Abstract
Molecular events that regulate cellular biosynthesis of steroid hormones have been a topic of intense research for more than half a century. It has been established that transport of cholesterol into the mitochondria forms the rate-limiting step in steroid hormone production. In current models, both the steroidogenic acute regulatory protein (StAR) and the translocator protein (TSPO) have been implicated to have a concerted and indispensable effort in this cholesterol transport. Deletion of StAR in mice resulted in a critical failure of steroid hormone production, but deletion of TSPO in mice was found to be embryonic lethal. As a result, the role of TSPO in cholesterol transport has been established only using pharmacologic and genetic tools in vitro. To allow us to explore in more detail the function of TSPO in cell type-specific experimental manipulations in vivo, we generated mice carrying TSPO floxed alleles (TSPOfl/fl). In this study we made conditional knockout mice (TSPOcΔ/Δ) with TSPO deletion in testicular Leydig cells by crossing with an anti-Mullerian hormone receptor type II cre/+ mouse line. Genetic ablation of TSPO in steroidogenic Leydig cells in mice did not affect testosterone production, gametogenesis, and reproduction. Expression of StAR, cytochrome P450 side chain cleavage enzyme, 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase type I, and TSPO2 in TSPOcΔ/Δ testis was unaffected. These results challenge the prevailing dogma that claims an essential role for TSPO in steroid hormone biosynthesis and force reexamination of functional interpretations made for this protein. This is the first study examining conditional TSPO gene deletion in mice. The results show that TSPO function is not essential for steroid hormone biosynthesis.
Biosynthesis of steroid hormones in steroidogenic tissues begins with the enzymatic cleavage of the side chain of cholesterol to form the first steroid pregnenolone (1). This reaction is catalyzed by CYP11A1 (cytochrome P450 side chain cleavage) enzyme that is located on the matrix side of the inner mitochondrial membrane (IMM) (2, 3). To effect translocation of a hydrophobic molecule like cholesterol from cellular stores into the mitochondria, only 2 highly conserved protein candidates fulfilled functional criteria: 1) the channel-like translocator protein (TSPO) (4, 5), previously known as the peripheral benzodiazepine receptor (PBR) that is present in the outer mitochondrial membrane (OMM); 2) the steroidogenic acute regulatory protein (StAR) (6) that is a mitochondrial cholesterol-binding protein. Clear evidence for the involvement of StAR in cholesterol translocation came from studies on different StAR mutations that cause congenital lipoid adrenal hyperplasia, ranging from an almost complete inability of newborn infants to synthesize steroids (7) to less severe mutations that retain partial StAR activity (8, 9). This phenotype was corroborated in StAR-knockout mice that showed a critical failure of steroid hormone production (10). Although confirmed, mechanistic events involved in the transfer of cholesterol mediated by StAR remains a topic of debate (11). According to the current model, there exists a functional interaction between StAR and TSPO that is required for cholesterol transport into the mitochondria (12).
TSPO/PBR was originally identified as a receptor showing high-affinity binding to diazepam (13). Although TSPO was found expressed at different levels in multiple tissue types, abundance in steroidogenic cells (14) focused attention on a potential discrete function. The 5 transmembrane helix channel-like structure of TSPO indicated a strong possibility for specific substrate translocation (15, 16). Interactions of TSPO with mitochondrial proteins like voltage-dependent anion channel (VDAC) and adenine nucleotide translocator (ANT) suggested its existence in contact sites between the OMM and IMM (17). Moreover, the presence of a putative cholesterol recognition amino acid consensus (CRAC) at the C-terminal end of TSPO suggested cholesterol binding (18). The first study linking TSPO to regulation of steroid hormone biosynthesis demonstrated that a small molecule ligand PK11195 stimulated steroid hormone production in the Y-1 mouse adrenal tumor cell line (4). This was followed by an identical study using the MA-10 Leydig cell line (19). Although the protein synthesis inhibitor cycloheximide could block trophic hormone-dependent transport from the OMM to IMM (20, 21), PK11195-induced steroid hormone production was not inhibited by cycloheximide (5). This suggested that TSPO action did not depend on acute synthesis of proteins like StAR to elicit a steroidogenic response (6, 22) and that its action was linked to cholesterol already situated within the OMM. In support of this conclusion, TSPO gene disruption in the R2C Leydig cell line resulted in failure of pregnenolone production (23). Moreover, TSPO antisense oligonucleotides reduced the steroidogenic capacity of an MA-10 Leydig cell line that overexpresses a mitochondria-affixed StAR-Tom20 fusion protein (24), suggesting a functional cooperation between StAR and TSPO (25).
In addition to its steroidogenic function, TSPO is also known to effect several other cellular actions. Experimental evidence suggested a direct or indirect TSPO involvement in cell proliferation, apoptosis, cellular respiration, heme synthesis, stress responses, photosensitization, and malignancy. Studies highlighting these functions have been extensively documented in review articles (26–28). A case for an essential function for TSPO in cell survival and development was made when TSPO gene-deleted mice were reported to be embryonic lethal (29).
To explore the precise function of TSPO in steroidogenic cells in vivo, we generated conditional knockout mice, with specific TSPO deletion in Leydig cells (TSPOcΔ/Δ). Our results demonstrate that TSPO is not essential for steroid hormone biosynthesis.
