Abstract
Our previous work has shown that Akt3 is required for mitochondrial biogenesis in primary human endothelial cells (ECs) and in Akt3-null mice; Akt3 affects subcellular localization of peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1α), the master regulator of mitochondrial biogenesis. The purpose of this study is to determine the mechanism by which Akt3 controls the subcellular distribution of PGC-1α and to explore the effect on mitochondrial biogenesis and turnover during angiogenesis. Here we use standard biochemical analyses and Akt3-knockdown strategies to show that Akt3 controls the stabilization of chromosome maintenance region-1 (CRM-1), the major nuclear export receptor. Site-directed mutagenesis and association analyses show that PGC-1α nuclear export is CRM-1 dependent. Akt3 knockdown and CRM-1 overexpression cause 3-fold reductions in PGC-1α target gene expression, compared to control levels. Akt3 inhibition causes autophagy, as measured by autophagosome formation, in a CRM-1-dependent, Akt1/mTOR-independent pathway. In vivo, Akt3-null and heterozygous mice show dose-dependent decreases in angiogenesis compared to wild-type littermates (∼5- and 2.5-fold decreases, respectively), as assessed by Matrigel plug assays. This correlates with an ∼1.5-fold decrease in mitochondrial Cox IV expression. Our studies suggest that Akt3 is a regulator of mitochondrial dynamics in the vasculature via regulation of CRM-1-dependent nuclear export.—Corum, D. G., Tsichlis, P. N., Muise-Helmericks, R. C. AKT3 controls mitochondrial biogenesis and autophagy via regulation of the major nuclear export protein CRM-1.
Keywords: angiogenesis, endothelial cells, mitophagy
The vascular system is central to myriad processes critical to human health, including proper gas exchange, nutrient delivery, and metabolic waste removal (1). Proper maintenance and renewal of the vasculature is therefore critical to ensuring these processes are not interrupted and optimal health is maintained. Angiogenesis, the creation of new vessels from the existing vasculature, is responsible for the bulk of new vessel formation in the adult. Defects in angiogenesis occur in a wide range of pathologies, including those where angiogenesis is aberrantly active such as macular degeneration and cancer, as well as in ischemic conditions such as stroke and coronary artery disease. It is of no surprise then that therapies targeting angiogenesis have wide-ranging applications and have become subjects of intense research.
Angiogenesis is a profoundly dynamic and anabolic process, requiring endothelial cells (ECs) to switch from a quiescent, lower-energy state to a high-energy state capable of growth, proliferation, and migration, finally resolving to a lower-energy state on establishment of the vessel (2, 3). How ECs cope with these stark changes in energy demand is an open question. Increased energy demand is typically met with increased mitochondrial biogenesis. Mitochondrial oxidative metabolism and respiratory capacity are controlled by a coordinated transcriptional program induced in response to high-energy demands such as exercise, fasting, and cold exposure (4). A key player in this regulation is peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), a transcriptional coactivator shown to control genes involved in mitochondrial biogenesis and oxidative metabolism. PGC-1α controls expression of both mitochondrial and nuclear encoded mitochondrial genes, directly affecting cellular oxidative capacity and fatty acid catabolism (5–8). PGC-1α is, therefore, thought to be a central regulator of bioenergetic functions and mitochondrial biogenesis.
Total mitochondrial content of a cell is controlled through a balance between mitochondrial biogenesis and mitochondrial turnover by the autophagosome (mitophagy). Mitophagy is the key quality control mechanism by which cells can sequester and degrade dysfunctional or aged mitochondria in order to minimize oxidative damage. In addition, mitophagy allows the cell to dynamically tailor mitochondrial content to match energy requirements, retaining only enough mitochondria to meet ATP requirements, as even healthy mitochondria are a source of oxidative stress (9). Under conditions of steady-state energy requirements, de novo biogenesis matches the rate of turnover, and overall content remains constant (10). When energy demand increases, the rate of biogenesis exceeds the rate of turnover, increasing mitochondrial content and respiratory capacity. However, once a cell's energy demands decreases, the rate of mitophagy can be increased to degrade superfluous mitochondria in order to ensure optimal cellular energetics, minimal oxidative stress, and long-term vascular health.
The vascular endothelial growth factor (VEGF)/phosphoinositol-3-kinase (PI3K)/protein kinase B (PKB; Akt) pathway is a well-established a proangiogenic pathway. In particular, PI3K/Akt have been shown to be important regulators of cellular survival, cell motility, and nitric oxide production (11, 12). Studies focusing on the role of Akt have demonstrated that Akt signaling plays a key role in vascular morphogenesis (13–16). Constitutive activation of Akt1 in the vasculature results in marked increases in angiogenesis (17), albeit resulting in “leaky” vasculature. Members of the Akt/PKB family of serine/threonine kinases exist in 3 nonredundant isoforms. The isoforms (Akt1–3) share 80% sequence homology and are all downstream effectors of VEGF via PI3K (18).
Though Akt-dependent signaling, especially as driven by Akt1 activation, is known to be important for angiogenesis, the roles of the individual isoforms are less well understood. In general, it is accepted that Akt family members share a degree of functional redundancy. However, there is growing evidence that these 3 kinases have isoform-specific targets and functions (19–25). The mild nature of the PKBα (Akt1), PKBβ (Akt2), and PKBγ (Akt3) knockouts supports the idea of redundancy in downstream function (26–28). However, it is important to note that all reports describe animals on a mixed genetic background, which can lead to the expression of genetic modifiers that can inhibit or enhance true, gene-specific phenotypes (29).
Previously we have compared Akt1 vs. Akt3 function in ECs, where these are the only Akt isoforms expressed (30), and found a direct link between Akt3 and mitochondrial biogenesis that is independent of Akt1 and not attributable to total kinase activity (2). Indeed, loss of Akt3 resulted in a nuclear to cytoplasmic shift of PGC-1α, down-regulation of PGC-1α-dependent gene expression, decreased respiration, and decreased mitochondrial content (2). Loss of Akt1 had no effect on mitochondrial gene expression or mitochondrial content. These findings suggest that Akt3 is a key regulator of endothelial cell energetics.
The purpose of the current study is to explore the mechanism by which Akt3 affects PGC-1α nuclear/cytoplasmic shuttling and to determine whether the decreased mitochondrial content in response to Akt3 knockdown was due to decreased biogenesis alone or due to a concomitant increase in autophagy. Understanding the molecular mechanisms responsible for the maintenance of cellular energy demands in regard to new vessel formation is a prerequisite to designing new angiogenic therapies for the treatment of a broad range of human diseases linked to mitochondrial function (31, 32).
MATERIALS AND METHODS
Mice
All procedures and animal care were approved by the institutional research committee and conformed to the animal care guidelines of the Medical University of South Carolina. Both Akt1 (33) and Akt3-null animals (28) were backcrossed onto C57Bl6 for 8 generations. C57Bl6 littermates were used as controls.
Cell culture, transfection, and transduction
Pooled, multiple-donor human umbilical vein endothelial cells (HUVECs; Lonza, Basel, Switzerland) were maintained at 37°C with 5% CO2 in endothelial basal medium 2 (Lonza) supplemented with EGM-2 SingleQuots. HUVECs were transfected using an Amaxa Nucleofection system (Lonza) in procedures described by the manufacturer. Briefly, 2 × 106 cells/cuvette were transfected, using no more than 5 μg/transfection. All transfections were monitored by expression of GFP using GFP expression vector pGFP-C1 (Clontech, Mountain View, CA, USA) or a GFP-directed RNAi (Lonza). Akt1 RNAi was purchased from Thermo Scientific (Pittsburgh, PA, USA), Akt3 RNAi from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Expression plasmids for GFP-tagged PGC-1α (Addgene Plasmid 17647; Addgene, Cambridge, MA, USA; ref. 34) and FLAG-tagged CRM-1 (Addgene Plasmid 4; ref. 35) were provided to Addgene by the respective researchers.
Mission shRNA lentiviral constructs directed against Akt1 and Akt3 were purchased from Sigma-Aldrich (St. Louis, MO, USA). A scrambled pLKO.1 shRNA vector was purchased from Addgene (Plasmid 1864). Lentiviruses were propagated by the Maine Medical Center Cell Culture and Viral Core Facility (Scarborough, ME, USA). For transduction of target cells, 1 × 106 cells were transduced using a final concentration of 1 μg/ml polybrene and either scrambled control or Akt1 or Akt3 shRNA lentiviruses. All transductions were monitored for appropriate knockdown by RT-PCR. Akt primer sequences are as follows: Akt1F 5′-ATGAGCGACGTGGCTATTGTGAAG-3′, Akt1R 5′-GAGGCCGTCAGCCACAGTCTGGAT-3′, Akt3F 5′-ATGAGCGATGTTACCATTGT-3′, Akt3R 5′-CAGTCTGTCTGCTACAGCCTG-3′.
