Abstract
Adrenal blood flow (ABF) is closely coupled to steroid hormone release. ACTH and angiotensin (Ang) II stimulate cortisol and aldosterone secretion; however, their effects on ABF remain poorly defined. We used the laser-Doppler technique to measure rat ABF. Anesthetized male Sprague-Dawley rats were cannulated for mean arterial pressure (MAP) measurement and drug infusion. The left adrenal gland was exposed for ABF measurement. ABF and MAP changes to ACTH and Ang II were determined. Bolus injections of Ang II (0.01–1000 ng/kg) increased ABF (maximal increase = 110 ± 18 perfusion units at 1000 ng/kg) and increased MAP at doses greater than 10 ng/kg (basal, 99.2 ± 1.4 mm Hg; 1000 ng/kg Ang II, 149.7 ± 3.9 mm Hg). ACTH (0.1–1000 ng/kg) increased ABF (maximum increase = 158 ± 33 perfusion units) without increasing MAP. ABF increases induced by Ang II and ACTH were ablated by the cytochrome 450 inhibitor miconazole (2 mg/kg). Bolus injections of endothelin-1 (1–1000 ng/kg) increased ABF only at 1 ng/kg and increased MAP at 1000 ng/kg. Bolus injections of sodium nitroprusside increased ABF at 1 and 10 μg/kg and decreased MAP at 10 μg/kg. Thus, laser-Doppler flowmetry is a useful tool for understanding ABF regulation by peptides that stimulate steroid hormone release. Our results demonstrate that Ang II and ACTH increases in ABF are mediated by a cytochrome P450 metabolite.
The adrenal gland is highly vascularized and receives a disproportionately high percentage of cardiac output for its weight and size (1–3). The high flow is probably related to endocrine function where extensive perfusion delivers stimulants and nutrients and exports steroid hormones into the systemic circulation and to target organs (4, 5). Thus, adrenal blood flow (ABF) may be an important mediator of adrenal steroid secretion. This notion is supported by the observation that increases in flow to perfused adrenal glands promotes steroidogenesis (3).
ABF is regulated by neural, humoral, and local mediators as well as changes in O2 tension (2, 4–6). Steroidogenic stimuli increase ABF, which likely facilitates steroidogenesis (3, 6–8). For example, ACTH stimulates cortisol and aldosterone secretion and increases ABF in perfused adrenal glands in vivo (9–12). However, ACTH has no effect on the vascular tone of isolated bovine adrenal arterioles in vitro (13, 14). In contrast, when zona glomerulosa (ZG) cells are present, ACTH causes relaxation (14). The ZG cell-mediated relaxations are inhibited by cytochrome P450 (CYP) inhibitors, the potassium channel blocker, iberiotoxin and the epoxyeicosatrienoic acid (EET) antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE). Similarly, EET relaxations of adrenal arteries are inhibited by iberiotoxin and 14,15-EEZE (14). Thus, ZG cells are essential for ACTH relaxation, and ACTH stimulates ZG cell EET release. EETs are CYP metabolites of arachidonic acid. They activate adrenal artery smooth muscle cell potassium channels to cause membrane hyperpolarization and relaxation (15). In contrast to ACTH, angiotensin (Ang) II dilates adrenal arterioles in vitro (16, 17). Ang II activates endothelial cell angiotensin type 2 receptors to release nitric oxide, and in the presence of ZG cells, Ang II stimulates ZG cell EET release to cause relaxation (15–17). However, in the intact adrenal gland, the contribution of these mechanisms to ABF regulation is not known.
Although the control of adrenal steroidogenesis and the respective underlying mechanisms are well characterized, our understanding of ABF regulation is lagging. This knowledge gap may exist, in part, due to the restrictive methods used to measure ABF. Early techniques to measure ABF included the hydrogen washout technique (18), rubidium fractionation (19), venous outflow (20, 21), and radiographic imaging (22). Later, radiolabeled, fluorescent-labeled, or colored microsphere distribution emerged as a reliable measure (23). These techniques are limited by the inability to provide continuous ABF measurement. The aim of this study was to determine the in vivo effects of ACTH and Ang II on ABF and the role of CYP metabolites in these responses. To accomplish this aim, we used laser-Doppler flowmetry as a continuous, real time measure of ABF in anesthetized rats.
Materials and Methods
Materials
ACTH, Ang II, endothelin (ET)-1, sodium nitroprusside (SNP), and miconazole were purchased from Sigma-Aldrich. Miconazole was dissolved in dimethylsulfoxide, followed by dilution in sterile saline. All other drugs used were dissolved in sterile saline.