Materials and Methods
Generation of TSPOfl/fl floxed mice
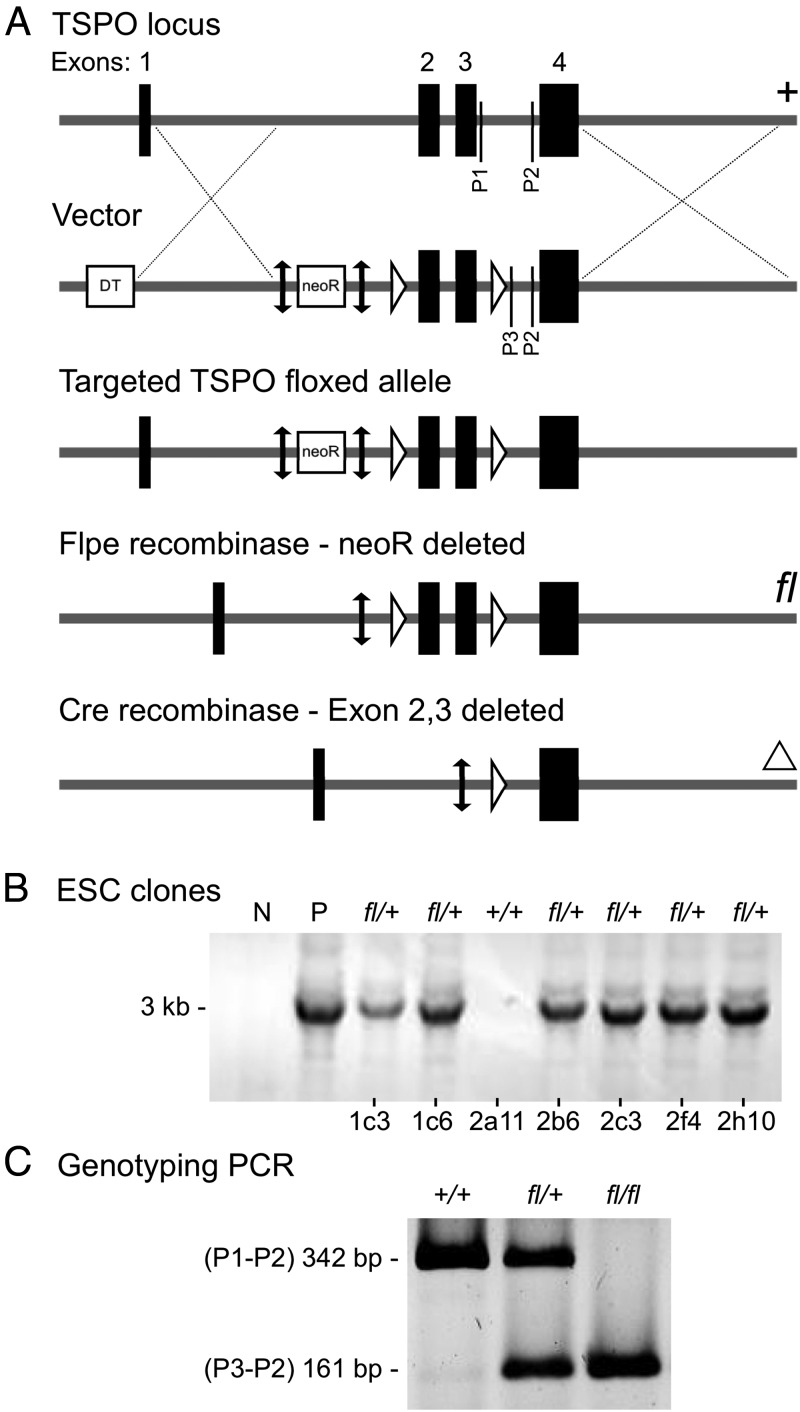
Steps involved in the generation of TSPOfl/fl mice were performed by the Mouse Biology Program at the University of California, Davis. In brief, a TSPO-targeting construct was generated to flank exons 2 and 3 of the TSPO locus with LoxP sites (Figure 1A) and electroporated for homologous recombination in C57BL/6 embryonic stem cells. Correctly targeted embryonic stem cell clones were selected using long-range PCR (Figure 1B), loss of allele outside loxP, and neo copy number (Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org); 2 clones, 1c3 and 1c6, were injected into blastocysts to generate chimeric mice. After confirmation of germline transmission, the 1c3 TSPOfl/+ line was bred with a Flpe-expressing mouse [129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym] to remove the neomycin resistance (neoR)-selectable marker (30). Then TSPOfl/fl female mice were crossed with anti-Mullerian hormone receptor type II (Amhr2)cre/+ (31) males and backcrossed to generate conditional deletions in TSPOfl/fl-Amhr2cre/+ mice (TSPOcΔ/Δ). The mouse colony was maintained by breeding TSPOcΔ/Δ males and TSPOfl/fl female mice to generate TSPOcΔ/Δ offspring of both sexes for experiments. TSPOfl/fl mice were bred as a separate colony to compare with age-matched TSPOcΔ/Δ mice. All animals used in this study were PCR genotyped using specific primers (Figure 1C and Supplemental Table 2). ROSA26-tdTomato (R26-tdTom) reporter females [B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze (32)] were used to confirm recombination induced by the Amhr2cre/+ male mice. R26-tdTom reporter mice were also bred to generate TSPOfl/fl-R26-tdTom homozygous mice to directly mark TSPO deletion. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and all mouse experiments were approved by the Institutional Animal Care and Use Committee of Cornell University.
Figure 1.
Generation of TSPO conditional knockout mice. A, Schematic showing recombination stages. Exons 2 and 3 were flanked with LoxP sites (open arrowheads), using a vector that also carries a neomycin resistance (neoR) selectable marker flanked by Frt sites (vertical double-headed black arrows). Correctly recombined embryonic stem cell (ESC) clones were used to generate mice through blastocyst injections. Germline transmitting TSPO-targeted mice were crossed with ubiquitous Flpe-expressing mice to remove neoR cassette. TSPOfl/fl mice were bred with Amhr2cre/+ knock-in mice, resulting in the deletion of exons 2 and 3 in target cells. Genotyping primers are indicated as P1, P2, and P3. B, Long-range PCR for selecting ES cell clones. Six correctly targeted clones were identified (N, negative control; P, positive control). C, Specific DNA primers (P1, P2, and P3) were used to genotype and identify the floxed and wild-type alleles in TSPO-targeted mice.
Phenotypic analysis
Testicular weights and seminal vesicle weights were measured to assess the testosterone-dependent development of male reproductive organs in 8- to 10-week-old mice. Cauda epididymal sperm counts were estimated using a hemocytometer as previously described (33). Breeding trials were conducted to quantify litter sizes from both TSPOfl/fl and TSPOcΔ/Δ males and TSPOcΔ/Δ females. At least 5 individual males/females for each genotype were examined, evaluating not more than 2 litters for each individual pair.
Histology
Tissues were fixed with 4% formaldehyde and embedded in paraffin, and 4-μm sections were prepared. Immunohistochemistry was performed using a rabbit monoclonal anti-TSPO antibody (Abcam) and a polymerized HRP-conjugated secondary antibody for diaminobenzidine-based chemistry. Alternate tissue sections were stained with hematoxylin and eosin to visualize morphology. For analysis of R26-tdTom reporter, fixed tissues were embedded in optimal cutting temperature compound, and frozen sections were prepared and mounted using Prolong Gold with 4′,6-diamidino-2-phenylindole (Life Technologies). Microscope images were acquired using either a DFC365 FX or an ICC50HD camera (Leica).
