Abstract
Hypoxia has been widely implicated in many pathological conditions, including those associated with inflammation and tumorigenesis. A number of recent studies have implicated hypoxia in the control of vasculogenesis and permeability, the basis for which is not fully understood. Here we examine the transcriptional regulation of angiogenesis and permeability by hypoxia in endothelial cells. Guided by a global profiling approach in cultured endothelial cells, these studies revealed the selective induction of human gravin (protein kinase A anchoring protein 12) by hypoxia. Analysis of the cloned gravin promoter identified a functional hypoxia-responsive region including 2 binding sites for hypoxia-inducible factor (HIF). Site-directed mutagenesis identified the most distal HIF-binding site as essential for the induction of gravin by hypoxia. Further studies examining gravin gain and loss of function confirmed strong dependence of gravin in control of microvascular endothelial tube formation, wherein gravin functions as a “braking” system for angiogenesis. Additional studies in confluent endothelia revealed that gravin functionally couples to control endothelial barrier function in response to protein kinase A (PKA) agonists. Taken together, these results demonstrate transcriptional coordination of gravin by HIF-1α and amplified PKA-dependent endothelial responses. These findings provide an important link between hypoxia and metabolic conditions associated with inflammation and angiogenesis.—Weissmüller, T., Glover, L. E., Fennimore, B., Curtis, V. F., MacManus, C. F., Ehrentraut, S. F., Campbell, E. L., Scully, M., Grove, B. D., Colgan, S. P. HIF-dependent regulation of AKAP12 (gravin) in the control of human vascular endothelial function.
Keywords: hypoxia-inducible factor, permeability, barrier function, angiogenesis
Physiological adaptation to limited oxygen availability (hypoxia) is currently an area of intense investigation. Indeed, hypoxia is coincident with a variety of inflammatory, cardiovascular, and infectious conditions (1). Among other influences, hypoxia has been shown to regulate tissue permeability and drive the formation of new blood vessels (angiogenesis) (2, 3). Central to both tissue barrier and angiogenesis are cytoskeleton rearrangements, wherein dynamic reorganization of actin filaments control both passive and active movement of solutes across cell monolayers (4). Our previous work has demonstrated that, as a major actin-binding protein, vasodilator-stimulated phosphoprotein (VASP) colocalizes with ZO-1 at tight junctions and plays a key role in establishing and maintaining barrier function (5).
Numerous vascular endothelial functions are attributable to the activation of G-protein-coupled receptors (GPCRs) by extracellular ligands that trigger cellular responses through the second messenger cyclic AMP (cAMP) (6). Signaling by cAMP occurs through activation of downstream effector proteins including cyclic nucleotide-gated ion channels, exchange proteins activated by cAMP (Epac), and the cAMP-dependent protein kinase A (PKA). Specificity of cAMP signaling is achieved by compartmentalization through PKA anchoring proteins (AKAPs). AKAPs represent a family of nearly 50 scaffolding proteins that anchor PKA and other proteins (e.g., protein phosphatases and phosphodiesterases) to defined locales within the cell and thereby control spatiotemporal cAMP signaling (7). AKAPs bind and regulate the activity of PKA, a tetrameric complex comprising a dimer of regulatory subunits of type I or type II and 2 catalytic subunits (7). Each regulatory subunit interacts with a catalytic subunit, which is released on binding to cAMP. AKAP binding is mediated by the dimerization and docking domain formed by the dimer of R subunits. The PKA-binding domain is structurally conserved within the AKAP family, and based on their preferential binding of type I or II regulatory subunits, AKAPs are classified as RI- or RII-specific AKAPs. Gravin (also called AKAP12 and SSeCKS) is an RII-specific AKAP that was originally identified as an endothelial autoantigen in myasthenia gravis (8). Gravin has been strongly implicated in the control of numerous endothelial functional responses (9–11).
In the present study, we identify gravin as a major endothelial hypoxia-inducible factor (HIF) target gene in control of endothelial function. For these purposes, endothelial cell models were utilized to define these principles. Studies of the cloned gravin promoter revealed a prominent role for HIF, and functional studies identified HIF-regulated gravin expression as an important link to endothelial barrier function and vascular tube formation.
MATERIALS AND METHODS
Cell culture and hypoxia
Human microvascular endothelial cell line 1 (HMEC-1) cells, a dermally derived immortalized microvascular endothelial cell line, were obtained and cultured as described previously (12). Where indicated, hypoxia was defined as pO2 20 torr and pCO2 35 torr, with the balance made up with N2 and water vapor, as described previously (12, 13).
Microarray analysis
HMEC-1 cells were incubated either in normoxic room air in a humidified incubator or incubated in a hypoxia chamber in preequilibrated culture medium. Following 8 h incubation, cells were washed with ice-cold PBS, and RNA was collected with Trizol and analyzed using an AffymetrixGeneChip Array (Affymetrix, Santa Clara, CA< USA) as described previously (14).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay buffer [10 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1 mM EDTA; 1% v/v Triton X-100; 0.1% SDS; and 1 protease inhibitor tablet/10 ml (Roche Diagnostics, Indianapolis, IN, USA)], and the lysates were cleared by centrifugation at 15,000 g for 20 min at 4°C. For immunoblotting, cleared protein was boiled in Laemmli's SDS sample buffer, resolved by electrophoresis on a 10% SDS-PAGE gel, and electroblotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). PVDF membranes were incubated in blocking buffer [Tris-buffered saline (TBS) and 5% nonfat dry milk] for 1 h at room temperature. Membranes were probed at 4°C overnight with a polyclonal anti-gravin antibody (15) or a polyclonal anti-HIF-1α antibody (clone NB100-479) and subsequently with a 1:10,000 dilution of horseradish peroxidase-linked anti-rabbit IgG (MP Biomedicals, Solon, OH, USA). Antibody staining was detected using the LumiGlo chemiluminescence detection system (KPL, Gaithersburg, MD, USA).
Immunofluorescence
For immunolocalization, HMEC-1 cells were cultured on acid-washed coverslips, exposed to experimental conditions, fixed (1% w/v formaldehyde, prepared from paraformaldehyde, in 0.1 M cacodylate buffer), and permeabilized (0.2% v/v Triton X-100 and 2% w/v BSA). Monolayers were incubated with an anti-gravin monoclonal antibody (1 μg/ml), as described previously (15), and, after washing, were incubated with a species-matched rhodamine-conjugated secondary antibody (1 μg/ml; Molecular Probes, Eugene, OR, USA), as indicated, for 30 min and counterstained with DAPI. The cells were then mounted in polyvinylalcohol and viewed with a fluorescence microscope (Olympus BH2; Olympus, Melville, NY, USA). As a control for background labeling, control cells were incubated with secondary antibody only.