Animal preparation
Male Sprague-Dawley rats (250–300 g) were anesthetized using pentobarbital (60 mg/kg ip followed by 30 mg/kg iv per hour as needed). The left femoral artery and vein were cannulated with PE-10 and PE-50 tubing, respectively, for mean arterial pressure (MAP) recording and drug infusion. An abdominal incision (below the left thoracic cage, a few millimeters left of the median) was made to expose the left adrenal gland. Protocols were approved by the Animal Care Committee of the Medical College of Wisconsin, and procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
ABF and MAP measurements
ABF was continuously monitored by laser-Doppler flowmetry (Periflux system 5000; Perimed). A stainless steel probe (PF-403, 1 mm diameter, 80 mm length, measuring depth <1 mm) was positioned and held perpendicularly over the exposed adrenal gland using a micromanipulator. ABF was determined as a measurement of laser-light Doppler shift and is reported as perfusion units (PU) (24, 25). In some instances, PU values were normalized to control values and expressed as change in PU. MAP was recorded using a Powerlab 4/25 data acquisition system (ADInstruments) and analyzed using Chart software (ADInstruments).
After surgical preparation, animals were stabilized for 1 hour. For physical manipulation of the aorta or adrenal vein, a metal lever was used to compress and occlude the vessels (10–30 seconds). To examine the effects of secretagogues and vasoactive agents, bolus iv injections were given in sequentially increasing doses. After each injection, ABF was continuously monitored for 5 to 10 minutes. In some experiments, an iv injection of miconazole (2 mg/kg) or vehicle was given and, after 10 minutes, followed by a single bolus of Ang II or ACTH (10 ng/kg).
Statistical analysis
Data are presented as mean ± SEM. Significance between 2 groups was evaluated by Student's t test. Significance between and within multiple groups was evaluated by ANOVA followed by the Student-Newman-Keuls (SNK) multiple-comparison test. P values < .05 were considered significant.
Results
Laser-Doppler flowmetry validation
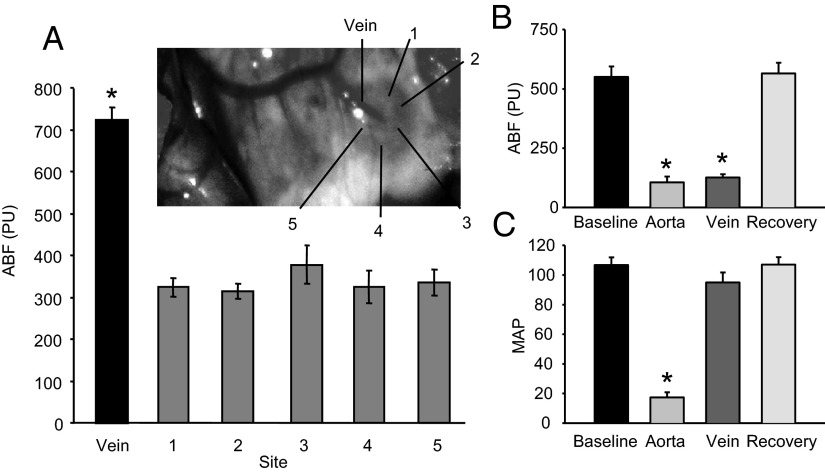
The probe was selectively oriented over the upper right corner, upper left corner, lower right corner, lower left corner, middle top, and the large vein (Figure 1A). High flow was observed with probe placement over the vein (736 ± 30 PU). A lower and similar flow was observed over all other regions (average = 356 ± 14 PU). Aorta occlusion decreased ABF from 550 ± 44 to106 ± 25 PU (Figure 1B) and MAP from 107 ± 5 to 17 ± 4 mm Hg (Figure 1B). Adrenal vein occlusion did not alter MAP but reduced ABF to 126 ± 14 PU (Figures 1, B and C). ABF and MAP returned to control values with occlusion release.
Figure 1.
Rat ABF measurement by laser-Doppler flowmetry. A, ABF above the major vein and upper right (1), upper left (2), middle front (3), lower left (4), and lower right (5) areas of the rat adrenal gland; n = 7. The inset shows measurement locations. B and C, ABF and MAP under baseline conditions, aortic occlusion (aorta), adrenal vein occlusion (vein), and occlusion release (recovery); n = 4–6. *, Significantly different from nonvein sites or from baseline values (ANOVA with SNK post hoc analysis).