Immunoblots
Samples were sonicated and boiled in Laemmli sample buffer, and total protein was quantified using a micro-BCA colorimetric assay (Pierce Chemical Co.). Proteins were then separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and immunoblotted. TSPO protein was detected using the same rabbit monoclonal anti-TSPO antibody (Abcam). StAR protein was detected using a polyclonal antiserum (a generous gift from Dr. Douglas Stocco, Texas Tech University Health Sciences Center) (6). In brief, membranes were blocked using 5% nonfat dry milk in Tris-buffered saline containing 0.2% Tween 20. Primary antibody incubation was multiplexed using rabbit anti-TSPO and mouse anti-β-actin; simultaneous detection was performed by using IRDye 700 and 800 for the respective primaries using a laser fluorescence scanner (Li-Cor). StAR band densities were quantified with intensity profile plots and calculating area under the curve (AUC) using ImageJ (US National Institutes of Health), and expressed as ratios to actin expression.
Hormone assays
Testosterone levels in plasma collected from 8- to 10-week-old TSPOfl/fl and TSPOcΔ/Δ male mice were measured using enzyme immunoassay (Oxford Biomedical Research). For stimulation tests, 7.5 IU of human chorionic gonadotropin (hCG) (EMD Biosciences) was administered ip to male mice, and blood was collected after 1 hour to measure testosterone in plasma. In female mice, estradiol and progesterone levels were measured by RIA (Serono Maia) as previously described (34).
RT-PCR and quantitative PCR
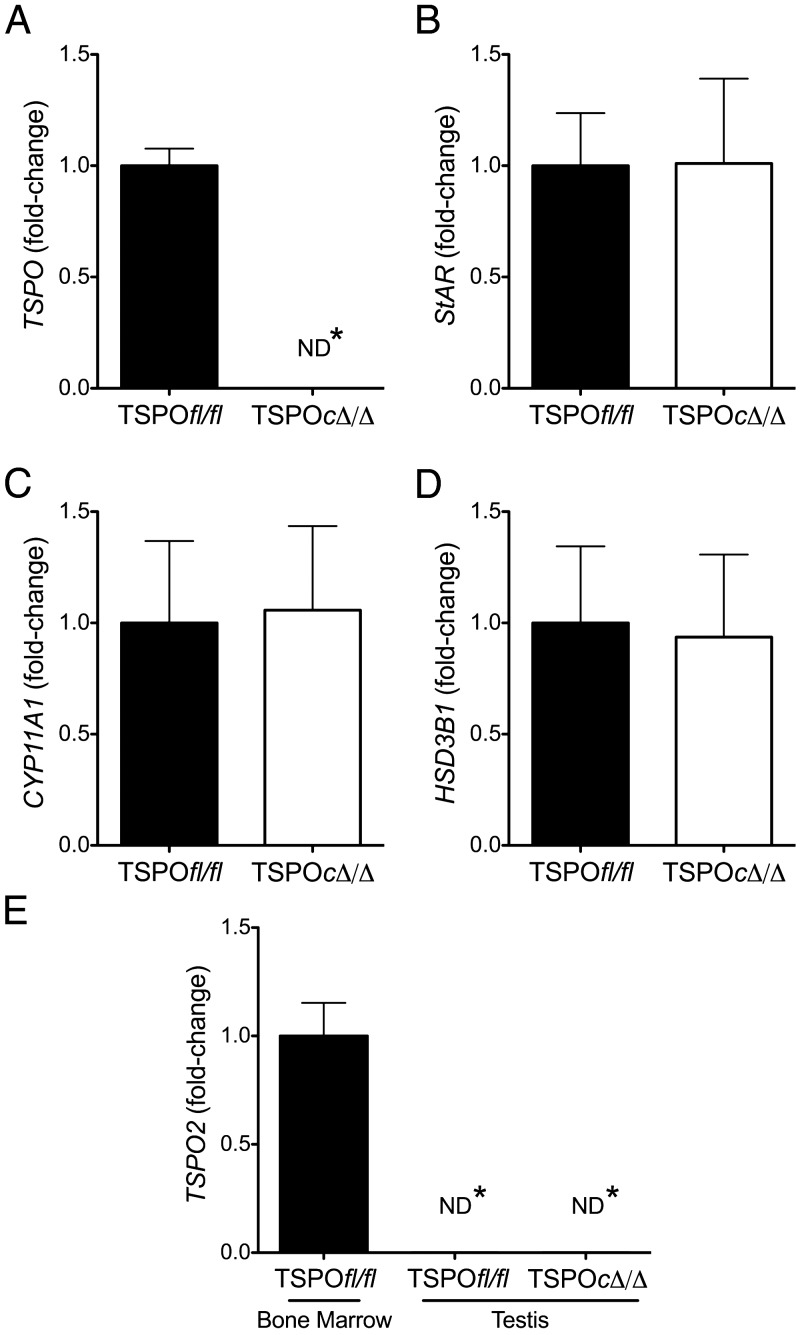
Total RNA was extracted from testes, ovary, and femur bone marrow of 8- to 10-week-old TSPOfl/fl and TSPOcΔ/Δ mice using Trizol (Life Technologies). Reverse transcription of 1.5 μg of total RNA was carried out using Multiscribe reverse transcriptase (Applied Biosystems). To examine TSPO mRNA expression in the testis by RT-PCR, we designed 3 sets of intron-spanning primers covering all 4 exons (Supplemental Table 3). Validated Taqman gene expression assays (Applied Biosystems) were used for quantitative PCR estimation of TSPO (Mm00437828_m1; spans exons 3 and 4), TSPO2 (Mm01281420_m1), StAR (Mm00441558_m1), CYP11A1 (Mm00490735_m1), and HSD3B1 (Mm00476184_g1). A relative efficiency plot was examined for validation, and all experimental samples were analyzed and normalized with expression level of the internal control gene, GAPDH (Mm99999915_g1). Relative quantification of fold-change was performed comparing Ct values from individual mice by applying the 2-ΔΔCt method (35).
Statistics
Numeric differences between parameters measured in TSPOfl/fl and TSPOcΔ/Δ mice were compared using a Student's t test; comparisons for more than 2 groups were performed using ANOVA and post hoc Tukey's test (P < .05 was considered significant). Data were confirmed to satisfy assumptions of normality and homogeneity of variance. All analyses were performed using Prism 5 (GraphPad).