Real-time PCR
cDNA was synthesized from 2 μg of total RNA with a Superscript First Strand Synthesis Kit purchased from Life Technologies (Grand Island, NY, USA) using Oligo(dT) following the manufacturer's instructions. Real-time PCR for CRM-1 was performed using a Brilliant CYBR green QPCR kit in combination with an Mx3000P Real-Time PCR system, both purchased from Stratagene (La Jolla, CA, USA). For Sirt3 and MCAD, real-time PCR was performed on the Light Cycler 480, obtained from Roche Diagnostics (Indianapolis, IN, USA). Real-time PCR was performed ≥3 independent times and at least in triplicate. Error bars showing the sd are shown. All primer sequences used in these analyses are as follows: CRM-1F 5′-AGGAAGGAGCAGTTGGTTCA-3′, CRM-1R 5′-TATTCCTTCGCACTGGTTCC-3′, S26F 5′-CTCCGGTCCGTGCCTCCAAG-3′, S26R 5′-CAGAGAATAGCCTGTCTTCAG-3′, Sirt3F 5′-CTTGTGCAGCGGGAAACT3-′, Sirt3R 5′-TCCTATGTTACCATTTATTGTGTGG-3′, MCADF 5′-AGGACCATTGATGTGTGC, MCADR 5′-CTGCTTTGGTCTTTATACCAGCTA.
Site-directed mutagenesis
Phosphospecific Akt-dependent sites on CRM-1 were determined using Scansite motif scanning software (http://scansite.mit.edu/motifscan_seq.phtml). Putative nuclear export sequences were determined using NES Finder 0.2 software (http://research.nki.nl/fornerodlab/NES-Finder.htm). Site-directed mutagenesis of the putative NES sequences in the GFP-PGC-1 vector and the putative Akt phosphorylation site in the Flag-CRM-1 vector were performed by DNAExpress (Montreal, QC, Canada). Sequence changes are as follows: PGC-1 NES 1 (+205): 5′-GATCTTCCTGAACTTGATCTT-3′, where C = A, changing leucine to an alanine; PGC-1 NES 2 (+394): 5′-CTCACAGAGACACTAGACAGTCTCCCTGTG-3′, where CT = GC and T = C, changing leucine or valine (respectively) to alanine; and CRM-1 Ser1054Ala: RQMS1154VP to RQMA1054VP (tct to gct).
Immunoprecipitation and Western blotting
Antibodies used for Western blotting and/or immunoprecipitations are anti-CRM-1 (Calbiochem, La Jolla, CA, USA), anti-p85 subunit of PI3 kinase (Millipopre-Upstate, Billerica, MA, USA), anti-FLAG M2 (Stratagene), p62/SQSTM1 (Santa Cruz Biotechnology), and anti-PGC-1α (Santa Cruz Biotechnology). Appropriate HRP-conjugated secondary antibodies were purchased from Invitrogen-Caltag (Camarillo, CA, USA). Proteins were visualized using Luminol Reagent 50 (Santa Cruz Biotechnology).
Treated cells were washed once with PBS and lysed in 1× RIPA Lysis Buffer (50 mM Tris-HCl, pH 7.5; 1% Triton X-100; 150 mM NaCl; 0.1% SDS; 1% sodium deoxycholate; and 40 mM NaF), supplemented with Complete Protease Inhibitors without EDTA (Roche, Palo Alto, CA, USA) and 200 μM sodium orthovanadate. For immunoprecipitations, 200–400 μg of total protein lysate was incubated with primary antibodies directed against CRM-1, or PGC-1α at 4°C overnight in Nonidet P-40 lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 40 mM NaF; 0.5 mM Na3PO4; and 1% Nonidet P-40) supplemented with Complete Protease Inhibitors without EDTA (Roche) and 200 μM Na3PO4. Antibodies were precipitated using protein A/G bound to Sepharose beads (Amersham, Piscataway, NJ, USA) for 2 h at 4°C. Beads were collected by centrifugation, washed, incubated in 25 μl of 5× loading buffer for 5 min at 90°C, then resolved by SDS-PAGE and subjected to immunoblotting as described below. Protein concentrations were determined by a BCA protein assay (Pierce, Rockford, IL, USA), resolved by SDS-PAGE, and transferred onto Immobilon-P PVDF membranes (Millipore). Western analyses followed standard procedures. Proteins were visualized using Luminol Reagent (Santa Cruz Biotechnology).
For protein stability, HUVECs were treated with cyclohexamide (Sigma-Aldrich) at a concentration of 10 μg/ml to inhibit translation for the times indicated. Total protein lysates were subjected to Western blot analysis as described. Densitometry of the 0 and 24 h time points was performed, and CRM-1 expression at the 24 h time point is shown as a relative to CRM-1 expression at time 0. Statistical analysis was performed (Student's t test) comparing CRM-1 expression at 24 h for SCR vs. Akt3i or CRM-1 WT vs. S1054A (n≥3 for each experiment).
Immunofluorescence
Antibodies used for immunofluorescence are as follows: anti-LC3-B (Cell Signaling, Danvers, MA, USA), anti-total LC3 (Sigma), anti-GFP (Molecular Probes, Eugene, OR, USA), anti-α-smooth muscle actin (α-SMA; Sigma), and CoxIV (Cell Signaling). All fluorescent tagged secondary antibodies were purchased from Molecular Probes and Invitrogen. Transfected or treated HUVECs were seeded onto polylysine-coated coverslips, fixed in 3.7% formaldehyde for 20 min, and washed briefly in 1× PBS. Cells were permeabilized in 0.1% Triton 100-X for 20 min, washed briefly in 1× PBS, and blocked for 30 min in 5% BSA-PBS while undergoing gentle agitation. Cells were subsequently incubated in primary antibody for 1 h at room temperature, washed in 1× PBS, and incubated with the appropriate fluorescent secondary antibody for 1 h at room temperature. After washing in 1× PBS, cell nuclei were stained with DAPI nuclear dye, and coverslips were mounted to slides using Fluorogel (Electron Microscopy Sciences, Hatfield, PA, USA). Coverslips were imaged on a Zeiss Axio Imager M2 fluorescent microscope (Carl Zeiss, Oberkochen, Germany).
Matrigel sections
Paraffin-embedded Matrigel sections were deparaffinized using Histo-Clear II (National Diagnostics, Atlanta, GA, USA) and rehydrated in an ethanol series with increasing amounts of water. On hydration, sections were permeablized in 0.1% Triton 100-X for 10 min. Following a wash in 1× PBS, sections were blocked for 1 h in 5% BSA-PBS and incubated with appropriate primary antibody overnight at 4°C. Sections were washed in 1× PBS and incubated with appropriate fluorescent secondary antibody for 1 h at room temperature. After a final wash in 1× PBS, sections were stained with DAPI nuclear dye. All sections were imaged using a Zeiss Axio Imager M2 fluorescent microscope.
Matrigel angiogenesis assay
For the in vivo Matrigel assay, 500 μl of growth factor-reduced Matrigel (BD Biosciences, Bedford, MA, USA) supplemented with FGF (1 μg/ml) and VEGF (200 ng/ml) was injected subcutaneously into Akt3−/− and wild-type C57Bl6 mice contralaterally. After 7 d, the mice were euthanized by cervical dislocation, and the Matrigel plugs were harvested. Following fixation in 4% paraformaldehyde overnight at 4°C, the Matrigel plugs were embedded in paraffin, sectioned on a microtome into ∼4-μm sections, and mounted onto slides. Sections were subsequently stained using antibodies directed against α-SMA and Cox IV and imaged by immunofluorescence as described.
For the in vitro Matrigel assay, HUVECs were transfected with scramble control RNAi, Akt3 RNAi, or FLAG-CRM-1 for 48 h prior to plating onto Matrigel matrix (BD Biosciences). Equal cell numbers were used for each assay. Each assay was repeated at least in triplicate. For quantitation branch points were counted from 6 fields/well, 3 wells/assay.
Cain's method for staining mitochondria
Deparaffinized Matrigel sections from Akt3-null and wild-type mice were placed in hot analine-acid fuschin solution for ∼10 min and incubated in 0.1% sodium carbonate solution until pale pink. Following a quick rinse in 1% hydrochloric acid, sections were rinsed in distilled water, and then counterstained in methyl blue. Sections were again rinsed in water, dipped in 1% HCl, rinsed once more in water, and then dehydrated in graded alcohols and xylene (36). Sections were mounted in Cytoseal XYL (Thermo Scientific, Kalamazoo, MI, USA) and imaged using an upright Olympus Bx40 microscope (Olympus America, Melville, NY, USA).