Cloning of gravin promoter
The gravin promoter (NM_005100, 648 bp encoding 227 bp of promoter upstream of gravin transcriptional start site) was isolated from HMEC-1 cell genomic DNA using standard PCR with Pfu DNA polymerase and then was cloned into a pGL-3 basic luciferase expression vector (Promega, Madison, WI, USA) using NheI (5′) and HindIII (3′) sites. Homology to published sequences was determined by sequencing through the University of Colorado genomics core sequencing facility.
Reporter assays were performed using HMEC-1 cells transfected with the indicated promoter constructs and exposed to normoxia or hypoxia as described previously (16). All activity was normalized with respect to a constitutive expressed Renilla reporter. Where indicated, single and double mutations were performed as described previously (17) using QuikChange (Stratagene, La Jolla, CA, USA). All of the mutants were cloned into a PGL-3 basic luciferase expression vector. For the mutation of the HIF sites, the original sequence ACGTG (position −124 to −120, termed SDM2) was changed to AATCG and GCGTG (position −183 to −179, termed SDM1) was altered to GATTT alone or in combination (termed SDM1/2).
Construction of gravin expression vector and generation of stable cells
The full-length gravin cDNA (5343 bp) was as originally described by Yan et al. (15). The cDNA was cloned into pcDNA3.1-zeo (Invitrogen, Carlsbad, CA, USA). Homology to published sequence was determined by sequencing through the University of Colorado genomics core sequencing facility. The plasmid was transfected into HMEC-1 cells using FuGene 6 (Roche Biochemicals). The stable cells were selected with 30 μg/ml of zeocin (Invitrogen) for 6 wk. The stable clones were verified by PCR and Western blot. The vector-only-transfected cells were used as negative controls.
Stable repression of gravin by siRNA
With the help of the siRNA Wizard (http://www.sirnawizard.com), a sequence was chosen within the coding region of the gene of interest. The chosen hairpin primer with the sequence 5′-CAAAAAGAAGACCAGAATGTGAAGACACTCTTGATGTCTTCACATTCTGGTCTTCG-3′ and 5′-TCGAAGACCAGAATGTGAAGACATCAAGAGTGTCTTCACATTCTGGTCTTCTT-3′ corresponds to the position (5622–5642) of the gravin gene. Primers were annealed for 2 min at 80°C to create the hairpin structure and ligated into the Bbs1/Bbs1-digested psiRNA-hH1neo G2 vector. After transformation using the Lyocomp GT116 Escherichia coli strain, cells were spread on a KanXgal agar plate with the advantage of white/blue selection. A recombinant white clone was grown, DNA was extracted, and HMEC-1 cells were transfected using an electroporation procedure. At 2 d after transfection, cells were selected with G418 (1 mg/ml), and stable transfectants were individualized after 2–3 wk. The control cell line was transfected with empty psiRNA-hH1 neoscr plasmid.
Paracellular permeability assays
Permeability to 70-kDa FITC-dextran in response to indicated concentrations of albuterol (Sigma-Aldrich, St. Louis, MO, USA) or the stable adenosine receptor agonist NECA (Sigma-Aldrich) and calculations of flux rates were performed exactly as described previously (18).
Endothelial tube formation assay
Wells on 24-well plates were coated with 100 μl Matrigel (Becton Dickenson, Franklin Lakes, NJ, USA) according to the manufacturer's recommendation. Control vector- and gravin loss/gain-of-function vector-transfected HMEC-1 cells were seeded at a density of 5 × 104 cells/well. Tube formation was inspected at 6 and 18 h using a phase-contrast light microscope (Nikon Eclipse E600) equipped with a digital camera (Nikon, Tokyo, Japan), as described previously (16). The differences in time of inspection are based on previous experience wherein tube formation saturates (16). Accommodation of the loss and gain of gravin function required analysis at these particular times. Tube length was quantified from digitized pictures as described elsewhere (19).
Data analysis
Data were compared by 2-factor ANOVA or by Student's t test where appropriate. Values are expressed as means ± sem from ≥3 separate experiments.
RESULTS
Gravin regulation by hypoxia
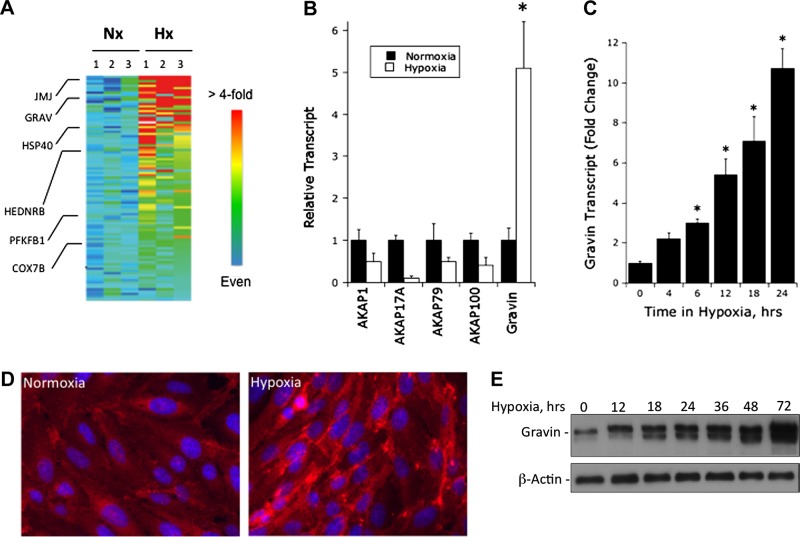
For a number of reasons, we sought to understand details of gravin regulation by hypoxia. First, gravin has been previously associated with hypoxia in rodent and human tissue models (20–22), and these studies have implicated gravin regulation by hypoxia. Second, recent work has suggested a link between gravin expression in tumors, which are known to be hypoxic, and VEGF expression (23, 24). Third, analysis of our published microarray studies (14) revealed a prominent induction of gravin by hypoxia in cultured human endothelial cells (5.1±1.1-fold induction, P<0.01; Fig. 1A). This level of induction on this array was comparable to or exceeded that of other hypoxia-inducible genes [e.g., jumonji-1 (JMJ), heat-shock protein 40 (HSP40), human endothelin receptor B (HEDNRB), fructose 6-phosphate 2-kinase (PFKB1) and cytochrome c oxidase subunit 7B (COX7B); see Fig. 1A]. Interestingly, the induction of gravin by hypoxia appeared to be specific for gravin, as other AKAP genes (e.g., AKAP1, AKAP17A, AKAP79, AKAP100) on this array were either not changed or significantly repressed by hypoxia (Fig. 1B).