Results
Design and validation for the TSPOfl/fl locus
The TSPO gene consists of 4 exons, of which exon 1 is noncoding. The translational start site is present in exon 2. Design of the TSPOfl/fl locus allows for cre-mediated deletion of exons 2 and 3 of the TSPO gene (Figure 1). Specific primers amplifying across intron 1 and exon 4 confirmed deletion of exons 2 and 3. The PCR product from the recombined locus was also sequenced to confirm the deletion (Supplemental Figure 1). Deletion of exons 2 and 3 also induces a 1-bp frame shift in the amino acid-coding sequence in exon 4. In addition, exons 1 and 4 do not have any in-frame translation start sites that can allow production of a partial TSPO peptide. Exon 4 contains 3 out-of-frame start codons, 2 of which have a stop within 2 amino acids; one can only produce a meaningless scrambled peptide if translated. The rabbit monoclonal antibody used to detect TSPO specifically recognizes amino acids 156–169 at the C-terminal end of exon 4. Therefore, use of this antibody can effectively validate absence of a partial TSPO peptide from exon 4 after recombination.
TSPOcΔ/Δ mice have normal testicular development
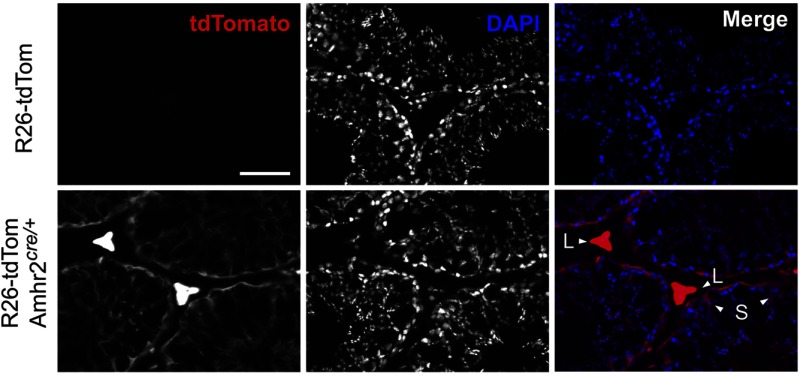
The knock-in anti-Mullerian hormone receptor type II (Amhr2) promoter-driven cre recombinase (Amhr2cre/+) mouse line (31) has been previously used to study Leydig cell and Sertoli cell conditional deletions (36). Using a ROSA26-tdTomato (R26-tdTom) reporter line, we confirmed specific recombination in Leydig and Sertoli cells in the testes of R26-tdTom-Amhr2cre/+ mice (Figure 2). In the testis, TSPO gene expression is restricted to Leydig and Sertoli cells (37). TSPOcΔ/Δ testis did not express TSPO protein in Leydig and Sertoli cells, evaluated by both immunohistochemistry (Figure 3A) and immunoblotting (Figure 3B). This deficiency of TSPO in Leydig and Sertoli cells did not affect spermatogenesis and seminiferous tubule morphology (Figure 3A). Sperm production, as evaluated by the number of sperm present in the cauda epididymis, was also not different between TSPOfl/fl and TSPOcΔ/Δ mice (Figure 3C). RT-PCR analysis for TSPO gene expression showed that a complete TSPO mRNA was not expressed in the TSPOcΔ/Δ testis. Intron-spanning primers that amplify TSPO between exons 1 and 2, exons 2 and 3, and exons 3 and 4, did not show a RT-PCR product in TSPOcΔ/Δ compared with TSPOfl/fl testis (Figure 3, D–F). Primers that amplified TSPO cDNA between exons 1 and 4 showed a reduction in size in TSPOcΔ/Δ consistent with the deletion of exon 2 and exon 3 that eliminates TSPO gene expression (Figure 3G). In the ovary, only partial recombination was induced by Amhr2cre/+ expression (Supplemental Figure 2), as reported in some previous studies (38, 39). This resulted in the partial loss of TSPO as a mosaic in TSPOcΔ/Δ ovaries (Supplemental Figure 3).
Figure 2.
Amhr2cre/+-mediated gene deletion in Leydig and Sertoli cells. Testis from ROSA26-tdTomato (R26-tdTom) reporter mice showing controls with no recombination and specific recombination in Leydig (L) and Sertoli (S) cells with Amhr2cre/+ expression (R26-tdTom-Amhr2cre/+ mice). Scale bar, 50 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Figure 3.
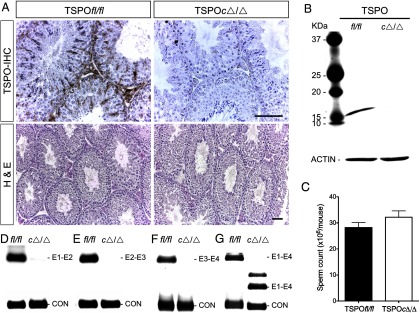
TSPO deletion in Leydig and Sertoli cells does not affect spermatogenesis. A, Immunohistochemical (IHC) localization showing complete absence of TSPO in Leydig and Sertoli cells of TSPOcΔ/Δ testes. Hematoxylin and eosin (H&E) staining showing unaltered seminiferous tubule morphology and spermatogenesis in TSPOcΔ/Δ testes (n = 5). Scale bars, 50 μm. B, Western blot showing absence of TSPO in TSPOcΔ/Δ testis tissue (n = 5); β-actin is shown as the loading control. C, Cauda epididymal sperm counts were not significantly different between TSPOfl/fl and TSPOcΔ/Δ mice (mean ± SEM; n = 5/group). D–F, Testis cDNA from TSPOfl/fl and TSPOcΔ/Δ mice examined for amplification products from exons 1 and 2 [250 bp] (D); exons 2 and 3 [241 bp] (E); exons 3 and 4 [424 bp] (F); exons 1–4 [711 bp in TSPOfl/fl and 361 bp in TSPOcΔ/Δ]. For all RT-PCR, glyceraldehydes-3-phosphate dehydrogenase was used as a control (CON).