Quantification of Cox IV staining
Matrigel sections from wild-type C57Bl6 mice, stained with antibody directed against Cox IV, were used to set the parameters (exposure time, gain, etc.) on a Zeiss Axio Imager M2. Matrigel sections from Akt3−/− mice were imaged under the exact same conditions as the wild-type mice. Fluorescence intensity was quantified from 4 Matrigel sections from 3 mice/genotype (n=12 sections/genotype) using ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA). Five random areas of equal size were analyzed for each image and for each genotype (n=60 random areas/genotype). Statistical analysis was performed (Student's t test) comparing the mean fluorescence intensities for each sample area analyzed between the genotypes. Fields either overcrowded or devoid of cells were excluded from the analysis.
RESULTS
Akt3-null mice fail to launch a meaningful angiogenic response
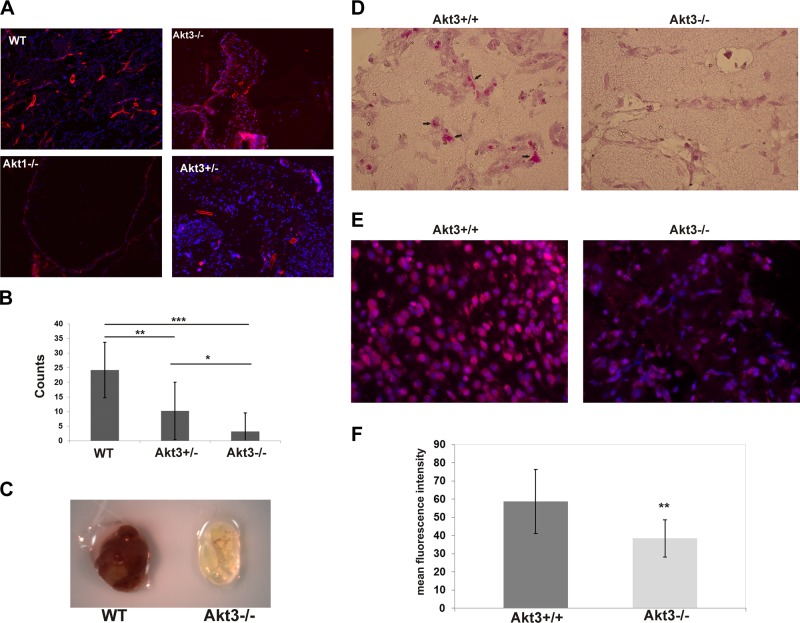
Previous reports characterizing Akt1-, Akt2-, and Akt3-knockout mice on mixed genetic backgrounds found mild, isoform-specific phenotypes, suggesting functional compensation by the other isoforms (26–28). To test the in vivo effect of Akt1 vs. Akt3 ablation on the angiogenic capacity of mice on pure genetic backgrounds, we performed Matrigel assays to assess for growth factor-induced vascular recruitment. It should be noted that on a pure C57Bl6 background, both the Akt1- and the Akt3-null animals have increased embryonic cell death, due at least in part to vascular phenotypes (data not shown and ref. 37). Surviving wild-type, Akt3-null, and Akt1-null animals were injected with Matrigel containing both VEGF and FGF, and vascularization of the Matrigel plugs was allowed to progress for 7 d. Resultant Matrigel plugs were stained with α-SMA to visualize established vessels by immunofluorescence. As shown in Fig. 1A, both Akt1- and Akt3-null animals fail to launch a meaningful angiogenic response by 7 d. However, phenotypically, the failure in angiogenesis between Akt3- and Akt1-null mice is in stark contrast to each other, suggesting unique, nonredundant requirements for each Akt isoform during angiogenesis. The plugs harvested from Akt3-null mice are replete with cells (Fig. 1A, top right), suggesting the failure is postrecruitment. In contrast, plugs harvested from Akt1-null mice (Fig. 1A, bottom left) show a failure in cellular infiltration of the plug, suggesting the failure is in cellular recruitment and/or decreased cell proliferation, both known functions of Akt1 (38). Given that the requirement of Akt3 in angiogenesis is not yet established, we focused on this isoform and further characterized its role in the vascular endothelium.
Figure 1.
Akt1- and Akt3-null animals have isoform-specific deficiencies in angiogenesis. A) Immunofluorescence of Matrigel plugs derived from wild-type, Akt3-null, Akt1-null, and Akt3-heterozygous animals stained with αSMA. B) Quantitation of vessels in the Matrigel plugs shown in A. *P < 0.05, **P < 0.01, ***P < 0.001. C) Representative image of wild-type and Akt3-null Matrigel plugs following harvest at 7 d. D) Images of wild-type and Akt3-null Matrigel sections stained using Cain's method for mitochondria. E) Immunofluorescence of mitochondrial CoxIV expression in cells within Matrigel plugs derived from wild-type and Akt3-null animals. DAPI nuclear stain is shown. F) Quantification of mean fluorescence intensity of CoxIV expression. **P < 0.01.
Our results suggest that Akt3 ablation leads to a gene-dose dependent decrease in angiogenesis. Figure 1A (bottom right) shows a representative image of a Matrigel plug harvested after 7 d from an Akt3 heterozygote stained with α-SMA. Quantitation of the α-SMA positive vessels (Fig. 1B) shows Akt3 heterozygotes have a statistically significant decrease in angiogenic response compared to wild-type littermates, yet a statistically significant increase in vessel number compared to Akt3-nulls. Akt3 heterozygote animals had a 2.5-fold reduction in vessel number compared to wild-type littermates, whereas the complete null animals showed at least a 5-fold reduction (Fig. 1B). A photograph of a typical Matrigel plug derived from wild-type and Akt3-null animals is shown in Fig. 1C. These results suggest Akt3 is required for growth-factor-induced angiogenic responses.
Akt3-null mice have decreased mitochondrial content during angiogenesis
Our previous work has shown the mitochondria in the brain of Akt3-null mice have altered phenotypes as visualized by EM. In addition to being fewer in number, they were also swollen and less electron dense (2, 39). To test whether the cells recruited during angiogenesis were similarly affected in Akt3-null mice, Matrigel sections were subjected to histological analysis using Cain's Method to specifically visualize mitochondria. Figure 1D shows Akt3-null mice present with an apparent decrease in the number of cells with positive staining compared to wild-type littermates (left panel, black arrows), suggesting Akt3-null mice have a mitochondrial deficit during angiogenesis.
To test whether this decreased mitochondrial content correlated with decreased mitochondrial specific gene expression, Matrigel sections were subjected to immunofluorescence using an antibody directed against mitochondrial marker Cox IV (Fig. 1E). Differences in fluorescence intensity were quantified by ImageJ software. Analysis of the images (Fig. 1F) revealed a statistically significant decrease (∼1.5-fold) in Cox IV staining in Akt3-null Matrigel sections as compared to wild-type controls. These findings support our original work (2) showing that Akt3 ablation results in a reduction in mitochondrial content and mitochondrial specific gene expression.
Akt3 controls the expression of CRM-1
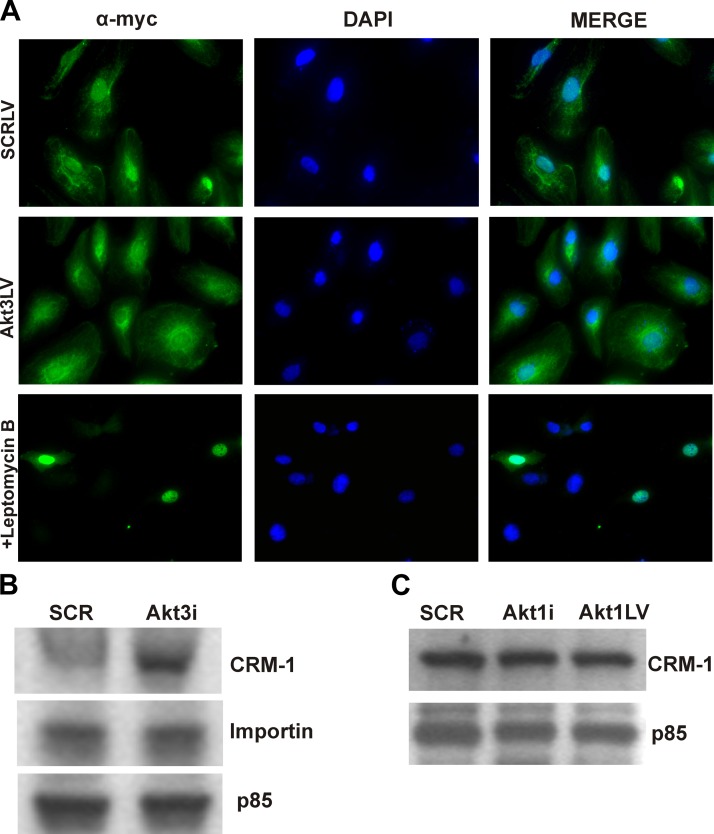
Our previous studies show that specifically Akt3 modulates mitochondrial biogenesis in HUVECs by controlling PGC-1α nuclear retention (2). To directly test whether PGC-1α nuclear export is controlled by major nuclear export receptor CRM-1 (exportin-1), HUVECs transfected with a myc-tagged PGC-1α construct were treated with nuclear export inhibitor, leptomycin B, a fungal antimycotic that covalently binds a critical cysteine residue within the cargo binding domain of CRM-1 (40). Figure 2A (top panels) shows the resulting immunofluorescence of ectopically expressed myc-tagged PGC-1α in HUVECs, indicating its nuclear and cytoplasmic localization. As previously demonstrated, Akt3 knockdown causes increased cytoplasmic localization of PGC-1α (2) (Fig. 2A, middle panels). Direct inhibition of CRM-1 by leptomycin B results in PGC-1α nuclear accumulation (Fig. 2A, bottom panels). These findings suggest that PGC-1α nuclear accumulation is, at least in part, regulated by CRM-1.