Effects of steroidogenic secretagogues
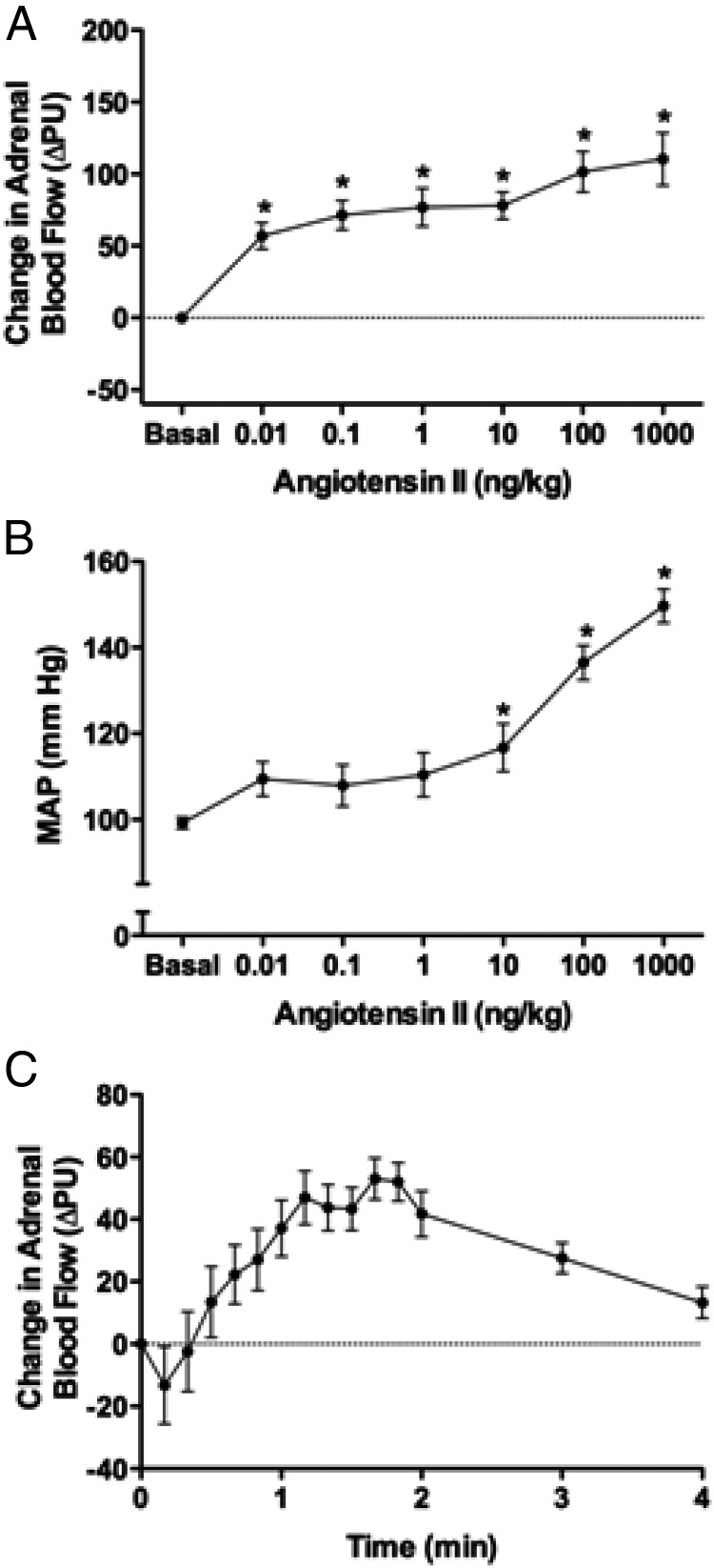
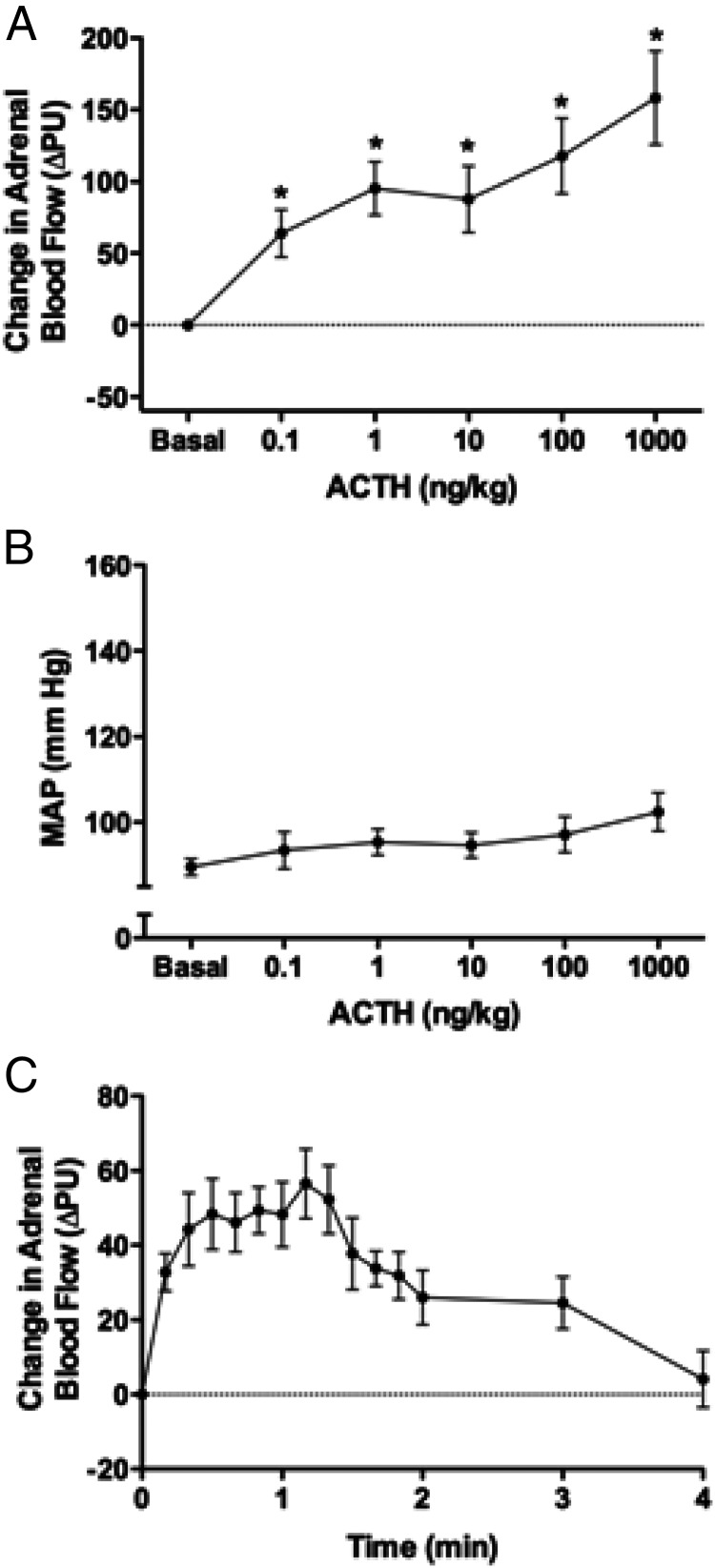
Bolus injections of Ang II (0.01–1000 ng/kg) increased ABF (maximal increase = 110 ± 18 PU with 1000 ng Ang II/kg) (Figure 2A). All doses increased ABF. Ang II increased MAP in a dose-dependent manner with maximal MAP of 149.7 ± 3.9 mm Hg with 1000 ng Ang II/kg (Figure 2B). The time course of ABF responses to Ang II (1 ng/kg) showed a small initial decrease followed by an increase that plateaued at 1 to 2 minutes (Figure 2C). ACTH similarly increased ABF (Figure 3A). All doses increased ABF (maximal increase = 158 ± 33 PU with 1000 ng ACTH/kg). ACTH did not alter MAP (Figure 3B). The time course of ABF responses to ACTH (1 ng/kg) shows that ABF increased and plateaued in 0.3 to 1.3 minutes (Figure 3C). For both Ang II and ACTH injection, ABF returned to basal levels within 4 minutes. The injection of saline alone did not alter ABF (Supplemental Figure 1, published on The Endocrine Society's Journals Online website at http://endo.endojournals.org).
Figure 2.