Testosterone production is not affected in TSPOcΔ/Δ mice
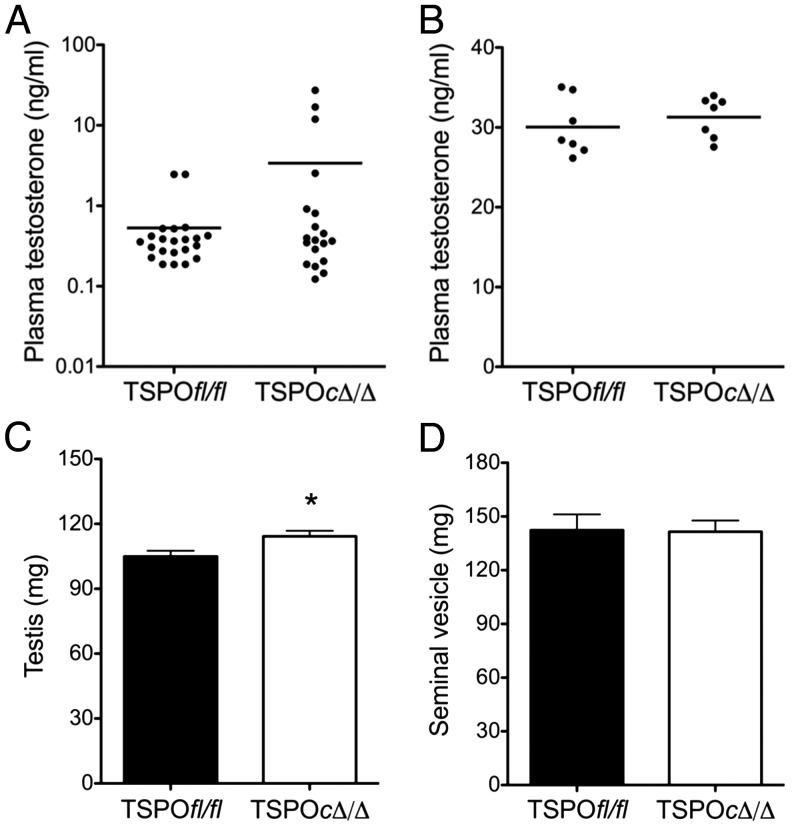
Testosterone produced by Leydig cells in the testis is critical for development of the male gonads, functional growth of accessory sex organs, and reproductive behavior (40). Deficiency of TSPO in Leydig cells did not affect physiological plasma testosterone levels (Figure 4A). The wide variation of plasma testosterone levels is expected due to the pulsatile pattern of testosterone production as a result of phasic LH release from the pituitary (41). Overall, the TSPOcΔ/Δ mice appeared to have a trend of higher plasma testosterone values. In order to directly examine Leydig cell potential for testosterone synthesis and release in TSPOcΔ/Δ mice, we stimulated mice with a single dose of hCG that has significant LH activity. One hour after hCG, we found plasma testosterone levels were highly elevated but values were not different between TSPOfl/fl and TSPOcΔ/Δ mice (Figure 4B). Testis weight and seminal vesicle weight are measures that directly correlate with testosterone levels. In TSPOcΔ/Δ mice we found that testis weight was significantly higher than that in TSPOfl/fl controls (P < .05), albeit the means differed by only 9.4 mg (Figure 4C). However, the seminal vesicle weight was not different between TSPOfl/fl and TSPOcΔ/Δ mice (Figure 4D).
Figure 4.
TSPO deletion in Leydig and Sertoli cells does not affect testosterone production. A, Plasma testosterone levels were not significantly different between TSPOfl/fl and TSPOcΔ/Δ mice (n = 19–22/group). B, When sampled 1 hour after hCG stimulation, plasma testosterone levels were highly elevated but not different between TSPOfl/fl and TSPOcΔ/Δ mice (n = 7/group). C, A modest but significant increase in testis weights was observed in TSPOcΔ/Δ mice compared with TSPOfl/fl mice (P < .05; mean ± SEM; n = 18/group). D, Seminal vesicle weights were not significantly different between TSPOfl/fl and TSPOcΔ/Δ mice (mean ± SEM; n = 18/group).
In female TSPOcΔ/Δ mice, estradiol and progesterone levels were unchanged compared with TSPOfl/fl cohorts (Supplemental Table 4). When we measured reproductive performance, we did not find any differences in mating (evaluated by vaginal plugs) and litter sizes produced by TSPOfl/fl and TSPOcΔ/Δ mice (Table 1).
Table 1.
Litter size from TSPOfl/fl and TSPOcΔ/Δ male and female mice
| Genetic Crosses | n | Litter Sizea ± SE |
|---|---|---|
| TSPOfl/fl × female TSPOfl/fl | 16 | 7.19 ± 0.56 |
| TSPOcΔ/Δ × female TSPOfl/fl | 10 | 7.40 ± 0.37 |
| Male TSPOfl/fl × female TSPOcΔ/Δ | 6 | 9.00 ± 1.13 |
No significant differences were detected for litter size between the 3 crosses.
Loss of TSPO does not affect expression of steroidogenic genes
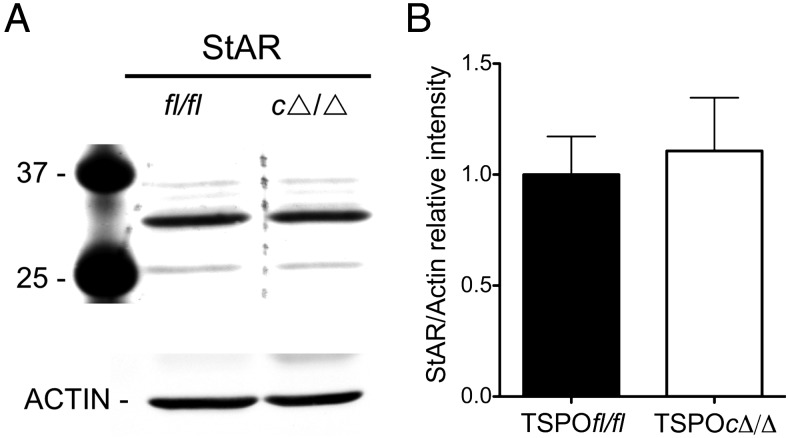
StAR protein expression was not different between TSPOfl/fl and TSPOcΔ/Δ testis (Figure 5). Expression levels for StAR, CYP11A1, and HSD3B1 (3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase type I) in the testis were examined by quantitative RT-PCR. TSPO was not expressed in the TSPOcΔ/Δ testis (Figure 6A). Expression levels for StAR, CYP11A1, and HSD3B1 were not different between TSPOfl/fl and TSPOcΔ/Δ testis (Figure 6, B–D). In the ovary, the apparent decrease in TSPO mRNA expression was not significant (Supplemental Figure 4A), and expression levels for StAR, CYP11A1, and HSD3B1 were not different between TSPOfl/fl and TSPOcΔ/Δ mice (Supplemental Figure 4, B–D).
Figure 5.
StAR expression is unchanged in TSPOcΔ/Δ testis. A, Representative Western blot showing no change in testicular StAR protein in TSPOcΔ/Δ compared with TSPOfl/fl mice; β-actin is shown as the loading control. B, Relative intensity of testicular StAR protein expression (ratios of StAR/β-actin band intensities) between TSPOfl/fl and TSPOcΔ/Δ mice was not significantly different (n = 3/group).
Figure 6.