Figure 1.
Induction of gravin by hypoxia. A) Affymetrix GeneChip analysis and gene family clustering identified gravin induction in HMEC-1 cells. Heat map depicts selected genes from gene chip analysis, where red indicates a >4-fold increase in transcript, and blue indicates no significant change in transcript level. Individual target transcripts are shown relative to gravin (GRAV), including jumonji protein (JMJ), heat-shock protein 40 (HSP40), human endothelin B receptor (HEDNRB), fructose 6-phosphate 2-kinase (PFKB1), and cytochrome c oxidase subunit 7B (COX7B). B) Relative clustering of the various AKAP gene family members. Note selective increase in gravin. *P < 0.01. C) Real-time quantitative PCR analysis of gravin mRNA expression in HMEC-1 cells following indicated periods of exposure to hypoxia. Data were calculated relative to β-actin and are expressed as mean ± sem fold change compared with control, n = 3. *P < 0.01. D) Localization of gravin following exposure to normoxia and hypoxia (24 h). Red indicates anti-gravin; blue indicates DAPI nuclear counterstain, Representative experiment from n = 5. E) Western blot analysis of gravin following incubation for indicated periods of hypoxia, with β-actin as a control. Representative experiment from n = 3.
Based on these observations, we sought to examine mechanisms of gravin regulation by hypoxia. Initially, we validated the microarray findings in more detail. As shown in Fig. 1C, analysis of endothelial mRNA by qPCR revealed a time-dependent induction of gravin by hypoxia (P<0.01 by ANOVA), with maximal changes of 11.3 ± 1.2-fold increase at 24 h (n=4, P<0.01) and no significant increase beyond 24 h. Localization of gravin in human endothelia (Fig. 1D) revealed a prominent increase in gravin following 24 h incubation in hypoxia, diffusely within the cytoplasm and prominently along the lateral border of endothelial cells. Extensions of these findings at the protein level by immunoblot revealed that total levels of gravin, like that of mRNA, were increased in a time-dependent manner by hypoxia (Fig. 1E). Densitometric analysis (relative to β-actin) revealed a maximal increase at 72 h, with a 6.9 ± 1.2-fold increase over normoxia (n=3, P<0.01) and no significant increase beyond 72 h. Such findings indicate that hypoxia prominently induces both gravin mRNA and protein.
Additional analysis was performed to define whether gravin induction by hypoxia required transcription. For these purposes, HMEC-1 cells were exposed to normoxia or hypoxia (18 h) in the presence or absence of the transcriptional inhibitor 5,6-dichlorobenzimidazole riboside (DRB; final concentration 10 μM) and assessed for changes in gravin expression by qPCR. In this instance, DRB prevented hypoxia-mediated gravin induction by 86 ± 10% (n=3, P<0.01), strongly indicating that hypoxia-mediated induction of gravin requires transcriptional activity.
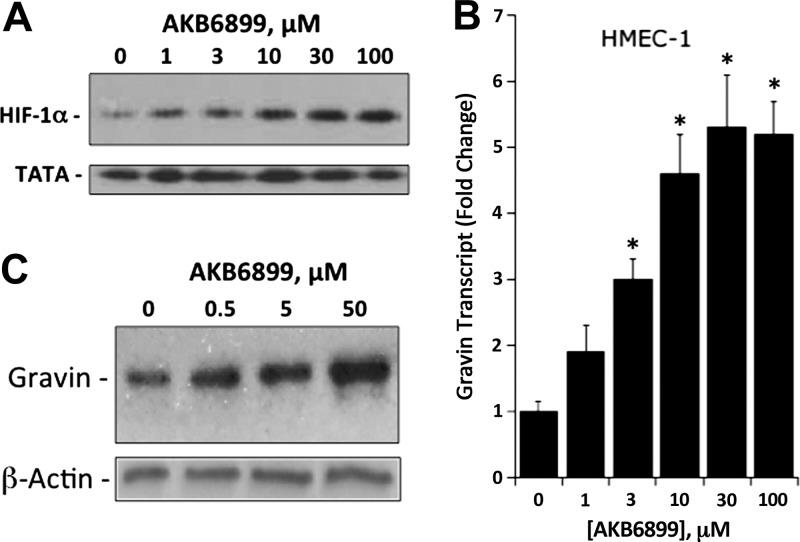
A major transcriptional pathway for gene regulation in hypoxia involves HIF, which is centrally regulated by a series of 3 prolyl hydroxylases (PHDs; ref, 25). To gain further insight into the transcriptional regulation of gravin in hypoxia, we determined whether the PHD inhibitor (HIF stabilizer) AKB6899 (26) influenced gravin expression. As shown in Fig. 2A, AKB6899 significantly stabilized HIF in room air (normoxia) at low micromolar concentrations. Exposure of HMEC-1 cells to AKB6899 for 12 h resulted in a concentration-dependent induction of gravin mRNA, as measured by qPCR (P<0.01 by ANOVA; Fig. 2B). Likewise, AKB6899 induced gravin protein, as demonstrated by Western blot analysis (Fig. 2C). Such findings implicate PHD-mediated regulation of gravin and strongly suggest a role for HIF in gravin transcriptional responses.
Figure 2.
Influence of PDH inhibitor (HIF stabilizer) AKB-6899 on gravin induction in normoxia. A) Validation of HIF-1α stabilization by AKB6899. HMEC-1 cells were exposed to indicated concentrations of AKB6899 for 6 h. Nuclear lysates were assessed for HIF-1a stabilization by Western blot relative to TATA binding protein as a control. Representative experiment from n = 3. B) Real-time quantitative PCR analysis of gravin mRNA expression in HMEC-1 cells following exposure to indicated concentrations of AKB6899 in normoxia. Data were calculated relative to the β-actin and are expressed as mean ± sem fold change compared with control, n = 3. *P < 0.01. C) Western blot analysis of gravin following incubation with indicated concentrations of AKB6899, with β-actin as a control. Representative experiment from n = 3.