A and B, Effect of Ang II on ABF (A) and MAP (B). Maximal changes in ABF and MAP are depicted; n = 10.*, Significantly different from basal (ANOVA with SNK post hoc analysis). C, Time course of ABF changes in response to a bolus injection of Ang II (1 ng/kg). The 10-second mean values for change in ABF are indicated for the first 2 minutes, followed by the 60-second mean values up to 4 minutes; n = 10.
Figure 3.
A and B, Effect of ACTH on ABF (A) and MAP (B). Maximal changes in ABF and MAP are depicted; n = 6. *, Significantly different from basal (ANOVA with SNK post hoc analysis). C, Time course of ABF changes to a bolus injection of ACTH (1 ng/kg). The 10-second mean values for change in ABF are indicated for the first 2 minutes, followed by the 60-second mean values up to 4 minutes; n = 6.
Effects of CYP inhibition
A single bolus injection of Ang II (10 ng/kg) or ACTH (10 ng/kg) produced significant increases in ABF of 75 ± 12 PU and 99 ± 27 PU, respectively (Figure 4, A and B). These increases were eliminated by pretreatment of the rats with the CYP inhibitor miconazole (2 mg/kg). Miconazole alone did not alter ABF.
Figure 4.
A and B, Effect of the CYP inhibitor miconazole (2 mg/kg) on Ang II (A) (10 ng/kg) and ACTH (B) (10 ng/kg) increases in ABF; n = 5–8. *, Significantly different from basal (Student's t test); φ, significantly different from Ang II or ACTH (Student's t test).