TSPO deletion does not affect expression of genes involved in testicular steroidogenesis. A, TSPO expression was undetectable in TSPOcΔ/Δ testis. StAR (B), CYP11A1 (C), and HSDB1 (D) expression levels were similar between TSPOfl/fl and TSPOcΔ/Δ testes. E, TSPO2 expression was not detectable in TSPOfl/fl and TSPOcΔ/Δ testes. Femur bone marrow was used as a positive control. (mean ± SEM; ND*, not detected; n = 6/group)
TSPO2 is not expressed in the testis
TSPO2 is a paralogous gene related to TSPO that remains to be completely characterized. We checked for TSPO2 expression in the testis and ovary to rule out the possibility for a redundant function. TSPO2 mRNA was not expressed in both the TSPOfl/fl and TSPOcΔ/Δ testis or ovary (Figure 6E and Supplemental Figure 4E).
Discussion
For almost 25 years, TSPO has been indicated as a required element in the model for steroid hormone biosynthesis (12). Our findings refute this prevailing dogma in proving that TSPO function is not required for steroidogenesis. In a Leydig cell-specific conditional deletion, we definitively demonstrate that deficiency of TSPO does not affect testosterone production in male mice.
We decided to target Leydig cells not only because they express TSPO at a high level but also because most functional explorations on TSPO that suggested a link to steroid hormone production were carried out using Leydig tumor cell lines (MA-10 or R2C) in vitro (19, 23, 25). Moreover, a phenotypic measure of potential Leydig cell dysfunction and testosterone deficiency could be carried out with precision by examining male reproductive development and fertility without overlapping responses in other organ systems and affecting mouse survival. In this study, complete deletion of Leydig cell TSPO in TSPOcΔ/Δ mice did not change plasma testosterone levels. Testosterone production in TSPOfl/fl and TSPOcΔ/Δ mice after stimulation with hCG was also identical. These findings clearly show that TSPO is not required for the steroidogenic pathway and that StAR does not require TSPO to accomplish cholesterol translocation to the IMM as previously suggested (25). Considering that StAR-knockout mice show failure to produce steroid hormones (10) despite the presence of functional TSPO, there is also no direct indication for an overlapping or sequential function for TSPO and StAR as previously modeled (12).
Given these unexpected findings, we strongly considered the possibility of a functionally redundant protein. Existence of a paralogous gene TSPO2 that is present in birds and mammals highlighted the possibility for a compensatory mechanism. TSPO2 has a 35% homology to TSPO but is localized to the endoplasmic reticulum and not the mitochondria (42). Despite its conserved cholesterol-binding property, TSPO2 expression was found to be restricted to the bone marrow with a function linked to cholesterol trafficking in erythropoiesis (42). In this study, TSPO2 expression was not detectable in TSPOfl/fl and TSPOcΔ/Δ testis or ovary, suggesting that there is no involvement for TSPO2 in this phenotype.
Binding of the acyl-coenzyme A-binding protein (previously known as diazepam-binding inhibitor/DBI) to TSPO was initially suggested to be a key regulator for its role in steroidogenesis (43). However, acyl-coenzyme A-binding protein -knockout mice showed a delayed adaptation in liver metabolism but did not have a phenotype associated with steroid hormone biosynthesis (44). Combining this evidence with what we discovered regarding TSPO in this study, it is evident that TSPO-associated mitochondrial pathways are poorly understood. Because we did not find even a small decline in testosterone biosynthesis in TSPOcΔ/Δ mice, it is clear that focus may need to shift from steroidogenesis to explore other functional pathways that would explain TSPO function and simultaneously link the various putative effects observed when modulating TSPO with small molecules (26).
Use of cell lines in nonphysiological in vitro systems have perhaps given raise to alternative pathways that suggest an essential role for TSPO in steroid hormone biosynthesis. Previous efforts have been made to integrate data on TSPO function to the StAR protein mechanism involved in steroidogenesis (45). It is possible that TSPO function could be rate limiting only in Leydig tumor cell models like MA-10 and R2C in which the rate of steroid hormone biosynthesis is much slower than in adrenal cells. Studies using adrenal Y-1 cells have suggested that minute amounts of newly synthesized intramitochondrial StAR are sufficient for maximal sustained steroidogenesis (46). However, this maximal activation preceded maximal StAR expression, leading to speculation that there might be other players in this process. Therefore, direct testing of adrenal steroidogenesis using an adrenal-specific TSPOcΔ/Δ model in vivo remains a necessary future direction. Moreover, partial loss of TSPO as a mosaic in TSPOcΔ/Δ ovaries had no effect on steroid hormone levels (estrogen and progesterone), and reproductive function. Although this indicates that TSPO is unlikely to be involved in ovarian steroidogenesis, there is still need for studies directly examining ovarian steroidogenesis after complete TSPO deletion in all steroidogenic cells.
In recent years, TSPO has gained immense interest as a therapeutic target for neurologic disorders (47, 48). Microglia and astrocytes overexpress TSPO in regions of traumatic brain injury (TBI), ischemic stroke, neuroinflammation, demyelination, and large amyloid deposits (reviewed in Ref. 48). Several studies showed that treatment with small-molecule TSPO ligands improved functional recovery in a variety of the above neurologic disorders (49, 50). One of the key mechanisms underlying protective effects has been highlighted as stimulation of mitochondrial steroid synthesis. We have also recently reported the efficacy of a TSPO ligand etifoxine in ameliorating the severity of multiple sclerosis in a mouse model (51). Therefore, evidence is undeniable that targeting TSPO using ligands might provide unique benefits in neurologic therapeutics. But because the precise function for TSPO in mediating these effects remains unclear, the nature of action mediated by these small molecule ligands, whether agonistic or antagonistic, is not known. As a result, it remains to be determined whether benefits are actually achieved by inhibition or by activation of TSPO function, whatever it may be, in these disease models.
Our results have raised many questions about the biological role of TSPO. Although TSPO structure has been modeled as a putative cholesterol transporter (48, 52), direct experimental proof for cholesterol translocation by TSPO does not exist. In the Rhodobacter sphaeroides TSPO structure (10-Å resolution), it is not possible to assign amino acid sequences (15). Therefore, orientation of CRAC in the TSPO 3-dmensional map remains unknown. This keeps possibilities open for CRAC function in TSPO either for cholesterol transport or simply for cholesterol association as seen for myelin P0 (53). Recent studies have also shown that CRAC sequences can function to target proteins to cholesterol-rich membrane rafts (54). VDAC, an integral member, together with TSPO of the multimeric protein complex known as the mitochondrial permeability transition pore, is a resident protein of membrane rafts (55). Therefore, it is conceivable that the CRAC sequence in TSPO merely explains its association with the mitochondrial permeability transition pore and promotes the proposed interaction between VDAC and TSPO in apoptosis (56). Our findings in this study do not support the model for TSPO involvement in cholesterol translocation for steroid hormone biosynthesis.
