] [PubMed] [Google Scholar]
- 51. Hagenauer MH, King AF, Possidente B, et al. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Horm Behav. 2011;60:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hagenauer MH, Ku JH, Lee TM. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus. Horm Behav. 2011;60:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039 [DOI] [PubMed] [Google Scholar]
- 54. Sieck GC, Ramaley JA, Harper RM, Taylor AN. Sleep-wakefulness changes at the time of puberty in the female rat. Brain Res. 1976;116:346–352 [DOI] [PubMed] [Google Scholar]
- 55. Sieck G, Ramaley J, Harper R, Taylor A. Puberty-related alterations in the organization of sleep-wakefulness states: differences between spontaneous and induced pubertal conditions. Exp Neurol. 1978;61:407–420 [DOI] [PubMed] [Google Scholar]
- 56. Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731 [DOI] [PubMed] [Google Scholar]
- 58. Mong JA, Devidze N, Goodwillie A, Pfaff DW. Reduction of lipocalin-type prostaglandin D synthase in the preoptic area of female mice mimics estradiol effects on arousal and sex behavior. Proc Natl Acad Sci USA. 2003;100:15206–15211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev. 2011;15:411–418 [DOI] [PubMed] [Google Scholar]
- 60. Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D. Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. Eur J Endocrinol. 2004;150:737–742 [DOI] [PubMed] [Google Scholar]
- 61. Silveyra P, Catalano PN, Lux-Lantos V, Libertun C. Impact of proestrous milieu on expression of orexin receptors and prepro-orexin in rat hypothalamus and hypophysis: actions of Cetrorelix and Nembutal. Am J Physiol Endocrinol Metab. 2007;292:E820–E828 [DOI] [PubMed] [Google Scholar]
- 62. Silveyra P, Cataldi NI, Lux-Lantos V, Libertun C. Gonadal steroids modulated hypocretin/orexin type-1 receptor expression in a brain region, sex and daytime specific manner. Regul Peptides. 2009;158:121–126 [DOI] [PubMed] [Google Scholar]
- 63. Deurveilher S, Cumyn EM, Peers T, Rusak B, Semba K. Estradiol replacement enhances sleep deprivation-induced c-Fos immunoreactivity in forebrain arousal regions of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1328–R1340 [DOI] [PubMed] [Google Scholar]
- 64. Lindberg E, Janson C, Bjornsson E, Hetta J, Boman G. Gender and sleep disturbance sleep disturbances in a young adult population : can gender differences be explained by differences in psychological status ? Sleep. 1997;20:381–387 [DOI] [PubMed] [Google Scholar]
- 65. Baker FC, Driver HS, Paiker J, Rogers GG, Mitchell D. Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. J Appl Physiol. 2002;92:1684–1691 [DOI] [PubMed] [Google Scholar]
- 66. Baker FC, Driver HS, Rogers GG, Paiker J, Mitchell D. High nocturnal body temperatures and disturbed sleep in women with primary dysmenorrhea. Am J Physiol. 1999;277:E1013–E1021 [DOI] [PubMed] [Google Scholar]
- 67. Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–735 [DOI] [PubMed] [Google Scholar]
- 69. Lee KA, McEnany G, Zaffke ME. REM sleep and mood state in childbearing women: sleepy or weepy? Sleep. 2000;23:877–885 [PubMed] [Google Scholar]
- 70. Parry BL, Mostofi N, LeVeau B, et al. Sleep EEG studies during early and late partial sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Psychiatry Res. 1999;85:127–143 [DOI] [PubMed] [Google Scholar]
- 71. Soares CN. Insomnia in women: an overlooked epidemic? Arch Womens Ment Health. 2005;8:205–213 [DOI] [PubMed] [Google Scholar]
- 72. Soares CN, Murray BJ. Sleep disorders in women: clinical evidence and treatment strategies. Psychiatr Clin North Am. 2006;29:1095–1113; abstract xi [DOI] [PubMed] [Google Scholar]
- 73. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93 [DOI] [PubMed] [Google Scholar]
- 74. Johnson EO, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117:e247–e256 [DOI] [PubMed] [Google Scholar]
- 75. Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab. 2001;86:517–520 [DOI] [PubMed] [Google Scholar]
- 76. de Sousa G, Schluter B, Buschatz D, et al. A comparison of polysomnographic variables between obese adolescents with polycystic ovarian syndrome and healthy, normal-weight and obese adolescents. Sleep Breath. 2010;14:33–38 [DOI] [PubMed] [Google Scholar]
- 77. Shechter A, Lesperance P, Ng Ying Kin NM, Boivin DB. Nocturnal polysomnographic sleep across the menstrual cycle in premenstrual dysphoric disorder. Sleep Med. 2012;13:1071–1078 [DOI] [PubMed] [Google Scholar]
- 78. Gupta R, Lahan V, Bansal S. Subjective sleep problems in young women suffering from premenstrual dysphoric disorder. N Am J Med Sci. 2012;4:593–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Evans JI, MacLean AW, Ismail AA, Love D. Concentrations of plasma testosterone in normal men during sleep. Nature. 1971;229:261–262 [DOI] [PubMed] [Google Scholar]
- 80. Roffwarg HP, Sachar EJ, Halpern F, Hellman L. Plasma testosterone and sleep: relationship to sleep stage variables. Psychosom Med. 1982;44:73–84 [DOI] [PubMed] [Google Scholar]
- 81. Cortes-Gallegos V, Castaneda G, Alonso R, et al. Sleep deprivation reduces circulating androgens in healthy men. Arch Androl. 1983;10:33–37 [DOI] [PubMed] [Google Scholar]
- 82. Luboshitzky R, Zabari Z, Shen-Orr Z, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86:1134–1139 [DOI] [PubMed] [Google Scholar]
- 83. Barrett-Connor E, Dam TT, Stone K, et al. The association of testosterone levels with overall sleep quality, sleep architecture, and sleep-disordered breathing. J Clin Endocrinol Metab. 2008;93:2602–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu PY, Yee B, Wishart SM, et al. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J Clin Endocrinol Metab. 2003;88:3605–3613 [DOI] [PubMed] [Google Scholar]
- 85. Matsumoto AM, Sandblom RE, Schoene RB, et al. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clin Endocrinol (Oxf). 1985;22:713–721 [DOI] [PubMed] [Google Scholar]
- 86. Sandblom RE, Matsumoto AM, Schoene RB, et al. Obstructive sleep apnea syndrome induced by testosterone administration. N Engl J Med. 1983;308:508–510 [DOI] [PubMed] [Google Scholar]
- 87. Schneider BK, Pickett CK, Zwillich CW, et al. Influence of testosterone on breathing during sleep. J Appl Physiol. 1986;61:618–623 [DOI] [PubMed] [Google Scholar]
- 88. Stewart DA, Grunstein RR, Berthonjones M, Handelsman DJ, Sullivan CE. Androgen blockade does not affect sleep-disordered breathing or chemosensitivity in men with obstructive sleep-apnea. Am Rev Respir Dis. 1992;146:1389–1393 [DOI] [PubMed] [Google Scholar]
- 89. Kunzel HE, Murck H, Stalla GK, Steiger A. Changes in the sleep electroencephalogram (EEG) during male to female transgender therapy. Psychoneuroendocrinology. 2011;36:1005–1009 [DOI] [PubMed] [Google Scholar]