Effects of ET-1 and SNP
We further investigated the effects of bolus injections of the vasoconstrictor ET-1 (1–1000 ng/kg) and the vasodilator SNP (1 and 10 μg/kg). ET-1 significantly increased ABF by 60 ± 9 PU (Supplemental Figure 2A) at 1 ng/kg, whereas higher doses were without effect. ET-1 increased MAP at 1000 ng/kg (basal, 92.2 ± 2.0 mm Hg; ET-1, 108.9 ± 6.8 mm Hg) (Supplemental Figure 2B). SNP increased ABF 84 ± 23 and 131 ± 16 PU at 1 and 10 μg/kg, respectively (Supplemental Figure 3A), whereas MAP was decreased at 10 μg/kg SNP (basal, 87.1 ± 3.0 mm Hg; SNP, 49.2 ± 9.3 mm Hg) (Supplemental Figure 3A).
Discussion
This study demonstrates the use of laser-Doppler flowmetry as a real-time, continuous measurement to evaluate mechanisms regulating ABF. To our knowledge, only one previous study used the laser-Doppler technique to measure ABF in anesthetized rats (26). We validated the technique by examining ABF from different areas of the gland under basal conditions and by examining the effects of physical vascular manipulations. ABF from different areas of the gland, without major blood vessels, showed uniform blood flow, whereas blood flow in the area of large surface veins showed increased perfusion. Physical occlusion of the aorta and adrenal vein decreased ABF, whereas only aortic occlusion altered MAP. These manipulations demonstrate the sensitivity of this method to volumetric changes in blood flow.
The depth of the Perimed laser-Doppler signal of 0.5 to 1 mm is greatly reduced in highly perfused organs such as the adrenal gland (http://www.perimed-instruments.com/brochures/Laser_Doppler_Probes_Perimed.pdf). Thus, adrenal regions targeted by the laser-Doppler probe include the capsular and subcapsular areas and the ZG with possible shallow penetrance into the zona fasciculata (ZF). It should not approach the depth of the zona reticularis or medulla. Importantly, arterioles of the capsular and subcapsular plexus that are responsive to vasoactive factors (1) are likely the predominant vasculature contributing to the adrenal laser-Doppler signal.
Bolus injections of Ang II caused dose-dependent increases in ABF. Ang II increased ABF at doses that did not change MAP. Thus, the increase in ABF was not secondary to increases in MAP alone. Others have observed ABF increases with Ang II infusions in anephric Sprague-Dawley rats (27). In contrast to our findings, another study reported that Ang II infusion decreased adrenal vascular conductance in rats using radioactive microspheres (28). In support of our current results, studies of isolated bovine adrenocortical arteries showed that low Ang II concentrations caused endothelium-dependent vasodilation via angiotensin type 2 receptors and enhanced ZG cell-mediated vasorelaxation (15–17, 29).
ACTH-mediated ABF regulation has been studied in several animal models (3). Studies are contradictory with regard to the stimulatory dose of ACTH and timing of the effect. Supraphysiological doses increased ABF in dogs (30, 31). One study observed an immediate response to ACTH infusion in isolated perfused glands (32), whereas another study reported a 2-minute delayed response to ACTH infusions in an in situ perfused canine adrenal gland model (33). We observed an immediate response to ACTH bolus injection with ABF increasing more than 40% within 1 minute. Increases in ABF of 99% were observed with 24-hour infusions of ACTH in fetal sheep (34). It should be noted that unlike Ang II, presser effects were not observed with ACTH injections. Signaling events mediating the rapid ABF responses to ACTH and Ang II were not investigated. However, rapid intracellular calcium responses have been observed in isolated bovine adrenal cortical cells with ACTH and Ang II stimulation (35, 36). We do not know whether this response is linked to CYP metabolite release.
Increases in ABF by Ang II and ACTH were ablated by CYP inhibition. These are the first data to demonstrate a role of CYP metabolites in regulating ABF in an in vivo model. In isolated adrenal arteries in the presence of ZG cells, ACTH and Nω-Nitro-L-arginine-resistant Ang II relaxations were similarly ablated by CYP inhibition (14, 15). EETs are produced by isolated rat ZG cells (37) and EETs and their hydrolysis products, the dihydroxyeicosatrienoic acids, cause relaxation of isolated adrenal arteries (15). In the intact adrenal gland, cellular sites of EET production could include both vascular endothelial cells and steroidogenic cells. However, the cellular source of the CYP metabolites must be independent of the vascular endothelium because ACTH alone does not cause relaxation of adrenal arteries. In this regard, it is possible that in addition to ZG cells, other adrenal steroidogenic cells also contribute to ABF regulation. Similar to ZG cells, ZF cells relax isolated adrenal arteries (14), but the mechanism of this relaxation was not identified. Bovine adrenal ZF cells produce dihydroxyeicosatrienoic acids, suggesting EET production (38). However, compared with ZG cells, the role of ZF cells in in our ABF changes are limited due to the restricted penetrance of the laser-Doppler probe. Combined with the current results, it is evident that paracrine signaling occurs between the ZG cells and the adrenal vasculature, and this interaction plays a significant role in ABF regulation.