From an evolutionary point of view, TSPO is an extremely conserved protein. A close functional relationship extending from bacteria to mammals was demonstrated by the ability of rat TSPO to substitute for photosynthetic R. sphaeroides TSPO in negatively regulating the expression of photosynthesis genes in response to oxygen (57). This activity for TSPO could be mediated, in part, by its conserved property of porphyrin binding (58, 59). These conserved functions need more rigorous exploration in mammalian systems for explaining TSPO actions.
In this manuscript, using an in vivo model, we have made the significant observation that TSPO is not essential for steroid hormone biosynthesis. Expression of TSPO in multiple tissue types, both steroidogenic (adrenal, testis, ovary, etc.,) and nonsteroidogenic (liver, heart, kidney, etc.,), warrants careful reexamination of its conserved functional properties. TSPOfl/fl mice generated in this study will be a key resource for producing different tissue-specific knockout models to decipher TSPO function in future studies.
Acknowledgments
We thank Richard R. Behringer (University of Texas M.D. Anderson Cancer Center) for providing Amhr2cre/+ mice used in these studies and Susan M. Quirk (Cornell University) for providing expert opinion and critically reviewing this manuscript. We also thank Sasha S. Wirth and her team at the University of California Davis Mouse Biology Program for providing expert service in generating TSPO floxed mice.
This study was supported by start-up funds from Cornell University (to V.S.). Generation of TSPOfl/fl mice was funded by the NIH grant (R01-NS059043) (to W.D.).
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 6 and For Counterpoint see page 15
- Amhr2
- Anti-Mullerian hormone receptor type II
- CRAC
- cholesterol recognition amino acid consensus
- CYP11A1
- cytochrome P450 side chain cleavage
- hCG
- human chorionic gonadotropin
- HSD3B1
- 3β-hydroxysteroid dehydrogenase/δ5-δ4 isomerase type I
- IMM
- inner mitochondrial membrane
- OMM
- outer mitochondrial membrane
- PBR
- peripheral benzodiazepine receptor
- StAR
- steroidogenic acute regulatory protein
- TSPO
- translocator protein
- VDAC
- voltage-dependent anion channel.
References
- 1. Simpson ER, Boyd GS. The cholesterol side-chain cleavage system of bovine adrenal cortex. Eur J Biochem. 1967;2(3):275–285 [DOI] [PubMed] [Google Scholar]
- 2. Yago N, Ichii S. Submitochondrial distribution of components of the steroid 11β-hydroxylase and cholesterol sidechain-cleaving enzyme systems in hog adrenal cortex. J Biochem. 1969;65(2):215–224 [PubMed] [Google Scholar]
- 3. Churchill PF, Kimura T. Topological studies of cytochromes P-450scc and P-45011β in bovine adrenocortical inner mitochondrial membranes. Effects of controlled tryptic digestion. J Biol Chem. 1979;254(20):10443–10448 [PubMed] [Google Scholar]
- 4. Mukhin AG, Papadopoulos V, Costa E, Krueger KE. Mitochondrial benzodiazepine receptors regulate steroid biosynthesis. Proc Natl Acad Sci USA. 1989;86(24):9813–9816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krueger KE, Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem. 1990;265(25):15015–15022 [PubMed] [Google Scholar]
- 6. Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269(45):28314–28322 [PubMed] [Google Scholar]
- 7. Lin D, Sugawara T, Strauss JF, 3rd, et al. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267(5205):1828–1831 [DOI] [PubMed] [Google Scholar]
- 8. Baker BY, Lin L, Kim CJ, et al. Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. J Clin Endocrinol Metab. 2006;91(12):4781–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Metherell LA, Naville D, Halaby G, et al. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2009;94(10):3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94(21):11540–11545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller WL. StAR search–what we know about how the steroidogenic acute regulatory protein mediates mitochondrial cholesterol import. Mol Endocrinol. 2007;21(3):589–601 [DOI] [PubMed] [Google Scholar]
- 12. Papadopoulos V, Miller WL. Role of mitochondria in steroidogenesis. Best Pract Res Clin Endocrinol Metab. 2012;26(6):771–790 [DOI] [PubMed] [Google Scholar]
- 13. Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci USA. 1977;74(9):3805–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Souza EB, Anholt RR, Murphy KM, Snyder SH, Kuhar MJ. Peripheral-type benzodiazepine receptors in endocrine organs: autoradiographic localization in rat pituitary, adrenal, and testis. Endocrinology. 1985;116(2):567–573 [DOI] [PubMed] [Google Scholar]
- 15. Korkhov VM, Sachse C, Short JM, Tate CG. Three-dimensional structure of TspO by electron cryomicroscopy of helical crystals. Structure. 2010;18(6):677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teboul D, Beaufils S, Taveau JC, et al. Mouse TSPO in a lipid environment interacting with a functionalized monolayer. Biochim Biophys Acta. 2012;1818(11):2791–2800 [DOI] [PubMed] [Google Scholar]
- 17. McEnery MW, Snowman AM, Trifiletti RR, Snyder SH. Isolation of the mitochondrial benzodiazepine receptor: association with the voltage-dependent anion channel and the adenine nucleotide carrier. Proc Natl Acad Sci USA. 1992;89(8):3170–3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Papadopoulos V. Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology. 1998;139(12):4991–4997 [DOI] [PubMed] [Google Scholar]
- 19. Papadopoulos V, Mukhin AG, Costa E, Krueger KE. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990;265(7):3772–3779 [PubMed] [Google Scholar]
- 20. Garren LD, Gill GN, Masui H, Walton GM. On the mechanism of action of ACTH. Recent Prog Horm Res. 1971;27:433–478 [DOI] [PubMed] [Google Scholar]
- 21. Crivello JF, Jefcoate CR. Mechanisms of corticotropin action in rat adrenal cells. I. The effects of inhibitors of protein synthesis and of microfilament formation on corticosterone synthesis. Biochim Biophys Acta. 1978;542(2):315–329 [DOI] [PubMed] [Google Scholar]
- 22. Alberta JA, Epstein LF, Pon LA, Orme-Johnson NR. Mitochondrial localization of a phosphoprotein that rapidly accumulates in adrenal cortex cells exposed to adrenocorticotropic hormone or to cAMP. J Biol Chem. 1989;264(4):2368–2372 [PubMed] [Google Scholar]
- 23. Papadopoulos V, Amri H, Li H, Boujrad N, Vidic B, Garnier M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J Biol Chem. 1997;272(51):32129–32135 [DOI] [PubMed] [Google Scholar]