Figure 2.
Akt3 knockdown results in increased CRM-1 protein levels. A) Immunofluorescence of HUVECs transfected with a myc-tagged PGC-1α construct (green) in cells transfected with a SCR control (top panels), Akt3 RNAi (middle panels), or treated with leptomycin B (20 ng/ml; bottom panels). B) Western blot analysis of total protein isolated from HUVECs transfected with either scrambled control (SCR) or Akt3 RNAi (Akt3i) and assessed for CRM-1 and importin expression. The p85 PI3K subunit is shown as an internal control. C) Western blot analysis of total protein isolated from HUVECs transfected with either scrambled controlor Akt1 RNAi (Akti) or transduced with a lentiviral construct expressing shAkt1 (Akt1LV) and assessed for CRM-1 expression. p85 is shown as an internal control.
Others have shown PGC-1α activity is modulated by post-translational modifications, such as acetylation or phosphorylation (41). Akt3 knockdown had no apparent effect on post-translational modifications of PGC-1α (data not shown), suggesting that Akt3 does not directly affect PGC-1α. To assess whether Akt3 was directly affecting CRM-1, Western blot analyses were performed. As shown in Fig. 2B, transfection of HUVECs with RNAi directed against Akt3 (Akt3i) resulted in increased CRM-1 protein levels as compared to a scrambled control RNAi (SCR). We did not detect differences in the expression of the major nuclear import protein, importin-1. Knockdown of Akt1 by either RNAi transfection or by shRNA lentiviral transduction does not affect CRM-1 protein expression (Fig. 2C), showing specificity to Akt3. Confirmation of Akt3 and Akt1 knockdown are shown in Supplemental Fig. S1. These findings suggest that Akt3 blockade results in increased CRM-1 expression and that this increased expression results in PGC-1α nuclear export.
PGC-1α is a CRM-1 cargo protein
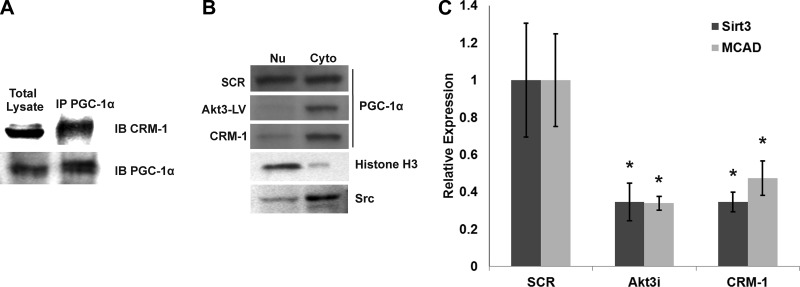
To assess whether CRM-1 associates with PGC-1α, coimmunoprecipitations were performed. As shown in Fig. 3A, immunoprecipitation of PGC-1α followed by Western blot analysis of CRM-1 shows potential interaction between these proteins. To test whether PGC-1α is a CRM-1-dependent cargo protein, HUVECs were transduced with an Akt3 shRNA lentivirus, a scrambled shRNA lentivirus, or transfected with a FLAG-tagged CRM-1 construct and subjected to nuclear/cytoplasmic fractionation. Western blot analysis of the nuclear/cytoplasmic fractions (Fig. 3B) shows that under control conditions, PGC1α is both cytoplasmic and nuclear, while both Akt3 down-regulation and CRM-1 overexpression result in a nuclear to cytoplasmic shift of PGC-1α. These results suggest that CRM-1 and PGC-1α associate, that PGC-1α is a CRM-1-dependent cargo protein, and that overexpression of CRM-1 increases PGC-1α cytoplasmic accumulation.
Figure 3.
PGC-1α is a CRM-1 cargo protein. A) Immunoprecipitation of PGC-1α followed by Western blot analysis of CRM-1 (top panel). Immunoprecipitation of PGC-1α followed by Western blot analysis of PGC-1α (bottom panel) shown as control for PGC-1α precipitation. B) Western blot analysis of PCG-1α in nuclear (Nu) and cytoplasmic (Cyto) fractions derived in HUVECs transduced with scrambled (SCR) or Akt3 shRNA lentiviruses (Akt3-LV), or transfection with a CRM-1 expression vector. Histone H3 shown as a nuclear control, and Src is shown as a cytoplasmic control. C) Real-time PCR analysis of Sirt3 and MCAD expression in HUVECs transfected with scrambled control RNAi (SCR), Akt3 RNAi (Akt3i), or FLAG-CRM-1. *P < 0.05.
Our previously published findings show that MCAD expression was controlled in an Akt3/PGC-1α-dependent manner (2). To validate the role of increased CRM-1-dependent transport on decreased PGC-1α-dependent transcription following Akt3 knockdown, we assessed mRNA levels of 2 PGC-1α-dependent nuclear encoded mitochondrial genes, MCAD and Sirt3 (42), under conditions of CRM-1 overexpression. As shown in Fig. 3C, both Akt3 inhibition and CRM-1 overexpression result in a statistically significant decrease in mRNA expression as determined by real-time PCR. Taken together, these results suggest that changes in CRM-1 protein levels, as modulated by Akt3 activity, are responsible for changes in PGC-1α-dependent nuclear encoded mitochondrial gene expression.
Akt3 controls CRM-1 protein stability
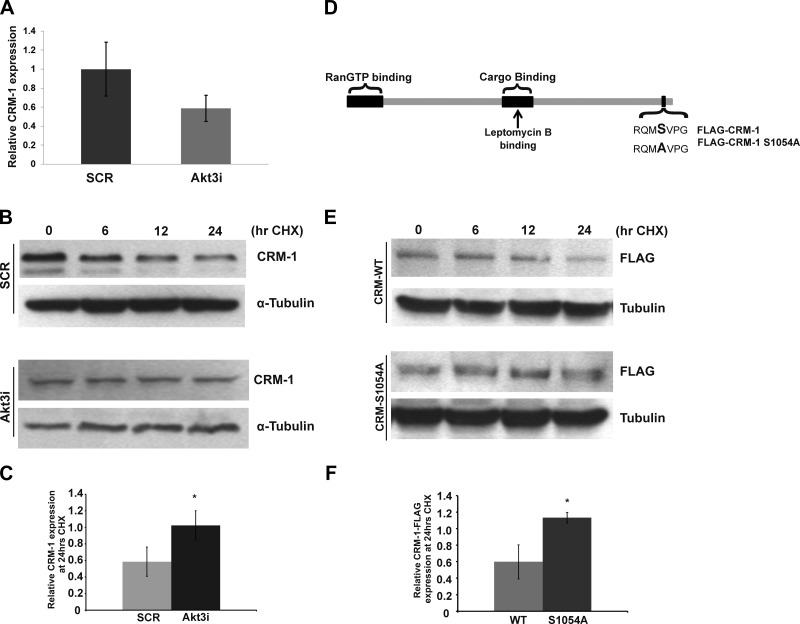
To address the mechanism of increased CRM-1 protein levels, we tested whether Akt3 affected CRM-1 mRNA levels by real-time PCR. Though there was a trend toward decreased mRNA expression following Akt3 knockdown, no statistically significant difference between Akt3 knockdown and control was detected (Fig. 4A). Next, we tested whether Akt3 knockdown resulted in changes in CRM-1 protein stability by treating HUVECs with cycloheximide to inhibit translation. Previously, Fornerod et al. (43) showed the half-life of CRM-1 to be ∼24 h. Therefore, we focused our analysis on the 24 h time point. Following transfection with SCR RNAi, we were able to show an approximate 50% decrease in CRM-1 protein levels at 24 h (Fig. 4B, top panel). However, following Akt3 knockdown, the CRM-1 protein level remains constant at the 24 h time point (Fig. 4B, bottom panel). These results are quantitated in Fig. 4C and show that CRM-1 protein levels are stabilized in the presence of Akt3 knockdown. These results suggest that Akt3 effects CRM-1 protein stability.