Cloning and studies of the gravin promoter
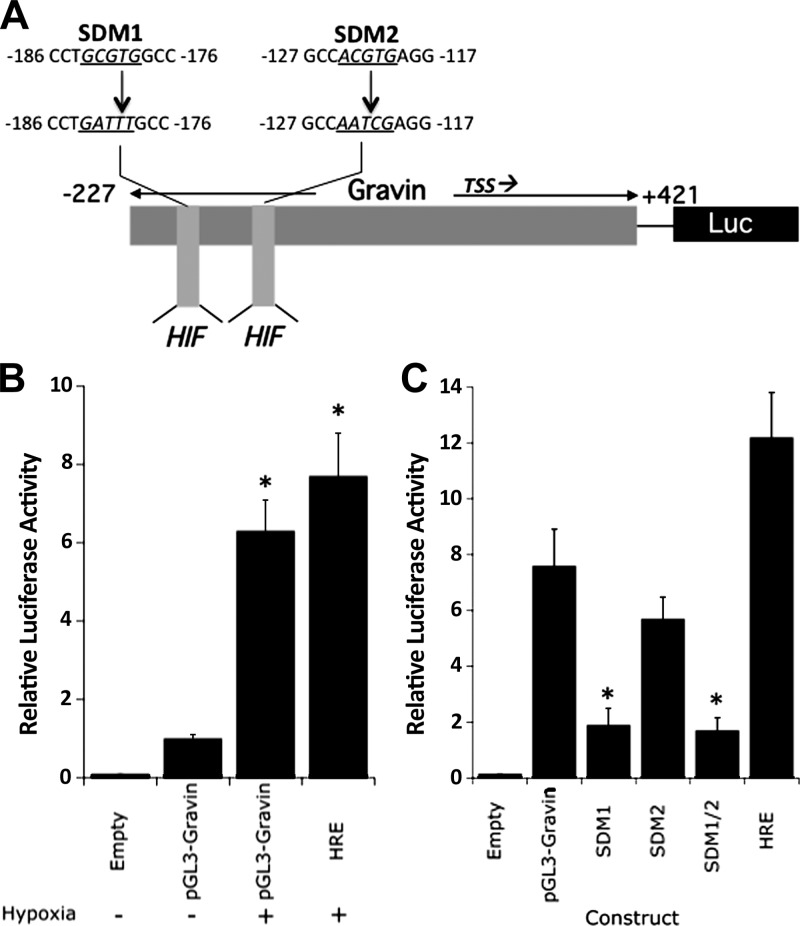
In view of the likelihood that hypoxia mediates transcriptional induction of gravin, attention was directed at the 5′-region of the gravin gene for potential hypoxia-regulated transcription factor sequences. Based on available public databases (27) and analysis of full-length cDNA (Genbank accession no. NM_005100), we cloned genomic fragments extending from positions −227 to +421 into the pGL3 luciferase reporter vector (see Fig. 3) and examined transcriptional activity in normoxia and hypoxia following transient transfection in HMEC-1 cells. As shown in Fig. 3B, analysis of luciferase reporter activity revealed that in constructs containing the 5′ promoter region spanning positions −227 to + 421, hypoxia induced gravin promoter activity by 6.3 ± 0.80-fold (P<0.01). By comparison, cells transfected with a canonical HIF response element (HRE) from the EPO enhancer (28) demonstrated a 7.7 ± 1.1-fold increase (P<0.01).
Figure 3.
Cloning and functional analysis of the gravin promoter. A) Map of cloned gravin promoter and luciferase constructs utilized here. Relative positions of each clone are annotated, as well as the transcription start site (TSS). Putative HIF binding sites (HIF) are shown, as are the sequences of the site-directed mutants SDM1 and SDM2. B) HMEC-1 monolayers were transfected with plasmids expressing sequence corresponding to full length gravin (pGL3-gravin) or the positive control plasmid encoding the HRE from the erythropoietin gene (HRE). Transfected cells were exposed to hypoxia or normoxia for 24 h and assessed for luciferase activity. Data are means ± sem, n = 3. *P < 0.01 vs. normoxia. C) HMEC-1 monolayers were transfected with plasmids expressing sequence corresponding to full length gravin (pGL3-gravin), SDM1, SDM2, or SDM1/2, or the positive control plasmid encoding the HRE from the erythropoietin gene (HRE). Transfected cells were exposed to hypoxia or normoxia for 24 h and assessed for luciferase activity. Data are means ± sem, n = 3. *P < 0.01 vs. normoxia.
Analysis of our cloned region of gravin revealed the existence of 2 potential binding sites for HIF at positions −117 and −176 relative to the transcription start site (Fig. 3A). HIF binding site mutations were introduced in the full-length promoter construct (see depiction of mutations in Fig. 3). As shown in Fig. 3C, mutagenesis of the more distal HIF consensus (depicted as SDM1) resulted in a 75 ± 3% decrease in luciferase activity under hypoxic conditions (P<0.01). The more proximal HRE (depicted as SDM2) was less active, wherein mutagenesis resulted in a nonsignificant 25 ± 6% decrease in activity (P=0.11). A double mutation in both SDM1 and SDM2 (depicted as SDM1/2) did not decrease activity beyond that of SDM1 alone, indicating that the HRE at position −179 is a major determinant of hypoxia responses for gravin transcriptional regulation.
Functional influence of gravin knockdown in endothelial cells
To gain insight into functional sequellae of gravin loss of function, we generated HMEC-1 lines with stable repression or stable overexpression of full length gravin and tested these lines for changes in 2 functional endpoints, namely, endothelial tube formation in Matrigel substrates (29) and endothelial barrier function measured as paracellular flux (12, 18).