- 24. Bose HS, Lingappa VR, Miller WL. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature. 2002;417(6884):87–91 [DOI] [PubMed] [Google Scholar]
- 25. Hauet T, Yao ZX, Bose HS, et al. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol. 2005;19(2):540–554 [DOI] [PubMed] [Google Scholar]
- 26. Gavish M, Bachman I, Shoukrun R, et al. Enigma of the peripheral benzodiazepine receptor. Pharmacol Rev. 1999;51(4):629–650 [PubMed] [Google Scholar]
- 27. Papadopoulos V. In search of the function of the peripheral-type benzodiazepine receptor. Endocr Res. 2004;30(4):677–684 [DOI] [PubMed] [Google Scholar]
- 28. Papadopoulos V, Baraldi M, Guilarte TR, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27(8):402–409 [DOI] [PubMed] [Google Scholar]
- 29. Papadopoulos V, Amri H, Boujrad N, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62(1):21–28 [DOI] [PubMed] [Google Scholar]
- 30. Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28(3–4):106–110 [PubMed] [Google Scholar]
- 31. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32(3):408–410 [DOI] [PubMed] [Google Scholar]
- 32. Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selvaraj V, Asano A, Page JL, et al. Mice lacking FABP9/PERF15 develop sperm head abnormalities but are fertile. Dev Biol. 2010;348(2):177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beam SW, Butler WR. Energy balance and ovarian follicle development prior to the first ovulation postpartum in dairy cows receiving three levels of dietary fat. Biol Reprod. 1997;56(1):133–142 [DOI] [PubMed] [Google Scholar]
- 35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods. 2001;25(4):402–408 [DOI] [PubMed] [Google Scholar]
- 36. Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J. Constitutive WNT/β-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod. 2010;82(2):422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morohaku K, Phuong NS, Selvaraj V. Developmental expression of translocator protein/peripheral benzodiazepine receptor in reproductive tissues. PLOS One. 2013;8(9):e74509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boerboom D, Paquet M, Hsieh M, et al. Misregulated Wnt/β-catenin signaling leads to ovarian granulosa cell tumor development. Cancer Res. 2005;65(20):9206–9215 [DOI] [PubMed] [Google Scholar]
- 39. Pangas SA, Li X, Robertson EJ, Matzuk MM. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol. 2006;20(6):1406–1422 [DOI] [PubMed] [Google Scholar]
- 40. Barkley MS, Goldman BD. A quantitative study of serum testosterone, sex accessory organ growth, and the development of intermale aggression in the mouse. Horm Behav. 1977;8(2):208–218 [DOI] [PubMed] [Google Scholar]
- 41. Coquelin A, Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am J Physiol. 1982;243(3):E257–E263 [DOI] [PubMed] [Google Scholar]
- 42. Fan J, Rone MB, Papadopoulos V. Translocator protein 2 is involved in cholesterol redistribution during erythropoiesis. J Biol Chem. 2009;284(44):30484–30497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papadopoulos V, Berkovich A, Krueger KE, Costa E, Guidotti A. Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology. 1991;129(3):1481–1488 [DOI] [PubMed] [Google Scholar]
- 44. Neess D, Bloksgaard M, Bek S, et al. Disruption of the acyl-CoA-binding protein gene delays hepatic adaptation to metabolic changes at weaning. J Biol Chem. 2011;286(5):3460–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jefcoate C. High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J Clin Invest. 2002;110(7):881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Artemenko IP, Zhao D, Hales DB, Hales KH, Jefcoate CR. Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J Biol Chem. 2001;276(49):46583–46596 [DOI] [PubMed] [Google Scholar]
- 47. Papadopoulos V, Lecanu L. Translocator protein (18 kDa) TSPO: an emerging therapeutic target in neurotrauma. Exp Neurol. 2009;219(1):53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rupprecht R, Papadopoulos V, Rammes G, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat Rev Drug Discov. 2010;9(12):971–988 [DOI] [PubMed] [Google Scholar]
- 49. Mills C, Makwana M, Wallace A, et al. Ro5–4864 promotes neonatal motor neuron survival and nerve regeneration in adult rats. Eur J Neurosci. 2008;27(4):937–946 [DOI] [PubMed] [Google Scholar]
- 50. Girard C, Liu S, Cadepond F, et al. Etifoxine improves peripheral nerve regeneration and functional recovery. Proc Natl Acad Sci USA. 2008;105(51):20505–20510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daugherty DJ, Selvaraj V, Chechneva OV, Liu XB, Pleasure DE, Deng W. A TSPO ligand is protective in a mouse model of multiple sclerosis. EMBO Mol Med. 2013;5(6):891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lacapère JJ, Papadopoulos V. Peripheral-type benzodiazepine receptor: structure and function of a cholesterol-binding protein in steroid and bile acid biosynthesis. Steroids. 2003;68(7–8):569–585 [DOI] [PubMed] [Google Scholar]
- 53. Luo X, Sharma D, Inouye H, et al. Cytoplasmic domain of human myelin protein zero likely folded as β-structure in compact myelin. Biophys J. 2007;92(5):1585–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xie HQ, Liang D, Leung KW, et al. Targeting acetylcholinesterase to membrane rafts: a function mediated by the proline-rich membrane anchor (PRiMA) in neurons. J Biol Chem. 2010;285(15):11537–11546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Herrera JL, Diaz M, Hernández-Fernaud JR, et al. Voltage-dependent anion channel as a resident protein of lipid rafts: post-transductional regulation by estrogens and involvement in neuronal preservation against Alzheimer's disease. J Neurochem. 2011;116(5):820–827 [DOI] [PubMed] [Google Scholar]
- 56. Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J Bioenerg Biomembr. 2008;40(3):199–205 [DOI] [PubMed] [Google Scholar]
- 57. Yeliseev AA, Krueger KE, Kaplan S. A mammalian mitochondrial drug receptor functions as a bacterial “oxygen” sensor. Proc Natl Acad Sci USA. 1997;94(10):5101–5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kessel D. Interactions between porphyrins and mitochondrial benzodiazepine receptors. Cancer Lett. 1988;39(2):193–198 [DOI] [PubMed] [Google Scholar]
- 59. Zeng X, Kaplan S. TspO as a modulator of the repressor/antirepressor (PpsR/AppA) regulatory system in Rhodobacter sphaeroides 2.4.1. J Bacteriol. 2001;183(21):6355–6364 [DOI] [PMC free article] [PubMed] [Google Scholar]