Figure 4.
Akt3 controls CRM-1 protein stability. A) Real-time PCR of mRNA isolated from HUVECs transfected with a scrambled control (SCR) or Akt3 RNAi (Akt3i) assessed for CRM-1 expression and shown as relative to S26. B) Western blot analysis of CRM-1 expression in total lysates derived from HUVECs transfected with SCR or Akt3i and treated cycloheximide for the times indicated. α-Tubulin shown as loading control. C) Quantification of relative CRM-1 expression following 24 h of cycloheximide treatment in HUVECs transfected with SCR or Akt3i. *P < 0.05. D) Diagram of CRM-1 indicating RanGTP and cargo-binding domains and the binding site of leptomycin B. Sequences showing the wild-type and mutated serine 1054 residues are shown. E) Western blot analysis of FLAG expression in HUVECs transfected with either wild-type or S1054A mutant FLAG-tagged CRM-1 constructs shown in C and treated with cyclohexamide for the times indicated. F) Quantification of relative CRM-1-FLAG expression following 24 h of cycloheximide treatment in HUVECs transfected with either wild-type or S1054A mutant FLAG-tagged CRM-1 plasmid. *P < 0.05.
In silico analysis of the CRM-1 amino acid sequence revealed a putative Akt phosphorylation site at serine 1054. To test whether phosphorylation of serine 1054 was important for the regulation of CRM-1 protein stability, site-specific mutagenesis was performed, mutating the serine to an alanine. HUVECs were transfected with either a FLAG-tagged wild-type CRM-1 expression vector (FLAG-CRM-1) or a FLAG-tagged Ser1054Ala CRM-1 expression vector (FLAG-CRM-1 S1054A), as diagramed in Fig. 4D, and tested for protein stability using cyclohexamide treatment. As shown in Fig. 4E and quantitated in Fig. 4F, in the presence of normal Akt3 function, Western blot analysis of ectopically expressed FLAG-CRM-1 again shows an approximate 50% reduction in expression 24 h after cyclohexamide treatment. However, mutation of serine 1054 (FLAG-CRM-1 S1054A) results in a statistically significant increase in protein compared to wild-type control. Our results suggest serine 1054 is required for the Akt3-dependent modulation of CRM-1 protein stability and that this stabilization may control PGC-1α nuclear retention under conditions of Akt3 knockdown.
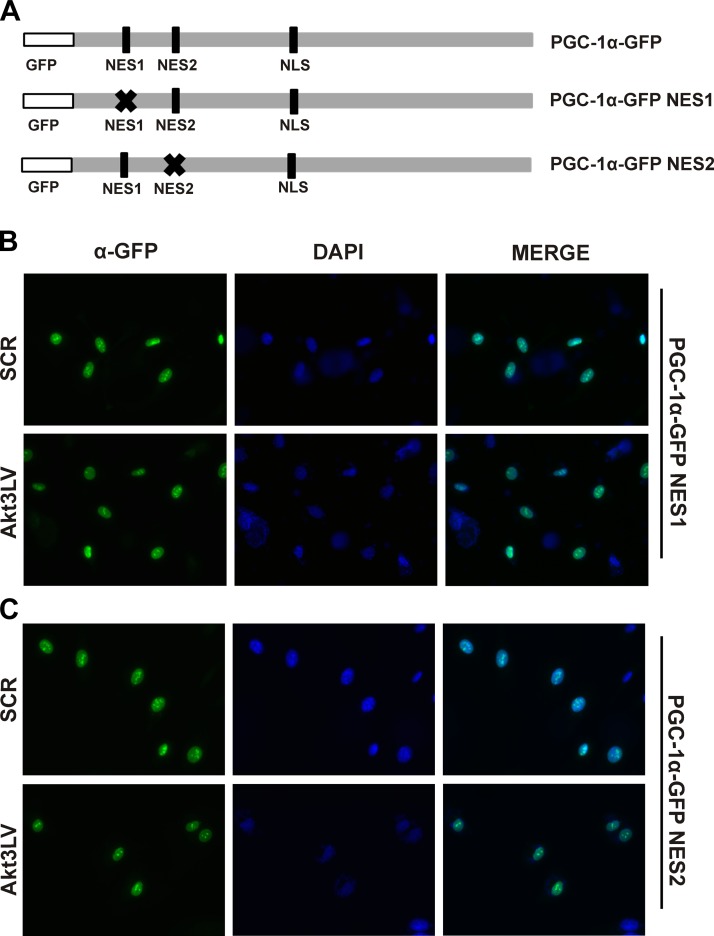
Mutation of putative PGC-1α nuclear export sequences blocks Akt3-dependent nuclear export
To further characterize the interactions between CRM-1 and PGC-1α, an in silico analysis of the PGC-1α amino acid sequence was performed to survey for candidate nuclear export sequences (NESs). These analyses revealed 2 putative NESs within the N-terminal portion of the protein (Fig. 5A). Previous reports have defined a nuclear localization signal contained within PGC-1α (44). Site-directed mutagenesis was performed on each of the candidate sites within a PGC-1α-GFP tagged construct, creating individual NES1 and NES2 mutants (GFP-PGC-NES1 and GFP-PGC-NES2, respectively). Each mutant construct was transfected separately into HUVECs transduced with an Akt3 shRNA expressing lentivirus and visualized by immunofluorescence. Mutation of either NES1 (Fig. 5B) or NES2 (Fig. 5C) blocked nuclear export under conditions of either control or Akt3 silencing. Our results suggest both sites are required for proper CRM-1-dependent transport of PGC-1α.
Figure 5.
PGC-1α contains 2 functional CRM-1 dependent nuclear export sequences. A) Stick diagram illustrating relative location of GFP tag, and points of site-directed mutagenesis on the PGC-1α-GFP construct used for the respective mutant constructs. B, C) Immunofluorescence of HUVECs transduced with scrambled control (SCR) or Akt3 shRNA (Akt3LV)-expressing lentiviruses and transfected with PGC-1α-GFP expression plasmids containing either an NES1 mutation (B) or NES2 mutation (C). DAPI nuclear stain is also shown.
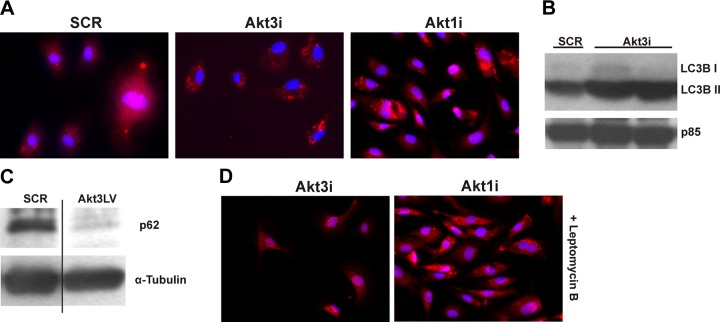
Akt3 blockade causes increased autophagy
Work from our laboratory has shown Akt3 is responsible for de novo mitochondrial biogenesis and overall mitochondrial content in ECs (2). To address whether resulting decreased mitochondrial content under conditions of Akt3 knockdown is due to decreased biogenesis alone or due to a concomitant increase in autophagy, we investigated whether Akt3 knockdown caused increased autophagic flux in HUVECs. HUVECs transfected with RNAi directed against Akt3, Akt1, or a scramble control were subjected to immunofluorescence using an antibody directed against LC3, a well-characterized marker of autophagy (45), in order to visualize autophagosome formation. Akt1 knockdown is used here as a control for the canonical Akt1/mTOR autophagy pathway. As shown in Fig. 6A, cells transfected with either Akt1 or Akt3 RNAi show increases in LC3-containing puncta as compared to SCR control. The increased LC3 processing was confirmed using Western blot analyses. As shown in Fig. 6B, Akt3 inhibition results in increased LC3B II levels. To confirm autophagic flux was increased, we assessed p62 (Sequestosome1) levels (46) following Akt3 knockdown. Indeed, Fig. 6C reveals p62 levels are decreased, suggesting autophagic flux is increased following Akt3 knockdown. To test whether Akt3-dependent autophagy was due to increased CRM-1 activity, transfected cells were treated with the CRM-1 inhibitor leptomycin B. As shown in Fig. 6D, cells transfected with an RNAi directed against Akt3 showed decreased autophagosome formation when treated with leptomycin B, whereas cells transfected with Akt1RNAi are unaffected by CRM-1 inhibition. These findings show that CRM-1 is required for Akt3-mediated autophagy.
Figure 6.