ET-1 is produced by endothelial and ZG cells of the adrenal gland (39). ET-1 constricts adrenal arteries through smooth muscle ET-A receptors and relaxes adrenal arteries through endothelial ET-B receptors (13, 40). ET-1 decreases vascular perfusion, probably through ET-A receptor activation (41). In our study, ET-1 induced a biphasic response. The low dose increased ABF 17.9%, whereas the higher dose was without effect. A similar biphasic pattern of vascular responses was also observed with ET-1 in the rat cerebral vascular bed (42). In a rat study using colored microspheres, decreased ABF and increased MAP responses were observed with ET-1 infusion (43). The presser effect observed at the high dose of ET-1 is likely due to the vasoconstrictor effect of ET-1. SNP increased ABF in a dose-dependent manner with parallel decreases in MAP. Similarly, others reported increased adrenal medullary blood flow during SNP hypotension (22, 44).
The adrenal vasculature is highly innervated with both pre- and postganglionic input. Innervation specifically of the subcapsular arteries importantly regulates ABF (26). When agonist administration altered MAP (ie, presser effects of high doses of Ang II and ET-1 and hypotensive response with SNP), a central baroreceptor-mediated sympathetic modulation of ABF should be considered.
A major limitation of the laser-Doppler technique is the need for the open abdominal approach to the adrenal gland that requires major surgical manipulation and deep anesthesia. This undoubtedly alters endogenous Ang II and ACTH levels and adrenal steroid hormone release. In addition, because the Doppler-laser probe measures blood flow to the capsular and cortical areas, comparison of cortical vs medullary flow cannot be determined.
Laser-Doppler flowmetry provides a fast, real-time, reproducible means to study ABF in the intact animal. We validated the method with physical as well as pharmacological means. Furthermore, the adrenal steroidogenic factors Ang II and ACTH increased ABF in a concentration-dependent manner, and this increase was blocked by CYP inhibition. This study indicates the importance of CYP metabolites in the regulation of ABF.
Acknowledgments
We thank Ms Gretchen Barg for secretarial assistance.
This work was supported by the National Heart, Lung, and Blood Institute (HL-83297 to W.B.C.), an American Heart Association Midwest Affiliate Postdoctoral Fellowship (to P.G.K.), and Deutsche Forschungsgemeinschaft (DFG KFO252/0 to I.A. and S.R.B.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ABF
- adrenal blood flow
- Ang
- angiotensin
- CYP
- cytochrome P450
- EET
- epoxyeicosatrienoic acid
- 14,15-EEZE
- 14,15-epoxyeicosa-5(Z)-enoic acid
- ET
- endothelin
- MAP
- mean arterial pressure
- PU
- perfusion units
- SNK
- Student-Newman-Keuls
- SNP
- sodium nitroprusside
- ZF
- zona fasciculata
- ZG
- zona glomerulosa.