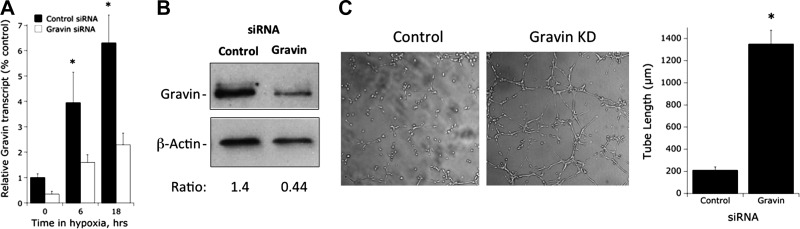
As shown in Fig. 4, we utilized siRNA to knock down gravin in cultured endothelial cells. Real-time PCR analysis revealed a 60 ± 8% repression in gravin transcript in both normoxia and hypoxia (P<0.01; Fig. 4A). Likewise, analysis of gravin protein by Western blot indicated a 65 ± 10% decrease in protein (representative blot shown in Fig. 4B). Using these cells, we tested the influence of gravin on endothelial tube formation in Matrigel substrates (29). As shown in Fig. 4C, the loss of gravin significantly enhanced spontaneous tube formation (6 h time point) when plated in this fashion (quantified as tube length, 6.1±0.8-fold increase with loss of gravin, P<0.01). Such findings indicate that gravin functions as an endogenous braking mechanism for endothelial tube formation.
Figure 4.
Characterization of gravin-knockdown endothelial line. A) Real-time quantitative PCR analysis of gravin mRNA expression in HMEC-1 cells following siRNA-mediated stable knockdown of gravin. Cells were exposed to indicated periods of hypoxia. Data were calculated relative to the β-actin and are expressed as mean ± sem fold change compared with control, n = 3, *P < 0.01. B) Western blot analysis of gravin following siRNA-mediated stable knockdown, with β-actin as a control. Representative experiment from n = 3. C) Influence of gravin knockdown on endothelial tube formation in Matrigel substrates. Left panels: images of a representative experiment assessing tube formation at 6 h. Right panel; quantification of tube length (means±sem) in control and gravin knockdown lines. Data are pooled from 3 experiments. *P < 0.01 vs. control.
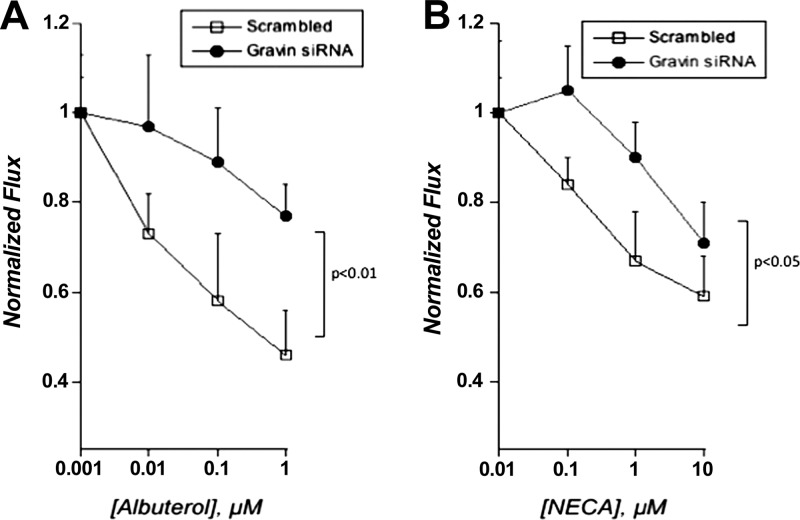
In parallel, we assessed the effect of gravin knockdown on endothelial permeability in response to agonists of the β-adrenergic and adenosine receptors. The rationale for these studies stems from previous observations that activation of protein kinase A results in increases in endothelial barrier function (i.e., decreased paracellular permeability; ref. 5). Given the role of gravin in clustering signaling proteins in a spatiotemportal manner (15), we reasoned that PKA signaling could be influenced by a change in gravin expression. As shown in Fig. 5, loss of gravin significantly decreased the influence of albuterol (β-adrenergic agonist) and NECA (adenosine receptor agonist) on endothelial permeability. Indeed, both albuterol and NECA significantly decreased endothelial permeability in a concentration-dependent manner in control HMEC-1 (by ANOVA, P<0.025). Knockdown of gravin attenuated this response to both albuterol (by ANOVA, P<0.01) and NECA (by ANOVA, P<0.05), indicating a prominent role for gravin in endothelial permeability responses.
Figure 5.
Influence of gravin knockdown on PKA agonist-induced changes to endothelial permeability. Influence of gravin knockdown on albuterol-stimulated (A) or NECA-stimulated (B) endothelial permeability. Endothelia were plated on permeable supports and exposed to indicated concentrations of albuterol or NECA, and permeability to FITC-dextran was quantified. Data are from 9 monolayers in each condition and expressed as means ± se. Results were analyzed by 2-way ANOVA.
Influence of gravin overexpression on endothelial functional responses
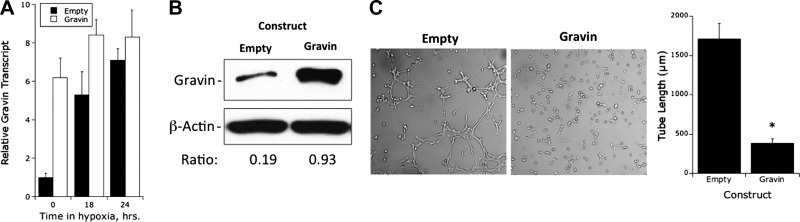
We next addressed how gravin overexpression, as a mimic of hypoxia, might affect endothelial tube formation and barrier responses. As depicted in Fig. 6, we successfully overexpressed full-length gravin in HMEC-1 cells. Analysis by real-time PCR indicated a baseline 6.3 ± 1.2-fold increase in transcript (P<0.01) with more modest increases with exposure to hypoxia. Analysis of gravin protein by Western blot revealed a 4.3 ± 1.6-fold increase in protein (representative blot shown in Fig. 6B). Assessment of gravin overexpression on endothelial tube formation revealed an opposite phenotype to that of gravin knockdown (see Fig. 4C) wherein the overexpression of gravin nearly abolished spontaneous tube formation (78±11% decrease, P<0.01). Notably, this analysis required a longer incubation period (18 h) compared to results shown in Fig. 4 (6 h time point) to allow significant tube formation in the control cells.
Figure 6.