Akt3 knockdown induces CRM-1-dependent autophagy. A) Immunofluorescence of LC3 (red) and DAPI (blue) in HUVECs transfected with scrambled control (SCR), Akt3 RNAi (Akt3i), or Akt1 RNAi (Akt1i). B) Western blot analysis of LC3 in total lysates of HUVEC cells transfected with scrambled control (SCR) or Akt3 RNAi (Akt3i). Two different Akt3 RNAi transfections are shown. PI3K p85 subunit is shown as an internal control. C) Analysis of p62 levels by Western blot for HUVECs transduced with SCR or Akt3LV shRNA lentiviruses. α-Tubulin is shown as loading control. D) Immunofluorescence of LC3 (red) and DAPI (blue) in HUVECs transfected with Akt3 RNAi (Akt3i) or Akt1 RNAi (Akt1i) and treated with leptomycin B (20 ng/ml).
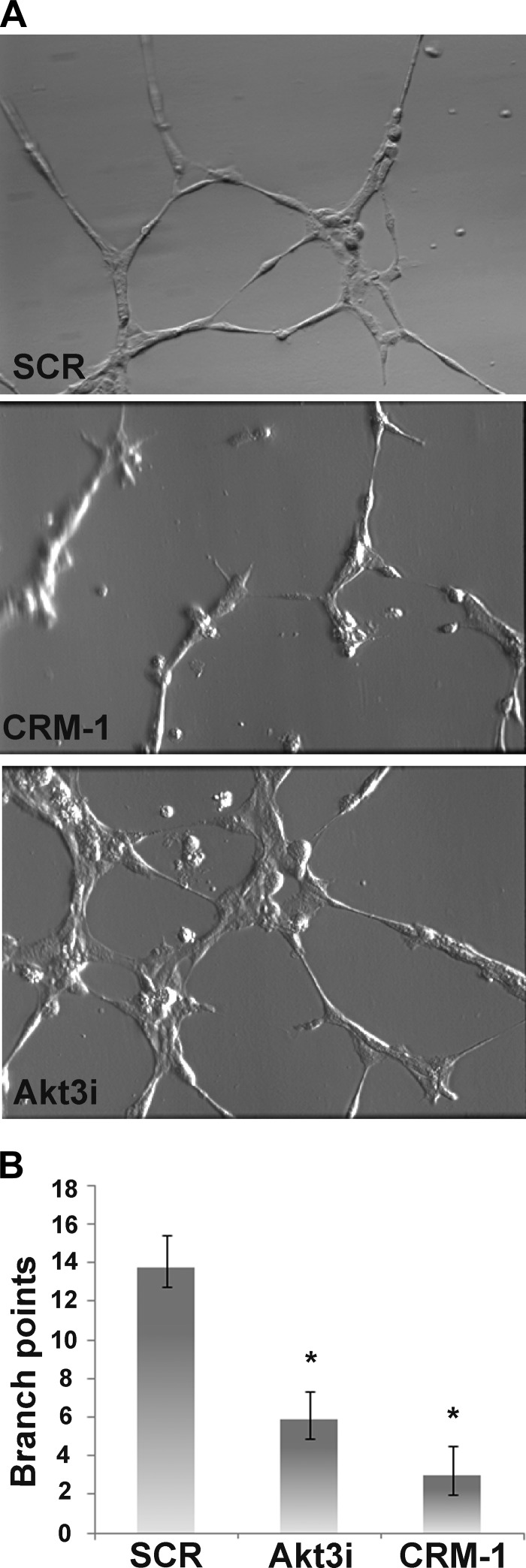
CRM-1 overexpression inhibits in vitro tubulogenesis
To test whether CRM-1 overexpression would inhibit angiogenesis, HUVECs were transfected with either FLAG-CRM-1, Akt3 RNAi, or scrambled control and subjected to an in vitro angiogenesis assay. Transfected cells were plated on Matrigel-coated culture wells in complete media (including serum and growth factors) and allowed to incubate for 6 h. Representative photographs of resulting branching networks are shown in (Fig. 7A). Control cells form narrow tubule-like structures with numerous interconnected branch points. Akt3 inhibition caused an overall reduction in formation of the narrow tubules; instead, cell-to-cell association was reduced, producing larger branching and fewer interconnections. CRM-1 overexpression resulted in a marked reduction in successful branching and in numerous single cells. A quantitation of branch points is shown in Fig. 7B. These findings suggest that CRM-1 overexpression inhibits angiogenesis, at least in vitro, thus linking nuclear export and mitochondrial function to angiogenesis.
Figure 7.
In vitro Matrigel cord formation is inhibited by Akt3 knockdown or by CRM-1 overexpression. HUVECs were transfected with scrambled control (SCR), Akt3 RNAi (Akt3i), or CRM-1 for 48 h, plated on Matrigel, and allowed to incubate for 6 h. A) Representative phase-contrast images. B) Quantitation of branch points. *P < 0.05.
DISCUSSION
Angiogenesis is a highly anabolic process, requiring ECs to grow, proliferate, migrate, and assemble into a functional vessel. The energy requirements of ECs in an established vessel are in stark contrast to those actively participating in an angiogenic event. Here we demonstrate an Akt3-dependent pathway whereby ECs can dynamically regulate mitochondrial content to match the changes in energy requirement during angiogenesis and quiescence. Indeed, Akt3-null animals fail to launch angiogenic responses to growth factor when challenged. Akt3 activity also appears to be dose dependent, as heterozyogous animals have an approximate 50% reduction in mature vasculature. Notably, we show the cells within the Matrigel plugs have a marked reduction in mitochondrial content. The deficit seems to be in the ability to form mature, stabilized vasculature. This phenotype is supported by the in vitro Matrigel assays, where knockdown of Akt3 causes larger, less narrow tubules, suggesting a problem with cell-cell interactions required for angiogenesis. In addition, the Akt3-null mice backcrossed onto a C57Bl6 pure background have increased in prenatal lethality at E12.5, due to an apparent loss of vascular integrity and hemorrhaging (data not shown). Whether this phenotype is due solely to the Akt3-dependent effect on mitochondrial content remains to be determined.
Our previous work has demonstrated Akt3 affects mitochondrial biogenesis by indirectly controlling the subcellular partitioning of PGC-1α (2). Here we show that Akt3 dependent changes in PGC-1α nuclear/cytoplasmic distribution are due to changes in the protein stability of the major nuclear export receptor, CRM-1. Loss of Akt3 increases CRM-1 protein stability, causing increased nuclear export of PGC-1α, and a concomitant down-regulation of PGC-1α-dependent mitochondrial genes encoded in the nucleus. Increased CRM-1 activity under conditions of Akt3 knockdown is required for the autophagy driven by Akt3 knockdown but is not required for canonical Akt/mTOR autophagy driven by mTOR repression via Akt1 knockdown. However, CRM-1 overexpression alone does not drive autophagy (data not shown), suggesting it is necessary but not sufficient. It has been shown that overexpression of PGC-1α can be protective against autophagy in neurons and in skeletal muscle (47, 48). However, blockade of PGC-1α expression does not induce autophagy. Indeed, PGC-1α-null animals present with normal mitochondrial numbers in heart and skeletal muscle but have a reduction in mitochondrial activity, decreased ATP output, and a reduced ability to adjust mitochondrial content in response to stimulation (5). This phenomenon is recapitulated here; CRM-1 overexpression alone causes decreased PGC-1α-dependent gene expression, yet has no effect on autophagy. Taken together, these results suggest Akt3 may affect angiogenesis via regulation of cellular energetics.
CRM-1 is responsible for the bulk of nuclear protein export in the cell, recognizing myriad cargo proteins. All cargo proteins contain a NES, defined as 3–5 hydrophobic amino acids (typically leucine) separated by 2 to 3 polar amino acids, respectively (e.g., LXXLXXXLXXL, where L = leucine; ref. 49). The NES of the cargo protein binds to a hydrophobic cleft on the apical surface of CRM-1 protein, whose donut-shaped conformation allows it to interact with a wide variety of cargo proteins (50). Interestingly both PGC-1α NESs are required for nuclear transport. Given their shortness in length and that the length of a particular NES dictates its strength; PGC-1α NESs may have to work in consort to promote CRM-1 binding.
Exactly how specific cargo proteins are selected by CRM-1 export is not known. There is an element of specificity in Akt3-dependent changes in nuclear export, as Akt3 knockdown does not result in the nuclear export of all CRM-1 dependent cargo proteins (data not shown). In addition to relative NES strength, we hypothesize that protein complexes, such as found for transcriptional coactivators like PGC-1α, may enable context-specific nuclear retention through sequestration, or by masking NESs altogether, to allow for a more complex regulation of nuclear export via CRM-1.