References
- 1. Bassett JR, West SH. Vascularization of the adrenal cortex: its possible involvement in the regulation of steroid hormone release. Microsc Res Tech. 1997;36:546–557 [DOI] [PubMed] [Google Scholar]
- 2. Ehrhart-Bornstein M, Hinson JP, Bornstein SR, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–143 [DOI] [PubMed] [Google Scholar]
- 3. Vinson GP, Pudney JA, Whitehouse BJ. The mammalian adrenal circulation and the relationship between adrenal blood flow and steroidogenesis. J Endocrinol. 1985;105:285–294 [DOI] [PubMed] [Google Scholar]
- 4. Breslow MJ, Tobin JR, Mandrell TD, Racusen LC, Raff H, Traystman RJ. Changes in adrenal oxygen consumption during catecholamine secretion in anesthetized dogs. Am J Physiol. 1990;259:H681–H688 [DOI] [PubMed] [Google Scholar]
- 5. Breslow MJ. Regulation of adrenal medullary and cortical blood flow. Am J Physiol. 1992;262:H1317–H1330 [DOI] [PubMed] [Google Scholar]
- 6. Nishijima MK, Breslow MJ, Raff H, Traystman RJ. Regional adrenal blood flow during hypoxia in anesthetized, ventilated dogs. Am J Physiol. 1989;256:H94–H100 [DOI] [PubMed] [Google Scholar]
- 7. Hinson JP, Vinson GP, Whitehouse BJ. The relationship between perfusion medium flow rate and steroid secretion in the isolated perfused rat adrenal gland in situ. J Endocrinol. 1986;111:391–396 [DOI] [PubMed] [Google Scholar]
- 8. Hinson JP, Vinson GP, Whitehouse BJ, Price GM. Effects of stimulation on steroid output and perfusion medium flow rate in the isolated perfused rat adrenal gland in situ. J Endocrinol. 1986;109:279–285 [DOI] [PubMed] [Google Scholar]
- 9. Carter AM, Richardson BS, Homan J, Towstoless M, Challis JR. Regional adrenal blood flow responses to adrenocorticotropic hormone in fetal sheep. Am J Physiol. 1993;264:E264–E269 [DOI] [PubMed] [Google Scholar]
- 10. Gerber JG, Nies AS. The failure of indomethacin to alter ACTH-induced adrenal hyperaemia or steroidogenesis in the anaesthetized dog. Br J Pharmacol. 1979;67:217–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sapirstein LA, Goldman H. Adrenal blood flow in the albino rat. Am J Physiol. 1959;196:159–162 [DOI] [PubMed] [Google Scholar]
- 12. Wright RD. Blood flow through the adrenal gland. Endocrinology. 1963;72:418–428 [DOI] [PubMed] [Google Scholar]
- 13. Zhang DX, Gauthier KM, Campbell WB. Characterization of vasoconstrictor responses in small bovine adrenal cortical arteries in vitro. Endocrinology. 2004;145:1571–1578 [DOI] [PubMed] [Google Scholar]
- 14. Zhang DX, Gauthier KM, Falck JR, Siddam A, Campbell WB. Steroid-producing cells regulate arterial tone of adrenal cortical arteries. Endocrinology. 2007;148:3569–3576 [DOI] [PubMed] [Google Scholar]
- 15. Kopf PG, Gauthier KM, Zhang DX, Falck JR, Campbell WB. Angiotensin II regulates adrenal vascular tone through zona glomerulosa cell-derived EETs and DHETs. Hypertension. 2011;57:323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gauthier KM, Zhang DX, Cui L, Nithipatikom K, Campbell WB. Angiotensin II relaxations of bovine adrenal cortical arteries: role of angiotensin II metabolites and endothelial nitric oxide. Hypertension. 2008;52:150–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gauthier KM, Zhang DX, Edwards EM, Holmes B, Campbell WB. Angiotensin II dilates bovine adrenal cortical arterioles: role of endothelial nitric oxide. Endocrinology. 2005;146:3319–3324 [DOI] [PubMed] [Google Scholar]
- 18. Monos E, Biró Z, Kovách AGB. The acute effect of hypophysectomy on tissue blood flow and oxygen consumption of the adrenal cortex and medulla in dogs. Acta Physiol Acad Sci Hung. 1969;36:379–389 [PubMed] [Google Scholar]
- 19. Kramer RJ, Sapirstein LA. Blood flow to the adrenal cortex and medulla. Endocrinology. 1967;81:403–405 [DOI] [PubMed] [Google Scholar]
- 20. Hume DM, Nelson DH. Adrenal cortical function in surgical shock. Surg Forum. 1955;5:568–575 [PubMed] [Google Scholar]
- 21. Schapiro S, Stjarne L. A method for collection of intermittent samples of adrenal vein blood. Proc Soc Exp Biol Med. 1958;99:414–415 [DOI] [PubMed] [Google Scholar]
- 22. Harrison RG, Hoey MJ. 1960 The adrenal circulation. Oxford: Blackwell Scientific Publications [Google Scholar]
- 23. Rutherford RB, Balis JV, Trow RS, Graves GM. Comparison of hemodynamic and regional blood flow changes at equivalent stages of endotoxin and hemorrhagic shock. J Trauma. 1976;16:886–897 [DOI] [PubMed] [Google Scholar]