Functional characterization of gravin-overexpressing endothelial line. A) PCR analysis of gravin mRNA expression in HMEC-1 cells following stable overexpression of gravin. Cells were exposed to indicated periods of hypoxia. Data were calculated relative to β-actin and are expressed as mean ± sem fold change compared with control, n = 4. *P < 0.01. B) Western blot analysis of gravin following stable overexpression of gravin relative to β-actin. Representative experiment from n = 3. C) Influence of gravin overexpression on endothelial tube formation in Matrigel. Left panels: images of a representative experiment assessing tube formation at 18 h. Right panel: of tube length (means±sem) in the control and gravin-overexpressing cell lines. Data are pooled from n = 3. *P < 0.01 vs. control.
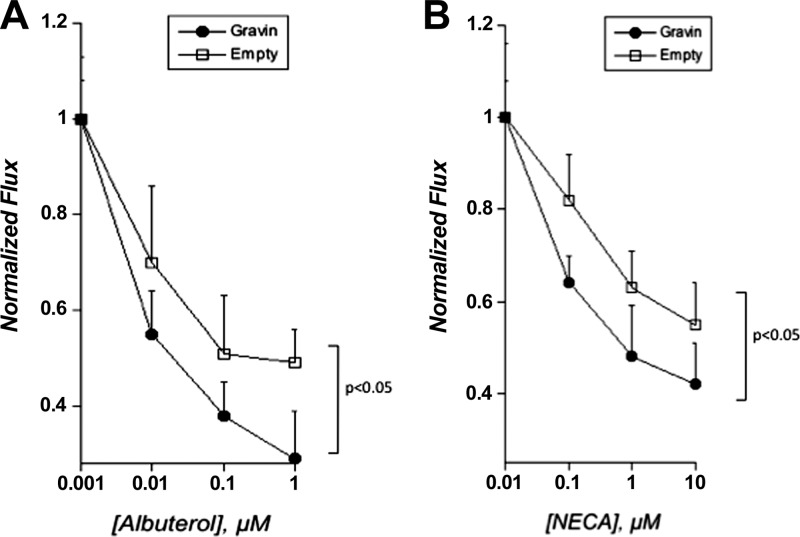
With regard to barrier regulation, an opposite phenotype to that noted in gravin knockdown cells was observed in cells overexpressing gravin. Indeed, as depicted in Fig. 7, overexpression of gravin-enhanced albuterol-induced (by ANOVA, P<0.05) and NECA-induced (by ANOVA, P<0.05) increases in endothelial barrier function. Taken together, these loss- and gain-of-function studies strongly implicate gravin in endothelial responses, including both angiogenesis and junctional permeability.
Figure 7.
Functional influence of gravin overexpression on PKA agonist-induced changes to endothelial permeability. Influence of gravin overexpression permeability in response to albuterol (A) or NECA (B). Endothelia were plated on permeable supports and exposed to indicated concentrations of albuterol or NECA, and permeability to FITC-dextran was quantified. Data are from 7–10 monolayers in each condition and are expressed as means ± se. Results were analyzed by 2-way ANOVA.
DISCUSSION
We report here the identification of the AKAP gravin as a major HIF target gene responsible for the fine-tuning of endothelial functional responses, including tube formation and GPCR-triggered changes to barrier function. Guided initially by an unbiased screen of hypoxia target genes, among other AKAPs, gravin was specifically induced in a time-dependent manner on exposure of endothelial cells to hypoxia. Extensions of these studies using a cloned gravin promoter identified a role for HIF in hypoxia-mediated regulation. Loss- and gain-of-function studies revealed a prominent role for gravin in both endothelial tube formation and GPCR-triggered changes to endothelial barrier function.
These new findings add significantly to our current base of knowledge related to GPCR signaling in hypoxia and control of PKA signaling. A common feature of this knowledge base is the control of both metabolism and fine-tuning of receptor coupling by HIF. In fact, multiple lines of evidence now significantly implicate HIF as a central coordinator of GPCR signaling in the hypoxic vasculature. For example, a significant adaptation to hypoxia is extracellular nucleotide metabolism and enhanced adenosine signaling, and each of the major metabolic, signaling, and transport components in this pathway is HIF dependent (6). The present studies support an additional role for HIF-regulated gravin as a mechanism to amplify GPCR signaling under such circumstances.
Given the temporal and robust hypoxia response observed in the induction of gravin, a candidate regulator was HIF, a member of the Per-ARNT-Sim family of basic helix-loop-helix transcription factors (30). HIF exists as an αβ heterodimer, the activation of which is dependent on stabilization of an O2-dependent degradation domain of the α subunit by the ubiquitin-proteasome pathway (30). A search of the cloned gravin gene promoter revealed 2 classic HREs (located at positions −117 and −176 relative to the transcription start site). However, the existence of a HIF binding consensus is not evidence for HIF-mediated response; rather, the HRE is defined as a cis-acting transcriptional regulatory sequence located within 5′-flanking, 3′-flanking or intervening sequences of target genes (31). Two approaches were used to define a role for HIF-1α in the induction of gravin. First, we cloned the gravin promoter into a luciferase construct and transient reporter construct transfections were used to establish evidence that the gravin gene promoter is hypoxia-inducible. In a second approach, site-directed mutagenesis within the luciferase reporter construct revealed a more prominent role for the distal HRE consensus sequence in this hypoxia response. Notably, neither of these mutations resulted in a complete loss of hypoxia inducibility. It is possible, for example, that other transcription factors regulate gravin promoter activity in hypoxia. Transcription factor binding analysis (e.g., TFSearch; ref. 32) in the region of these HRE indicated consensus sites for upstream-stimulating factor (USF) and cAMP response element binding protein (CREB). While we have not directly addressed this issue, both USF and CREB have been shown to interact directly with HIF to regulate promoter activity (33, 34), providing the possibility that other transcriptional regulators contribute to hypoxia-mediated induction of gravin. It is also important to note that gravin has been shown to directly regulate HIF. Indeed, Choi et al. (20) showed in retinal microvascular cells that gravin enhances the interaction of the HIF-1α subunit to both pVHL and PHD2, thereby stabilizing the degradation of HIF in hypoxia. They further reported that gravin and VEGF were expressed reciprocally and that gravin regulated VEGF expression by promoting the association of HIF-1α with pVHL and PHD2 and inducing HIF instability. The differences between this previous study and the current one indicate that regulation of gravin expression is likely complex and may be dictated by multiple conditions including cell and tissue type. Despite this, the identification of consensus sites for HIF binding in the gravin promoter that regulate hypoxia-dependent gravin expression strongly supports a role for HIF in the regulation of gravin expression in vascular endothelium. It is possible, therefore, that this HIF-gravin axis could serve as a counterbalance in the regulation of endothelial HIF target genes.