As mentioned, increasing amounts of data support the nonredundant roles for the Akt isoforms. Since the kinase domains among the 3 Akt isoforms are identical and phosphorylate the same consensus sequence, a standing hypothesis within the Akt field is that substrate specificity is accomplished through discrete subcellular partitioning and context-specific changes in expression (18). In vitro analyses support this idea, since substrate specificity of purified activated kinases is not generally recapitulated using in vitro kinase assays. Activated kinases can equally phosphorylate substrates in vitro although knockdown studies suggest specificity in intact cells. In support of this in ECs, Akt1 is generally nuclear (51) and Akt3 can be both nuclear and cytoplasmic (data not shown), suggesting that these two kinases may be differentially located within nuclear subdomains: CRM-1 accessible to direct Akt3 phosphorylation but not to Akt1.
Here we present data that CRM-1 protein stability is controlled specifically by Akt3 (Fig. 4). Interestingly, Ding et al. (52) have recently shown Akt3 to regulate ACAT-1 in macrophages by a mechanism similar to what we present here. Loss of Akt3 in macrophages caused an accumulation of ACAT-1 protein due to decreased protein degradation via a ubiquitin-proteasome pathway (UPP), contributing to accelerated progression of atherosclerosis in ApoE−/− mice (52). Taken together, one could hypothesize that an emergent role for Akt3 is to modulate specific protein degradation via UPP and autophagy. Exactly how Akt3 phosphorylation controls protein the half-life of CRM-1 remains to be determined. Future studies will characterize the specific pathways required from CRM-1 degradation.
Interestingly, all 3 Akt isoforms contain a conserved CRM-1 dependent NES. In the case of Akt1, this NES has been shown to be involved in the regulation of Akt1-dependent cell migration in some cancer cells (53). Whether nuclear cytoplasmic export of either Akt isoform is controlled by Akt3 remains to be determined. Under conditions of Akt3 ablation in vivo, we do not detect issues with cell recruitment in the in vivo Matrigel assays of Akt3-null mice (Fig. 1A). However, we do in similar Matrigel assays performed using Akt1-null animals, suggesting that Akt3 is not affecting Akt1 nuclear retention or activity. Taken together, Akt3 appears to specifically control CRM-1 protein stability and PGC-1α nuclear retention causing an Akt1-independent effect on mitochondrial biogenesis in primary ECs.
In addition to showing Akt3-mediated changes in PGC-1α-dependent mitochondrial biogenesis requires increased CRM-1 activity, we also show that Akt3 requires CRM-1 in a novel autophagic pathway. One of the PGC-1α-dependent genes down regulated by Akt3 inhibition and CRM-1 overexpression is Sirt3 (Fig. 4), a class III HDAC specific to the mitochondria. Sirt3 is known to interact with multiple electron transport chain (ETC) complexes to facilitate ETC efficiency (54). In addition, Sirt3 has known roles in maintenance of both mitochondrial DNA integrity (30) and the mitochondrial antioxidant pool (9, 54) and may then be critical to mitochondrial health. Its reduction, at least in part, is thought to contribute to increased mitochondrial fission, a necessary event in mitochondrial turnover (55). Though CRM-1 is not sufficient to cause autophagy, CRM-1 inhibition of Sirt3 may play an important role in the increased autophagic response to Akt3 knockdown in primary ECs.
There is a great deal of interest in the use of angiogenesis as a new therapeutic tool. There is also strong evidence that defects in mitochondrial dynamics lead to a variety of disease states, including cardiomyopathy, cancer, and neurodegenerative diseases, such as Alzheimer's disease (56–58). Emerging theories regarding Alzheimer's pathology are positing that a major contributor to pathology is the loss of small vessels within the brain due to oxidative stress, causing ischemic pockets within the brain and neuronal loss (57). Interestingly, it is thought that Akt3 is the predominant isoform of Akt expressed in the brain, particularly within the hippocampus, the area of the brain most affected during Alzheimer's progression. Indeed, data from our lab have shown mitochondrial dysfunction in the brains of Akt3-null mice (2). Here we show the requirement for Akt3 in angiogenesis and in the control of mitochondrial dynamics in the vascular endothelium through control of autophagy and biogenesis via modulation of the subcellular localization of the master regulator of nuclear mitochondrial gene expression, PGC-1α. Akt3 is required for the appropriate expression levels of the major nuclear export receptor, CRM-1. Understanding the molecular mechanisms responsible for the maintenance of cellular energy demands in regard to new vessel formation is a prerequisite to designing new angiogenic therapies for the treatment of a broad range of human diseases linked to mitochondrial function (31, 32).
Supplementary Material
Acknowledgments
The authors thank Yong Gong for help with Matrigel assays, Amy Phelps [Center of Biomedical Research Excellence (COBRE) Histology Core Facility, Medical University of South Carolina] for help with sectioning, and C. Beeson and R. Schnellman for their helpful suggestions.
This work was supported in part by U.S. National Heart, Blood and Lung Institute grants HL084565 (to R.M.H.) and 2T32HL007260-36 (to D.G.C.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- α-SMA
- α-smooth muscle actin
- Akt1/2/3
- protein kinase Bα/β/γ (PKBα/β/γ)
- EC
- endothelial cell
- ETC
- electron transport chain
- HUVEC
- human umbilical vein endothelial cell
- NES
- nuclear export sequence
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator-1α
- PI3K
- phosphoinositol-3-kinase
- PKB
- protein kinase B (Akt)
- UPP
- ubiquitin-proteasome pathway
REFERENCES
- 1. Larrivee B., Freitas C., Suchting S., Brunet I., Eichmann A. (2009) Guidance of vascular development: lessons from the nervous system. Circ. Res. 104, 428–441 [DOI] [PubMed] [Google Scholar]
- 2. Wright G. L., Maroulakou I. G., Eldridge J., Liby T. L., Sridharan V., Tsichlis P. N., Muise-Helmericks R. C. (2008) VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J. 22, 3264–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carmeliet P., Jain R. K. (2000) Angiogenesis in cancer and other diseases. Nature 407, 249–257 [DOI] [PubMed] [Google Scholar]
- 4. Liang H., Ward W. F. (2006) PGC-1alpha: a key regulator of energy metabolism. Adv. Physiol. Educ. 30, 145–151 [DOI] [PubMed] [Google Scholar]
- 5. Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O., Ahmad F., Matsui T., Chin S., Wu P. H., Rybkin I. I., Shelton J. M., Manieri M., Cinti S., Schoen F. J., Bassel-Duby R., Rosenzweig A., Ingwall J. S., Spiegelman B. M. (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 1, 259–271 [DOI] [PubMed] [Google Scholar]
- 6. Lin J., Wu P. H., Tarr P. T., Lindenberg K. S., St-Pierre J., Zhang C. Y., Mootha V. K., Jager S., Vianna C. R., Reznick R. M., Cui L., Manieri M., Donovan M. X., Wu Z., Cooper M. P., Fan M. C., Rohas L. M., Zavacki A. M., Cinti S., Shulman G. I., Lowell B. B., Krainc D., Spiegelman B. M. (2004) Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell 119, 121–135 [DOI] [PubMed] [Google Scholar]
- 7. Lehman J. J., Barger P. M., Kovacs A., Saffitz J. E., Medeiros D. M., Kelly D. P. (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 106, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., Lowell B. B., Bassel-Duby R., Spiegelman B. M. (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 [DOI] [PubMed] [Google Scholar]
- 9. Youle R. J., Narendra D. P. (2011) Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tal R., Winter G., Ecker N., Klionsky D. J., Abeliovich H. (2007) Aup1p, a yeast mitochondrial protein phosphatase homolog, is required for efficient stationary phase mitophagy and cell survival. J. Biol. Chem. 282, 5617–5624 [DOI] [PubMed] [Google Scholar]
- 11. Hers I., Vincent E. E., Tavare J. M. (2011) Akt signalling in health and disease. Cell. Signal. 23, 1515–1527 [DOI] [PubMed] [Google Scholar]
- 12. Karar J., Maity A. (2011) PI3K/AKT/mTOR pathway in angiogenesis. Front. Mol. Neurosci. 4, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J., Somanath P. R., Razorenova O., Chen W. S., Hay N., Bornstein P., Byzova T. V. (2005) Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat. Med. 11, 1188–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitamura T., Asai N., Enomoto A., Maeda K., Kato T., Ishida M., Jiang P., Watanabe T., Usukura J., Kondo T., Costantini F., Murohara T., Takahashi M. (2008) Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat. Cell Biol. 10, 329–337 [DOI] [PubMed] [Google Scholar]