- 24. Bonner RF, Nossal R. Principles of laser-Doppler flowmetry. In: Shepherd AP, Oberg PA, eds. Laser Doppler Blood Flowmetry. Boston: Kluwer Academic Publishers; 1990:17–46 [Google Scholar]
- 25. Roman RJ, Mattson DL, Cowley AW., Jr Measurement of regional blood flow in the kidney using laser-Doppler flowmetry. In: Wang DH, ed. Methods in Molecular Medicine. Totowa, NJ: Humana Press; 2000:407–426 [DOI] [PubMed] [Google Scholar]
- 26. Carlsson S, Jónsdóttir IH, Skarphedinsson JO, Thorén P. Evidence for an adrenergic innervation of the adrenal cortical blood vessels in rats. Acta Physiol Scand. 1993;149:23–30 [DOI] [PubMed] [Google Scholar]
- 27. Simmons JC, Freeman RH. l-arginine analogues inhibit aldosterone secretion in rats. Am J Physiol Regul Integr Comp Physiol. 1995;268:R1137–R1142 [DOI] [PubMed] [Google Scholar]
- 28. Schuijt MP, de Vries R, Saxena PR, Jan Danser AH. Prostanoids, but not nitric oxide, counterregulate angiotensin II mediated vasoconstriction in vivo. Eur J Pharmacol. 2001;428:331–336 [DOI] [PubMed] [Google Scholar]
- 29. Kopf PG, Campbell WB. Endothelial metabolism of angiotensin II to angiotensin III, not angiotensin (1–7), augments the vasorelaxation response in adrenal cortical arteries [published online October 3, 2013]. Endocrinology. doi:10.1210/en.2013-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. L'age M, Gonzalez-Luque A, Yates FE. Adrenal blood flow dependence of cortisol secretion rate in unaesthetized dogs. Am J Physiol. 1970;219:281–287 [DOI] [PubMed] [Google Scholar]
- 31. Stark E, Varga B, Acs Z, Papp M. Adrenal blood flow response to adrenocorticotrophic hormone and other stimuli in the dog. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965;285:296–301 [DOI] [PubMed] [Google Scholar]
- 32. Lake RB, Gann DS. Dynamic response of the intact canine adrenal to infused ACTH. Ann Biomed Eng. 1972;1:56–68 [DOI] [PubMed] [Google Scholar]
- 33. Urquhart J, Li CC. The dynamics of adrenocortical secretion. Am J Physiol. 1968;214:73–85 [DOI] [PubMed] [Google Scholar]
- 34. Carter AM, Richardson BS, Homan J, Towstoless M, Challis JR. Regional adrenal blood flow responses to adrenocorticotropic hormone in fetal sheep. Am J Physiol Endocrinol Metab. 1993;264:E264–E269 [DOI] [PubMed] [Google Scholar]
- 35. Chiyo T, Yamazaki T, Aoshika K, Kominma S, Ohta Y. Corticosterone enhances ACTH-induced calcium signals in bovine adrenocortical cells Endocrinology. 2003;144:3376–3381 [DOI] [PubMed] [Google Scholar]
- 36. Csukas S, Hanke CJ, Rewolinski D, Campbell WB. Prostaglandin E2-induced aldosterone release is mediated by an EP2 receptor. Hypertension. 1998;31:575–581 [DOI] [PubMed] [Google Scholar]
- 37. Campbell WB, Brady MT, Rosolowsky LJ, Falck JR. Metabolism of arachidonic acid by rat adrenal glomerulosa cells: Synthesis of hydroxyeicosatetraenoic acids and epoxyeicosatrienoic acids. Endocrinology. 1991;128:2183–2194 [DOI] [PubMed] [Google Scholar]
- 38. Nishimura M, Hirai A, Omura M, Tamura Y, Yoshida S. Arachidonic acid metabolites by cytochrome P-450 dependent monooxygenase pathway in bovine adrenal fasciculata cells. Prostaglandins. 1989;38:413–430 [DOI] [PubMed] [Google Scholar]
- 39. Hinojosa-Laborde C, Lange DL. Endothelin regulation of adrenal function. Clin Exp Pharmacol Physiol. 1999;26:995–999 [DOI] [PubMed] [Google Scholar]
- 40. Mazzuca MQ, Khalil RA. Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol. 2012;84:147–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mazzocchi G, Malendowicz LK, Musajo FG, Gottardo G, Markowska A, Nussdorfer GG. Role of endothelins in regulation of vascular tone in the in situ perfused rat adrenals. Am J Physiol Endocrinol Metab. 1998;274:E1–E5 [DOI] [PubMed] [Google Scholar]
- 42. Willette RN, Sauermelch C, Ezekiel M, Feuerstein G, Ohlstein EH. Effect of endothelin on cortical microvascular perfusion in rats. Stroke. 1990;21:451–458 [DOI] [PubMed] [Google Scholar]
- 43. Kemp PA, Gardiner SM, March JE, Rubin PC, Bennett T. Assessment of the effects of endothelin-1 and magnesium sulphate on regional blood flows in conscious rats, by the coloured microsphere reference technique. Br J Pharmacol. 1999;126:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jordan D, Shulman SM, Miller ED. Esmolol hydrochloride, sodium nitroprusside, and isoflurane differ in their ability to alter peripheral sympathetic responses. Anesth Analg. 1993;77:281–290 [DOI] [PubMed] [Google Scholar]