PKA-dependent changes in permeability tracked with the expression of gravin. Indeed, loss- and gain-of-function studies in endothelium revealed that gravin is a critical control point for consolidated PKA signaling, as both adenosine- and β-adrenergic-mediated barrier regulation were strongly influenced by gravin expression. Previous studies have indicated that a significant target for PKA activation in endothelial junctional permeability is VASP, a 46-kDa actin binding protein that localizes to endothelial cell-cell junctions in response to PKA stimulation (5). Since both VASP and gravin bind PKA, it is likely that they complex to provide a signaling scaffold in the organization of the actin cytoskeleton. Such a mechanism highlights the multifunctional role of gravin in regulating junctional barriers. Kwon et al. (35) reported similar results in that gravin knockdown resulted in both loss of junctional proteins at intercellular junctions in cultured HUVEC monolayers and decreased vascular integrity and increased vessel leakage in a zebrafish model, but proposed a mechanism linking gravin expression to PAK2 and AF6 activity in the regulation of junctional permeability. You et al. (36, 37) also reported a role for gravin in junctional regulation, but in their studies linked gravin expression to cytokine mediated increase in endothelial permeability through a mechanism that involved gravin-PKC interactions. Further studies are necessary to understand more precisely how gravin regulates barrier function under different physiological situations, but it appears that gravin, through its interaction with multiple signaling partners, is likely to be a critical player in regulating barrier function in a context-dependent manner. Also notable is the observation that gravin controls the angiogenic phenotype, as measured by endothelial tube formation. Loss- and gain-of-function studies revealed that the expression of gravin serves as a “braking mechanism” for endothelial tube formation. Both hypoxia and overexpression of gravin attenuated the kinetics of this response, while gravin knockdown significantly enhanced endothelial tube formation. This observation is consistent with multiple studies that implicate increased PKA activity (i.e., consistent with gravin gain of function) with attenuated endothelial cell motility in vitro and angiogenesis in vivo (38). These findings are likely related to the influence of cAMP/PKA on αvβ3 integrin function (38). Moreover, given that gravin is commonly down-regulated in tumors (9), gravin-dependent regulation of the angiogenic response may provide, at least in part, a mechanism for increased angiogenesis associated with a number of tumor types.
In summary, several lines of evidence implicate HIF-regulated gravin in GPCR-mediated endothelial function. First, inflammation-associated changes in metabolism (e.g., hypoxia) at the vascular surface likely serve as a feed-forward mechanism to induce gravin as a means to promote PKA-mediated signaling. Second, such signals promote vascular integrity and diminish fluid loss through enhanced endothelial barrier function (e.g., via amplified PKA activation). Third, gravin likely serves to constrain angiogenesis as a control mechanism within the vasculature. Such findings may be critical to the dynamic reorganization of endothelial cell-cell junctions during inflammation and may serve as an endogenous fine-tuning mechanism of the vasculature under such conditions.
Acknowledgments
This work was supported by U.S. National Institutes of Health grants HL60569, DK50189, and DK095491 and by funding from the Crohn's and Colitis Foundation of America.
The authors declare no conflicts of interest. Author contributions: T.W. and S.P.C. designed the research, analyzed data, and wrote the paper; L.E.G. and C.F.M. designed the research and analyzed data; B.F., V.F.C., S.F.E., E.L.C., and M.S. performed experiments and analyzed data. B.D.G. designed the research, provided vital reagents, and analyzed data.
Footnotes
- AKAP
- protein kinase A anchoring protein
- cAMP
- cyclic AMP
- CREB
- cAMP response element binding protein
- DRB
- 5,6-dichlorobenzimidazole riboside
- GPCR
- G-protein-coupled receptor
- HIF
- hypoxia-inducible factor
- HMEC-1
- human microvascular endothelial cell line 1
- HRE
- HIF response element
- PHD
- prolyl hydroxylase
- PKA
- protein kinase A
- PVDF
- polyvinylidene difluoride
- USF
- upstream-stimulating factor
- VASP
- vasodilator stimulated phosphoprotein
REFERENCES
- 1. Kominsky D. J., Campbell E. L., Colgan S. P. (2010) Metabolic shifts in immunity and inflammation. J. Immunol. 184, 4062–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glover L. E., Colgan S. P. (2011) Hypoxia and metabolic factors that influence inflammatory bowel disease pathogenesis. Gastroenterology 140, 1748–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Semenza G. L. (2011) Oxygen sensing, homeostasis, and disease. NEJM 365, 537–547 [DOI] [PubMed] [Google Scholar]
- 4. Ochoa C. D., Stevens T. (2012) Studies on the cell biology of interendothelial cell gaps. Am. J. Physiol. Lung Cell. Mol. Physiol. 302, L275–L286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Comerford K. M., Lawrence D. W., Synnestvedt K., Levi B. P., Colgan S. P. (2002) Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 16, 583–585 [DOI] [PubMed] [Google Scholar]
- 6. Colgan S. P., Eltzschig H. K. (2012) Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu. Rev. Physiol. 74, 153–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Troger J., Moutty M. C., Skroblin P., Klussmann E. (2012) A-kinase anchoring proteins as potential drug targets. Br J. Pharmacol. 166, 420–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gordon T., Grove B., Loftus J. C., O'Toole T., McMillan R., Lindstrom J., Ginsberg M. H. (1992) Molecular cloning and preliminary characterization of a novel cytoplasmic antigen recognized by myasthenia gravis sera. J. Clin. Invest. 90, 992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gelman I. H. (2010) Emerging roles for SSeCKS/gravin/AKAP12 in the control of cell proliferation, cancer malignancy, and barriergenesis. Genes Cancer 1, 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gelman I. H. (2012) Suppression of tumor and metastasis progression through the scaffolding functions of SSeCKS/gravin/AKAP12. Cancer Metastasis Rev. 31, 493–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee H. S., Han J., Bai H. J., Kim K. W. (2009) Brain angiogenesis in developmental and pathological processes: regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 276, 4622–4635 [DOI] [PubMed] [Google Scholar]