- 15. Phung T. L., Ziv K., Dabydeen D., Eyiah-Mensah G., Riveros M., Perruzzi C., Sun J., Monahan-Earley R. A., Shiojima I., Nagy J. A., Lin M. I., Walsh K., Dvorak A. M., Briscoe D. M., Neeman M., Sessa W. C., Dvorak H. F., Benjamin L. E. (2006) Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 10, 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Z. Z., Tschopp O., Hemmings-Mieszczak M., Feng J., Brodbeck D., Perentes E., Hemmings B. A. (2003) Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278, 32124–32131 [DOI] [PubMed] [Google Scholar]
- 17. Sun J. F., Phung T., Shiojima I., Felske T., Upalakalin J. N., Feng D., Kornaga T., Dor T., Dvorak A. M., Walsh K., Benjamin L. E. (2005) Microvascular patterning is controlled by fine-tuning the Akt signal. Proc. Natl. Acad. Sci. U. S. A. 102, 128–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez E., McGraw T. E. (2009) The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8, 2502–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cozzone D., Frojdo S., Disse E., Debard C., Laville M., Pirola L., Vidal H. (2008) Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia 51, 512–521 [DOI] [PubMed] [Google Scholar]
- 20. Davies M. A., Stemke-Hale K., Tellez C., Calderone T. L., Deng W., Prieto V. G., Lazar A. J., Gershenwald J. E., Mills G. B. (2008) A novel AKT3 mutation in melanoma tumours and cell lines. Br. J. Cancer 99, 1265–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dummler B., Hemmings B. A. (2007) Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 35, 231–235 [DOI] [PubMed] [Google Scholar]
- 22. Endersby R., Zhu X., Hay N., Ellison D. W., Baker S. J. (2011) Nonredundant functions for Akt isoforms in astrocyte growth and gliomagenesis in an orthotopic transplantation model. Cancer Res. 71, 4106–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grabinski N., Bartkowiak K., Grupp K., Brandt B., Pantel K., Jucker M. (2011) Distinct functional roles of Akt isoforms for proliferation, survival, migration and EGF-mediated signalling in lung cancer derived disseminated tumor cells. Cell. Signal. 23, 1952–1960 [DOI] [PubMed] [Google Scholar]
- 24. Koseoglu S., Lu Z., Kumar C., Kirschmeier P., Zou J. (2007) AKT1, AKT2 and AKT3-dependent cell survival is cell line-specific and knockdown of all three isoforms selectively induces apoptosis in 20 human tumor cell lines. Cancer Biol. Ther. 6, 755–762 [DOI] [PubMed] [Google Scholar]
- 25. Maroulakou I. G., Oemler W., Naber S. P., Klebba I., Kuperwasser C., Tsichlis P. N. (2008) Distinct roles of the three Akt isoforms in lactogenic differentiation and involution. J. Cell. Physiol. 217, 468–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B., 3rd, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001) Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 [DOI] [PubMed] [Google Scholar]
- 27. Chen W. S., Xu P. Z., Gottlob K., Chen M. L., Sokol K., Shiyanova T., Roninson I., Weng W., Suzuki R., Tobe K., Kadowaki T., Hay N. (2001) Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15, 2203–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Easton R. M., Cho H., Roovers K., Shineman D. W., Mizrahi M., Forman M. S., Lee V. M., Szabolcs M., de Jong R., Oltersdorf T., Ludwig T., Efstratiadis A., Birnbaum M. J. (2005) Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol. Cell. Biol. 25, 1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papaioannou V. E., Behringer R. (2005) Mouse Phenotypes: A Handbook of Mutation Analysis, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 30. Fieber C. B., Eldridge J., Taha T. A., Obeid L. M., Muise-Helmericks R. C. (2006) Modulation of total Akt kinase by increased expression of a single isoform: requirement of the sphingosine-1-phosphate receptor, Edg3/S1P3, for the VEGF-dependent expression of Akt3 in primary endothelial cells. Exp. Cell. Res. 312, 1164–1173 [DOI] [PubMed] [Google Scholar]
- 31. Ferrara N., Alitalo K. (1999) Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 5, 1359–1364 [DOI] [PubMed] [Google Scholar]
- 32. Isner J. M., Asahara T. (1999) Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J. Clin. Invest. 103, 1231–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mao C., Tili E. G., Dose M., Haks M. C., Bear S. E., Maroulakou I., Horie K., Gaitanaris G. A., Fidanza V., Ludwig T., Wiest D. L., Gounari F., Tsichlis P. N. (2007) Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J. Immunol. 178, 5443–5453 [DOI] [PubMed] [Google Scholar]
- 34. Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 35. Wang W., Budhu A., Forgues M., Wang X. W. (2005) Temporal and spatial control of nucleophosmin by the Ran-Crm1 complex in centrosome duplication. Nat. Cell Biol. 7, 823–830 [DOI] [PubMed] [Google Scholar]
- 36. Cain A. J. (1948) An easily controlled method for staining mitochondria. Q. J. Microsc. Sci. 89, 229–231 [PubMed] [Google Scholar]
- 37. Gentile C., Muise-Helmericks R. C., Drake C. J. (2013) VEGF-mediated phosphorylation of eNOS regulates angioblast and embryonic endothelial cell proliferation. Dev. Biol. 373, 163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xue G., Hemmings B. A. (2013) PKB/Akt-dependent regulation of cell motility. J. Natl. Cancer Inst. 105, 393–404 [DOI] [PubMed] [Google Scholar]
- 39. Tschopp O., Yang Z. Z., Brodbeck D., Dummler B. A., Hemmings-Mieszczak M., Watanabe T., Michaelis T., Frahm J., Hemmings B. A. (2005) Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132, 2943–2954 [DOI] [PubMed] [Google Scholar]
- 40. Hutten S., Kehlenbach R. H. (2007) CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 17, 193–201 [DOI] [PubMed] [Google Scholar]
- 41. Lerin C., Rodgers J. T., Kalume D. E., Kim S. H., Pandey A., Puigserver P. (2006) GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell. Metab. 3, 429–438 [DOI] [PubMed] [Google Scholar]
- 42. Bell E. L., Guarente L. (2011) The SirT3 divining rod points to oxidative stress. Mol. Cell 42, 561–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fornerod M., van Deursen J., van Baal S., Reynolds A., Davis D., Murti K. G., Fransen J., Grosveld G. (1997) The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 16, 807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chang J. S., Huypens P., Zhang Y., Black C., Kralli A., Gettys T. W. (2010) Regulation of NT-PGC-1alpha subcellular localization and function by protein kinase A-dependent modulation of nuclear export by CRM1. J. Biol. Chem. 285, 18039–18050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barth S., Glick D., Macleod K. F. (2010) Autophagy: assays and artifacts. J. Pathol. 221, 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bjorkoy G., Lamark T., Pankiv S., Overvatn A., Brech A., Johansen T. (2009) Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 452, 181–197 [DOI] [PubMed] [Google Scholar]
- 47. Scarpulla R. C. (2011) Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 1813, 1269–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wareski P., Vaarmann A., Choubey V., Safiulina D., Liiv J., Kuum M., Kaasik A. (2009) PGC-1α and PGC-1β regulate mitochondrial density in neurons. J. Biol. Chem. 284, 21379–21385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kutay U., Guttinger S. (2005) Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 15, 121–124 [DOI] [PubMed] [Google Scholar]
- 50. Guttler T., Gorlich D. (2011) Ran-dependent nuclear export mediators: a structural perspective. EMBO J. 30, 3457–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adini I., Rabinovitz I., Sun J. F., Prendergast G. C., Benjamin L. E. (2003) RhoB controls Akt trafficking and stage-specific survival of endothelial cells during vascular development. Genes Dev. 17, 2721–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ding L., Biswas S., Morton R. E., Smith J. D., Hay N., Byzova T. V., Febbraio M., Podrez E. A. (2012) Akt3 deficiency in macrophages promotes foam cell formation and atherosclerosis in mice. Cell Metab. 15, 861–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saji M., Vasko V., Kada F., Allbritton E. H., Burman K. D., Ringel M. D. (2005) Akt1 contains a functional leucine-rich nuclear export sequence. Biochem. Biophys. Res. Commun. 332, 167–173 [DOI] [PubMed] [Google Scholar]
- 54. Haigis M. C., Deng C. X., Finley L. W., Kim H. S., Gius D. (2012) SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res. 72, 2468–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Twig G., Elorza A., Molina A. J., Mohamed H., Wikstrom J. D., Walzer G., Stiles L., Haigh S. E., Katz S., Las G., Alroy J., Wu M., Py B. F., Yuan J., Deeney J. T., Corkey B. E., Shirihai O. S. (2008) Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 27, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu M., Gu L., Sulkin M. S., Liu H., Jeong E. M., Greener I., Xie A., Efimov I. R., Dudley S. C., Jr. (2013) Mitochondrial dysfunction causing cardiac sodium channel downregulation in cardiomyopathy. J. Mol. Cell. Cardiol. 54, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marchesi V. T. (2011) Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 25, 5–13 [DOI] [PubMed] [Google Scholar]
- 58. Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J. (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.