- 12. Eltzschig H. K., Ibla J. C., Furuta G. T., Leonard M. O., Jacobson K. A., Enjyoji K., Robson S. C., Colgan S. P. (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 198, 783–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Furuta G. T., Turner J. R., Taylor C. T., Hershberg R. M., Comerford K. M., Narravula S., Podolsky D. K., Colgan S. P. (2001) Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J. Exp. Med. 193, 1027–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacManus C. F., Campbell E. L., Keely S., Burgess A., Kominsky D. J., Colgan S. P. (2011) Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. FASEB J. 25, 1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yan X., Walkiewicz M., Carlson J., Leiphon L., Grove B. (2009) Gravin dynamics regulates the subcellular distribution of PKA. Exp. Cell Res. 315, 1247–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kong T., Westerman K. A., Faigle M., Eltzschig H. K., Colgan S. P. (2006) HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 20, 2242–2250 [DOI] [PubMed] [Google Scholar]
- 17. Louis N. A., Hamilton K. E., Kong T., Colgan S. P. (2005) HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB J. 19, 950–959 [DOI] [PubMed] [Google Scholar]
- 18. Lennon P. F., Taylor C. T., Stahl G. L., Colgan S. P. (1998) Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J. Exp. Med. 188, 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kiosses W. B., Hood J., Yang S., Gerritsen M. E., Cheresh D. A., Alderson N., Schwartz M. A. (2002) A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ. Res. 90, 697–702 [DOI] [PubMed] [Google Scholar]
- 20. Choi Y. K., Kim J. H., Kim W. J., Lee H. Y., Park J. A., Lee S. W., Yoon D. K., Kim H. H., Chung H., Yu Y. S., Kim K. W. (2007) AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha. J. Neurosci. 27, 4472–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee S. W., Kim W. J., Choi Y. K., Song H. S., Son M. J., Gelman I. H., Kim Y. J., Kim K. W. (2003) SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat. Med. 9, 900–906 [DOI] [PubMed] [Google Scholar]
- 22. Zan L., Wu H., Jiang J., Zhao S., Song Y., Teng G., Li H., Jia Y., Zhou M., Zhang X., Qi J., Wang J. (2011) Temporal profile of Src, SSeCKS, and angiogenic factors after focal cerebral ischemia: correlations with angiogenesis and cerebral edema. Neurochem. Int. 58, 872–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y., Gao L., Gelman I. H. (2006) SSeCKS/gravin/AKAP12 attenuates expression of proliferative and angiogenic genes during suppression of v-Src-induced oncogenesis. BMC Cancer 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Su B., Zheng Q., Vaughan M. M., Bu Y., Gelman I. H. (2006) SSeCKS metastasis-suppressing activity in MatLyLu prostate cancer cells correlates with vascular endothelial growth factor inhibition. Cancer Res. 66, 5599–5607 [DOI] [PubMed] [Google Scholar]
- 25. Colgan S. P., Taylor C. T. (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 7, 281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clambey E. T., McNamee E. N., Westrich J. A., Glover L. E., Campbell E. L., Jedlicka P., de Zoeten E. F., Cambier J. C., Stenmark K. R., Colgan S. P., Eltzschig H. K. (2012) Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc. Natl. Acad. Sci. U. S. A. 109, E2784–E2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamashita R., Suzuki Y., Wakaguri H., Tsuitani K., Nakai K., Sugano S. (2006) DBTSS: DataBase of Human Transcription Start Sites, progress report 2007. Large-scale collection and characterization of promoters of human and mouse genes 5′-end SAGE for the analysis of transcriptional start sites. Nucleic Acids Res. 34, D86–D89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sheta E. A., Trout H., Gildea J. J., Harding M. A., Theodorescu D. (2001) Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene 20, 7624–7634 [DOI] [PubMed] [Google Scholar]
- 29. Feoktistov I., Ryzhov S., Zhong H., Goldstein A. E., Matafonov A., Zeng D., Biaggioni I. (2004) Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension 44, 649–654 [DOI] [PubMed] [Google Scholar]
- 30. Semenza G. L. (2011) Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb. Symp. Quant. Biol. 2011, 22. [DOI] [PubMed] [Google Scholar]
- 31. Semenza G. L. (1999) Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15, 551–578 [DOI] [PubMed] [Google Scholar]
- 32. Heinemeyer T., Wingender E., Reuter I., Hermjakob H., Kel A. E., Kel O. V., Ignatieva E. V., Ananko E. A., Podkolodnaya O. A., Kolpakov F. A., Podkolodny N. L., Kolchanov N. A. (1998) Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res. 26, 362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu J., Stiehl D. P., Setzer C., Wichmann D., Shinde D. A., Rehrauer H., Hradecky P., Gassmann M., Gorr T. A. (2011) Interaction of HIF and USF signaling pathways in human genes flanked by hypoxia-response elements and E-box palindromes. Mol. Cancer Res. 9, 1520–1536 [DOI] [PubMed] [Google Scholar]
- 34. Thamotharan S., Raychaudhuri N., Tomi M., Shin B. C., Devaskar S. U. (2013) Hypoxic adaptation engages the CBP/CREST-induced coactivator complex of CREB-HIF-1alpha in transactivating murine neuroblastic glucose transporter. Am. J. Physiol. Endocrinol. Metab. 304, E583–E598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwon H. B., Choi Y. K., Lim J. J., Kwon S. H., Her S., Kim H. J., Lim K. J., Ahn J. C., Kim Y. M., Bae M. K., Park J. A., Jeong C. H., Mochizuki N., Kim K. W. (2012) AKAP12 regulates vascular integrity in zebrafish. Exp. Mol. Med. 44, 225–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. You Q. H., Sun G. Y., Wang N., Chen S., Luo Q. L. (2010) Role of src-suppressed C kinase substrate in rat pulmonary microvascular endothelial hyperpermeability stimulated by inflammatory cytokines. Inflamm. Res. 59, 949–958 [DOI] [PubMed] [Google Scholar]
- 37. You Q. H., Sun G. Y., Wang N., Shen J. L., Wang Y. (2010) Interleukin-17F-induced pulmonary microvascular endothelial monolayer hyperpermeability via the protein kinase C pathway. J. Surg. Res. 162, 110–121 [DOI] [PubMed] [Google Scholar]
- 38. Dormond O., Ruegg C. (2003) Regulation of endothelial cell integrin function and angiogenesis by COX-2, cAMP and protein kinase A. Thromb. Haemost. 90, 577–585 [DOI] [PubMed] [Google Scholar]